Magnetic Porous Hydrogel-Enhanced Wearable Patch Sensor for Sweat Zinc Ion Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of the Fe3O4 Nanoparticles
2.4. Preparation of Multifunctional Hydrogels
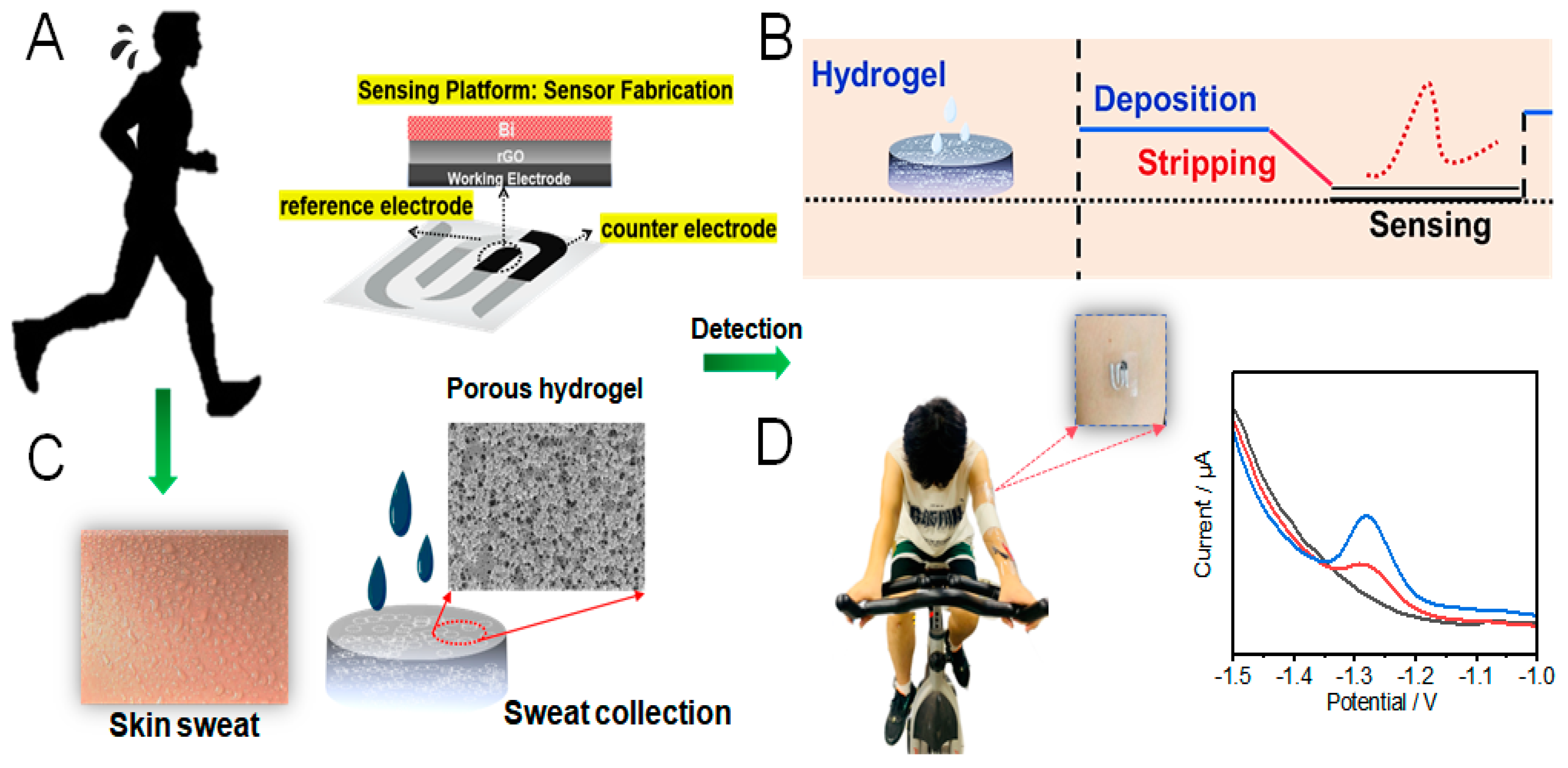
2.5. Preparation of Electrochemical Sensing Platform
2.6. On-Body Test of Sweat Zn2+ by Hydrogel-Based Flexible Patch Sensor
3. Results and Discussion
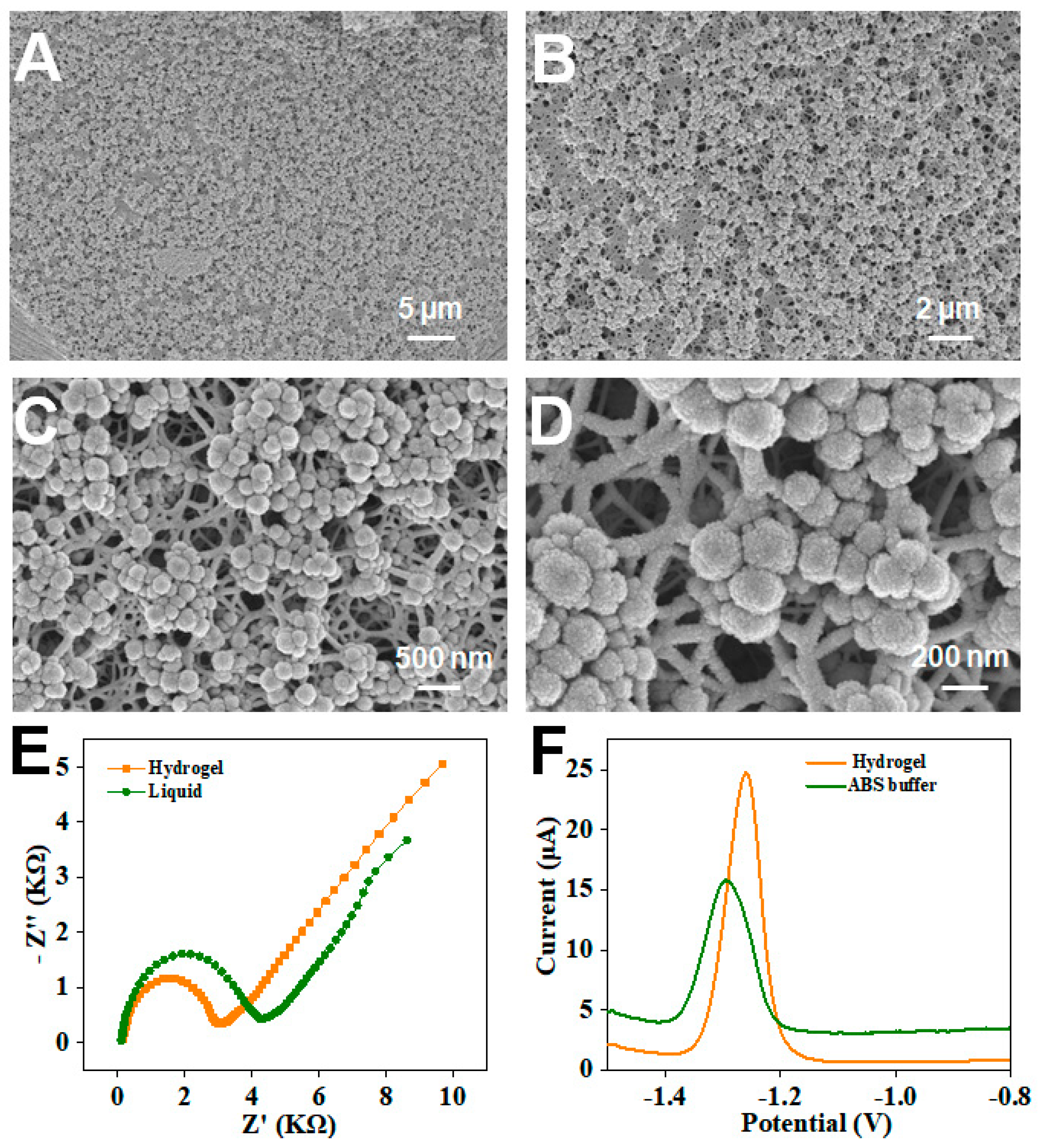
3.1. Uniform Structural Analysis of Magnetic Porous Hydrogel
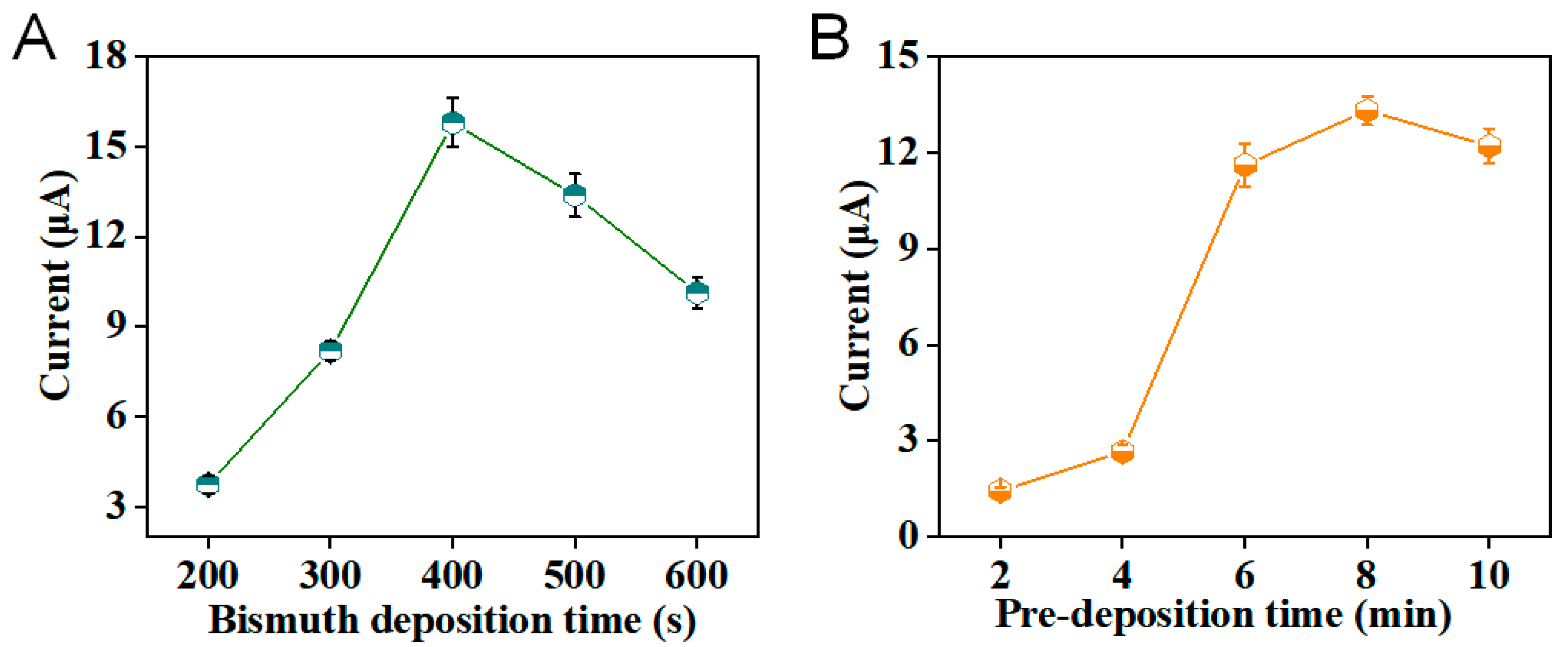
3.2. Optimization of rGO/Bi Film Sensing Platform
3.3. rGO/Bi Film Flexible Patch Sensor for Zn2+ Sensing
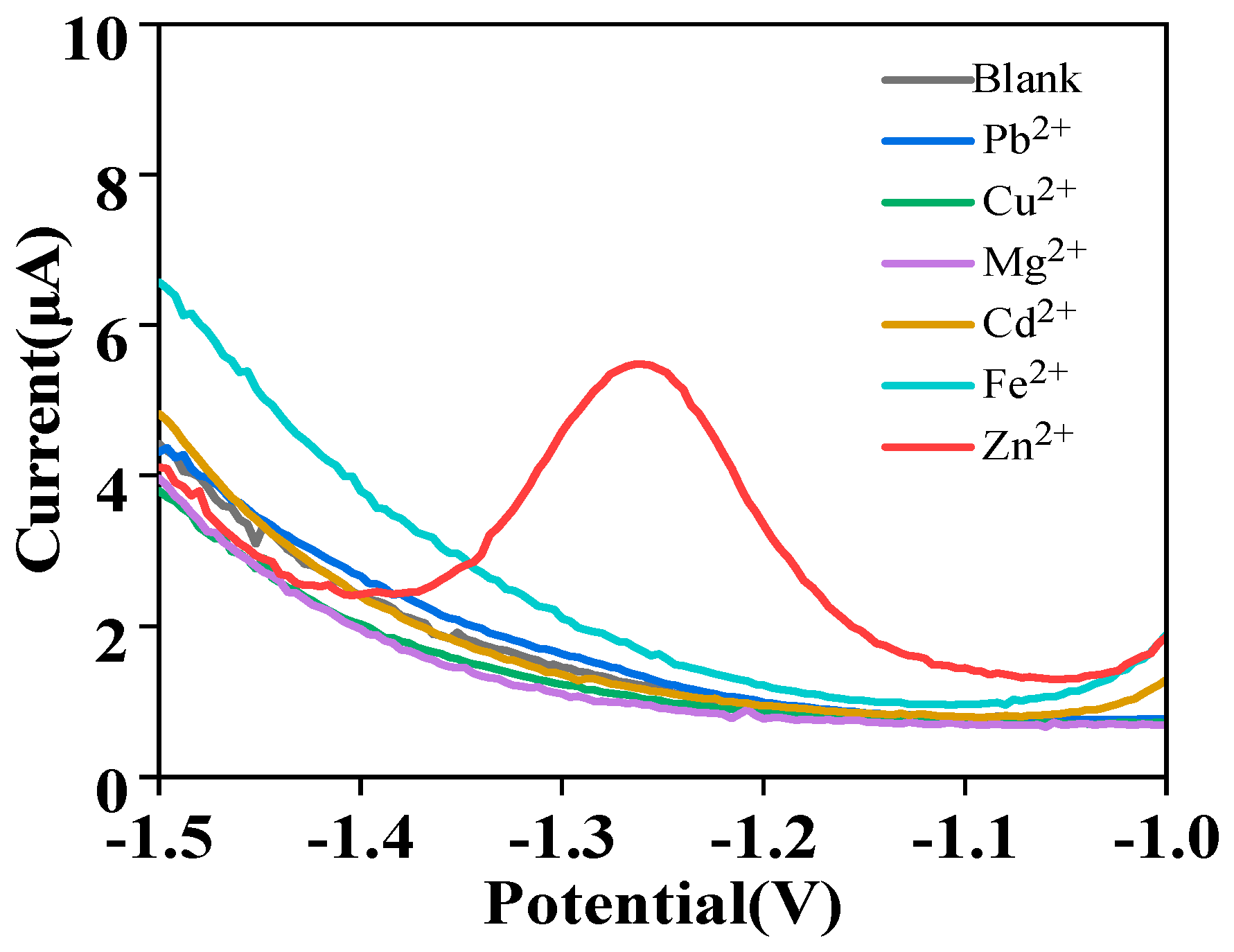
3.4. Interference
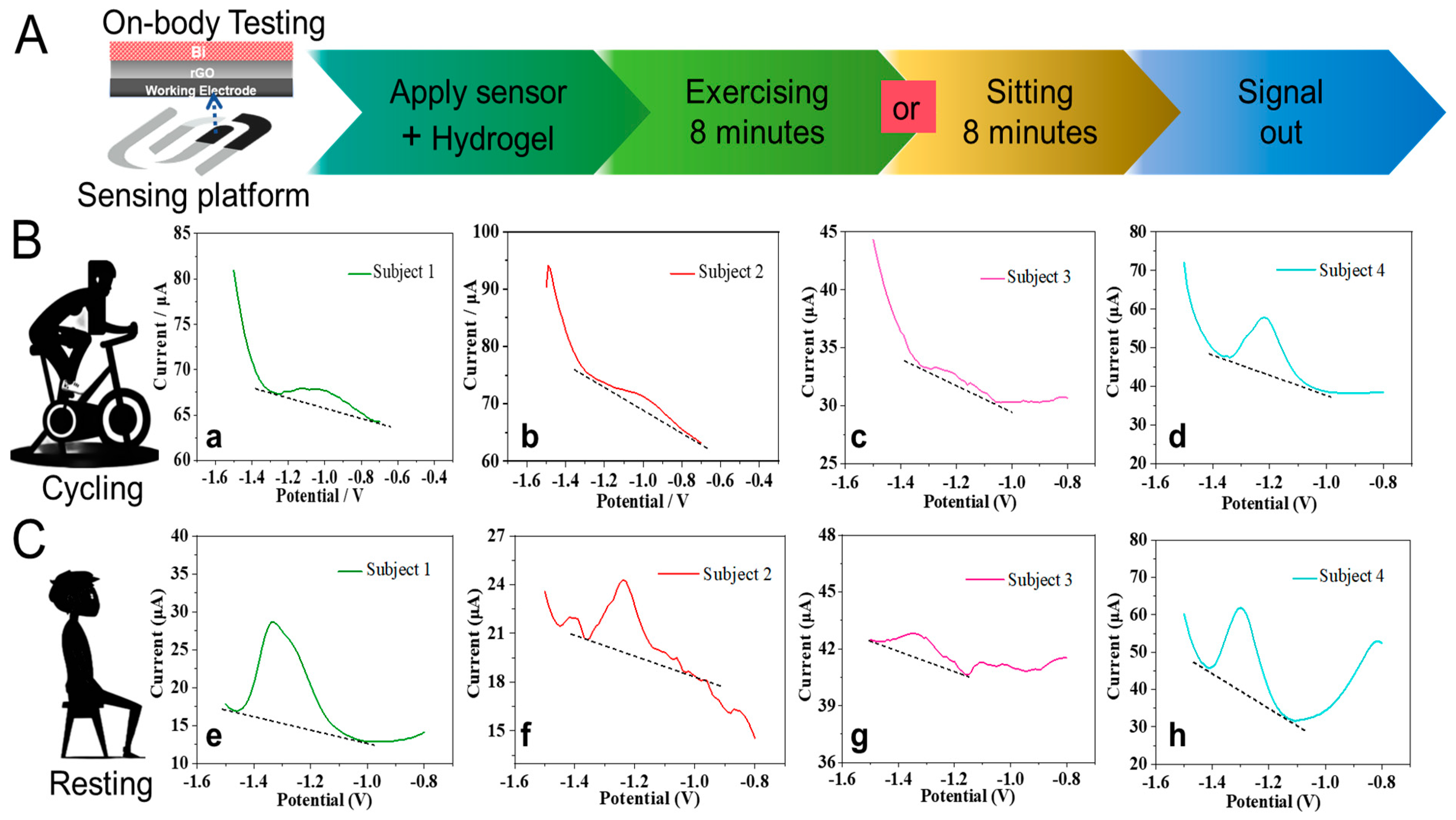
3.5. On-Body Characterization of Flexible Zn2+ Patch Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Fang, Y.S.; Chen, J. Wearable biosensors for non-invasive sweat diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, W.; Lee, C.H. Electrochemically active materials and wearable biosensors for the in-situ analysis of body fluids for human healthcare. NPG Asia Mater. 