ZnO Hexagonal Nano- and Microplates Modified with Nanomaterials as a Gas-Sensitive Material for DMS Detection—Extended Studies
Abstract
:1. Introduction
2. Materials and Methods
3. Results
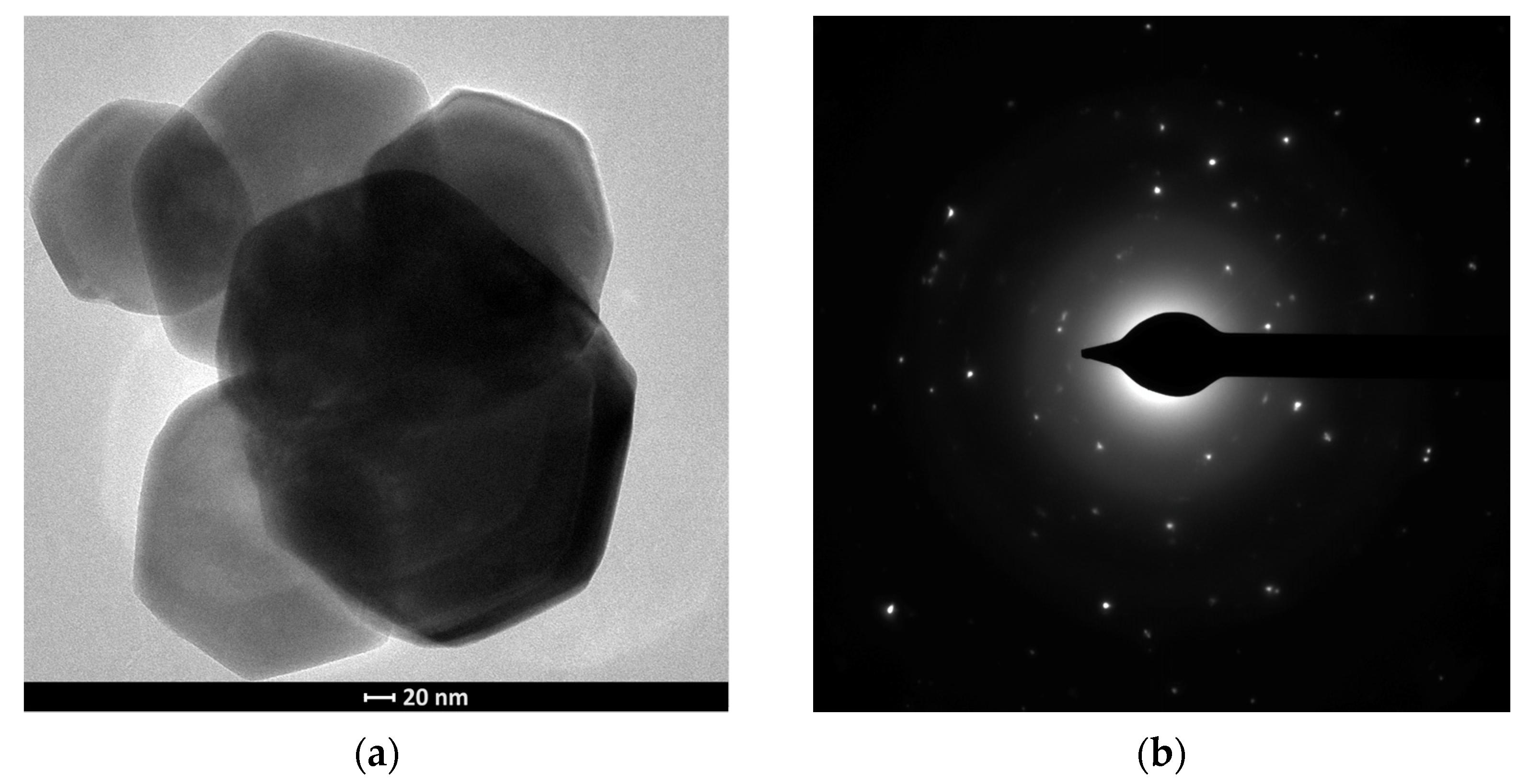