2021, 13, 22. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.J.; Min, J.H.; Shun Pak, O.; Zhu, L.L.; Wang, M.Q.; Tu, J.B.; Kogan, A.; Zhang, H.X.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E.; Kerr, K.J.; Bray, R.I. Arsenic, cadmium, lead, and mercury in sweat: A systematic review. J. Environ. Public Health 2012, 2012, 184745. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, P.; Rahman, M.I.; Perumal, A.; Nallaiyan, R.; Basha, S.H.; Dakshanamoorthy, A. Non-invasive, non-enzymatic, biodegradable and flexible sweat glucose sensor and its electrochemical studies. ChemistrySelect 2020, 5, 11305–11321. [Google Scholar] [CrossRef]
- Omokhodion, F.O.; Howard, J.M. Trace elements in the sweat of acclimatized persons. Clin. Chim. Acta 1994, 231, 23–28. [Google Scholar] [CrossRef]
- Stauber, J.L.; and Florence, T.M. A comparative study of copper, lead, cadmium and zinc in human sweat and blood. Sci. Total Environ. 1988, 74, 235–247. [Google Scholar] [CrossRef]
- Deng, X.; Li, W.; Wang, Y.; Ding, G. Recognition and separation of enantiomers based on functionalized magnetic nanomaterials. TrAC Trends Anal. Chem. 2020, 124, 115804. [Google Scholar] [CrossRef]
- Kim, J.; de Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixao, T.R.L.C.; Wang, J. Wearable Temporary Tattoo Sensor for Real-Time Trace Metal Monitoring in Human Sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.J.; Tai, L.C.; Ota, H.; Wu, E.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef]
- Tang, W.X.; Yin, L.; Sempionatto, J.R.; Moon, J.M.; Teymourian, H.; Wang, J. Touch-Based Stressless Cortisol Sensing. Adv. Mater. 2021, 33, 2008465. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Moon, J.M.; Wang, J. Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for Predicting Blood Glucose Concentrations. ACS Sens. 2021, 6, 1875–1883. [Google Scholar] [CrossRef]
- Crew, A.; Cowell, D.C.; Hart, J.P. Development of an anodic stripping voltammetric assay, using a disposable mercury-free screen-printed carbon electrode, for the determination of zinc in human sweat. Talanta 2008, 75, 1221–1226. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef]
- Chen, S.H.; Yu, J.G.; Chen, Z.; Huang, Z.; Song, Y.H. Simultaneous electrochemical sensing of heavy metal ions based on a g-C3N4/CNT/NH2-MIL-88(Fe) nanocomposite. Anal. Methods 2021, 13, 5830. [Google Scholar] [CrossRef]
- Tang, W.; Gu, Z.; Chu, Y.; Lv, J.; Fan, L.; Liu, X.; Wang, F.; Ying, Y.; Zhang, J.; Jiang, Y.; et al. Magnetically-oriented porous hydrogel advances wearable electrochemical solidoid sensing heavy metallic ions. Chem. Eng. J. 2023, 453, 139902. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef]
- Dai, C.F.; Khoruzhenko, O.; Zhang, C.; Zhu, Q.L.; Jiao, D.; Du, M.; Breu, J.; Zhao, P.; Zheng, Q.; Wu, Z.L. Magneto-Orientation of Magnetic Double Stacks for Patterned Anisotropic Hydrogels with Multiple Responses and Modulable Motions. Angew. Chem. Int. Ed. 2022, 61, e202207272. [Google Scholar] [CrossRef]
- Sergeeva, A.; Feoktistova, N.; Prokopovic, V.; Gorin, D.; and Volodkin, D. Design of Porous Alginate Hydrogels by Sacrificial CaCO3 Templates: Pore Formation Mechanism. Adv. Mater. Interfaces 2015, 2, 1500386. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.A.; and Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Yin Nyein, H.Y.; Bariya, M.; Tran, B.; Heera Ahn, C.; Janatpour Brown, B.; Ji, W.B.; Davis1, N.; Javey, A. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Mukherjee, S.; Dickey, M.D.; Velev, O.D. Harvesting and manipulating sweat and interstitial fluid in microfluidic devices. Lab Chip 2024, 24, 1244–1265. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sheu, S.C.; Chen, C.W.; Huang, S.C.; Li, B.R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Wang, L.Q.; Zhou, Z.M.; Niu, J.G.; Peng, J.Y.; Wang, T.; Hou, X.H. Emerging innovations in portable chemical sensing devices: Advancements from microneedles to hydrogel, microfluidic, and paper-based platforms. Talanta 2024, 278, 126412. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; LvZeng, Z.; Lu, K.; Chen, Y.; Shen, Y.; Jing, K.; Yang, H.; Tang, W. Magnetic Porous Hydrogel-Enhanced Wearable Patch Sensor for Sweat Zinc Ion Monitoring. Sensors 2024, 24, 5627. https://doi.org/10.3390/s24175627
Chu Y, LvZeng Z, Lu K, Chen Y, Shen Y, Jing K, Yang H, Tang W. Magnetic Porous Hydrogel-Enhanced Wearable Patch Sensor for Sweat Zinc Ion Monitoring. Sensors. 2024; 24(17):5627. https://doi.org/10.3390/s24175627
Chicago/Turabian StyleChu, Yao, Zhengzhong LvZeng, Kaijie Lu, Yangyang Chen, Yichuan Shen, Kejia Jing, Haifeng Yang, and Wanxin Tang. 2024. "Magnetic Porous Hydrogel-Enhanced Wearable Patch Sensor for Sweat Zinc Ion Monitoring" Sensors 24, no. 17: 5627. https://doi.org/10.3390/s24175627
APA StyleChu, Y., LvZeng, Z., Lu, K., Chen, Y., Shen, Y., Jing, K., Yang, H., & Tang, W. (2024). Magnetic Porous Hydrogel-Enhanced Wearable Patch Sensor for Sweat Zinc Ion Monitoring. Sensors, 24(17), 5627. https://doi.org/10.3390/s24175627