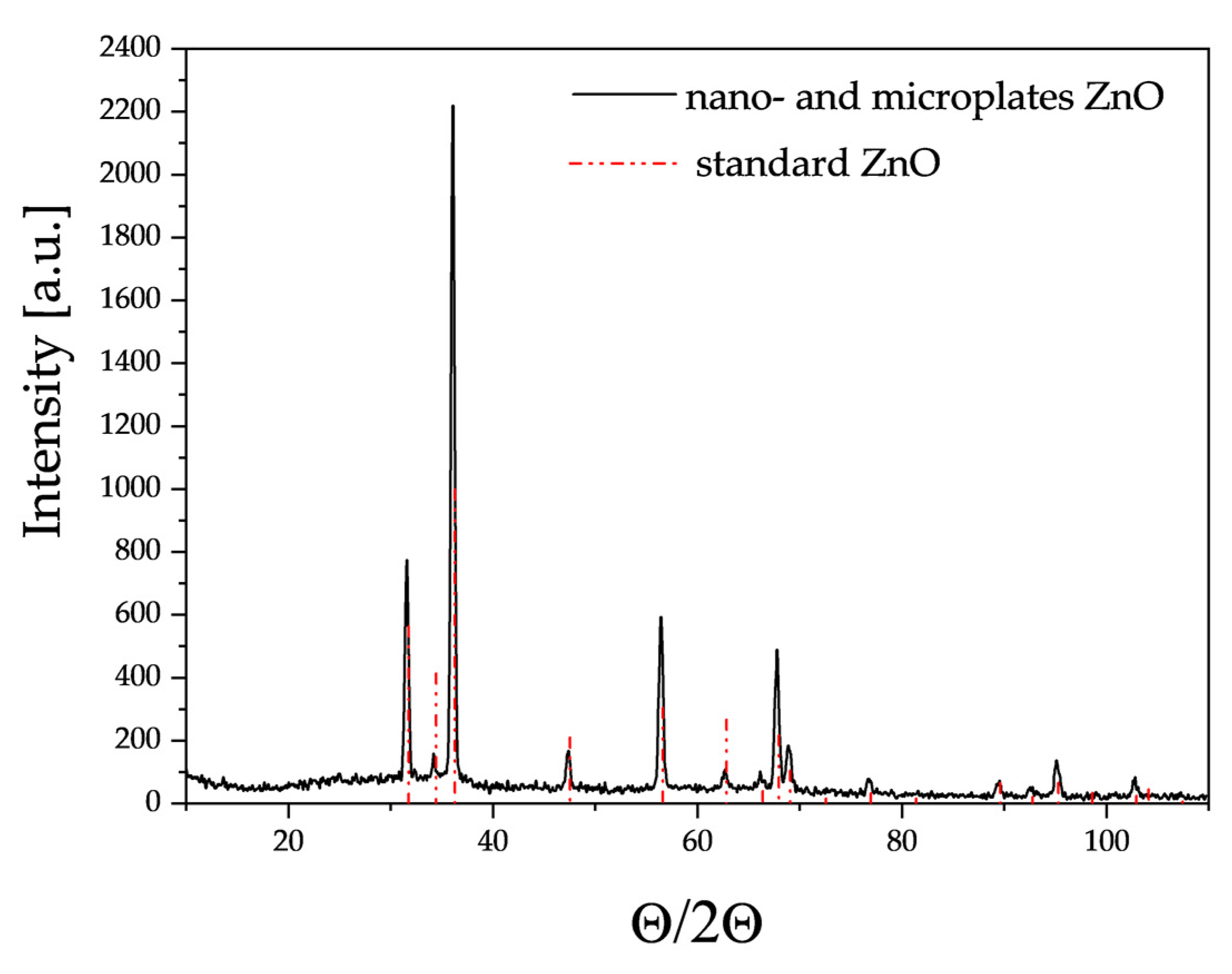
3.1. Characterisation of Materials
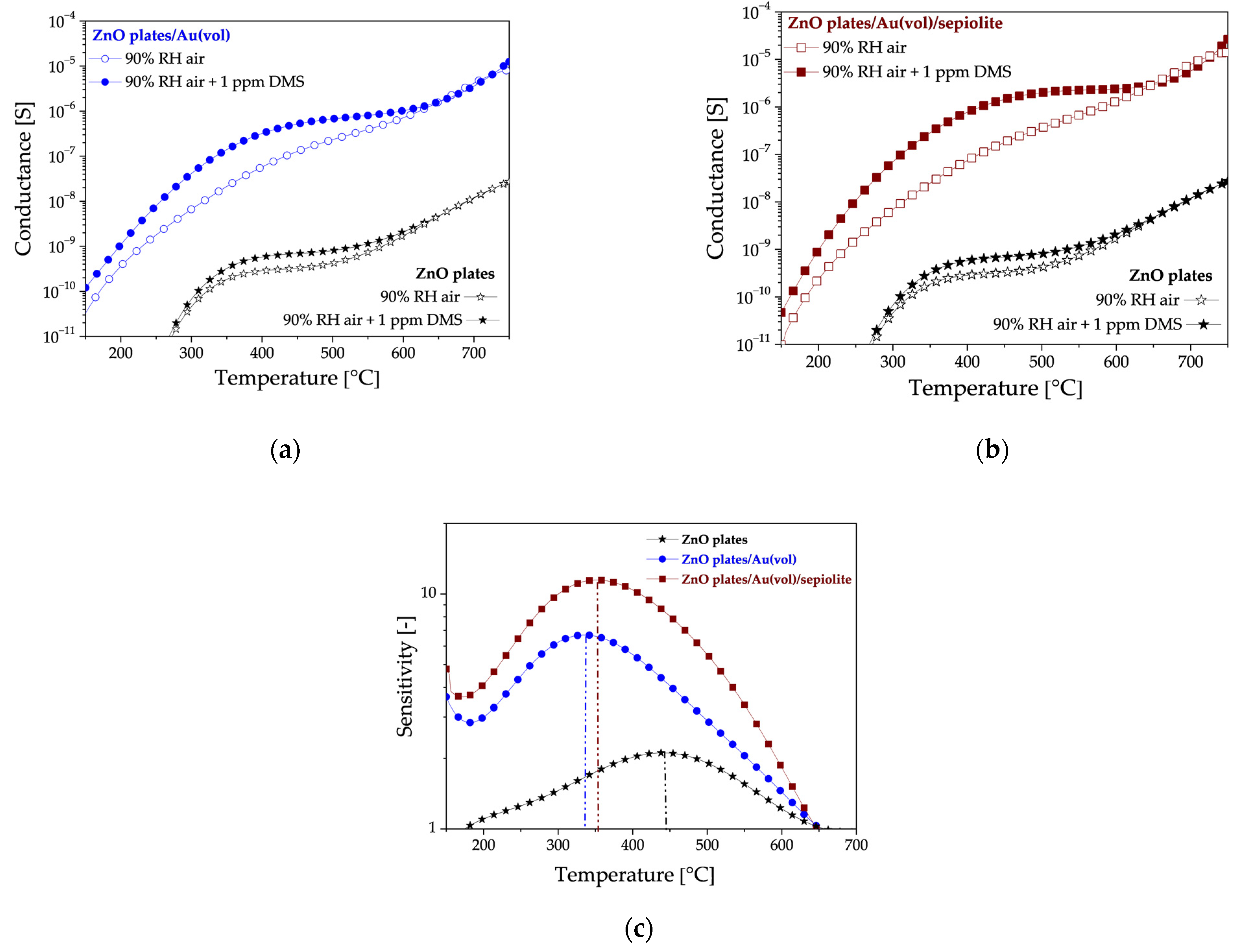
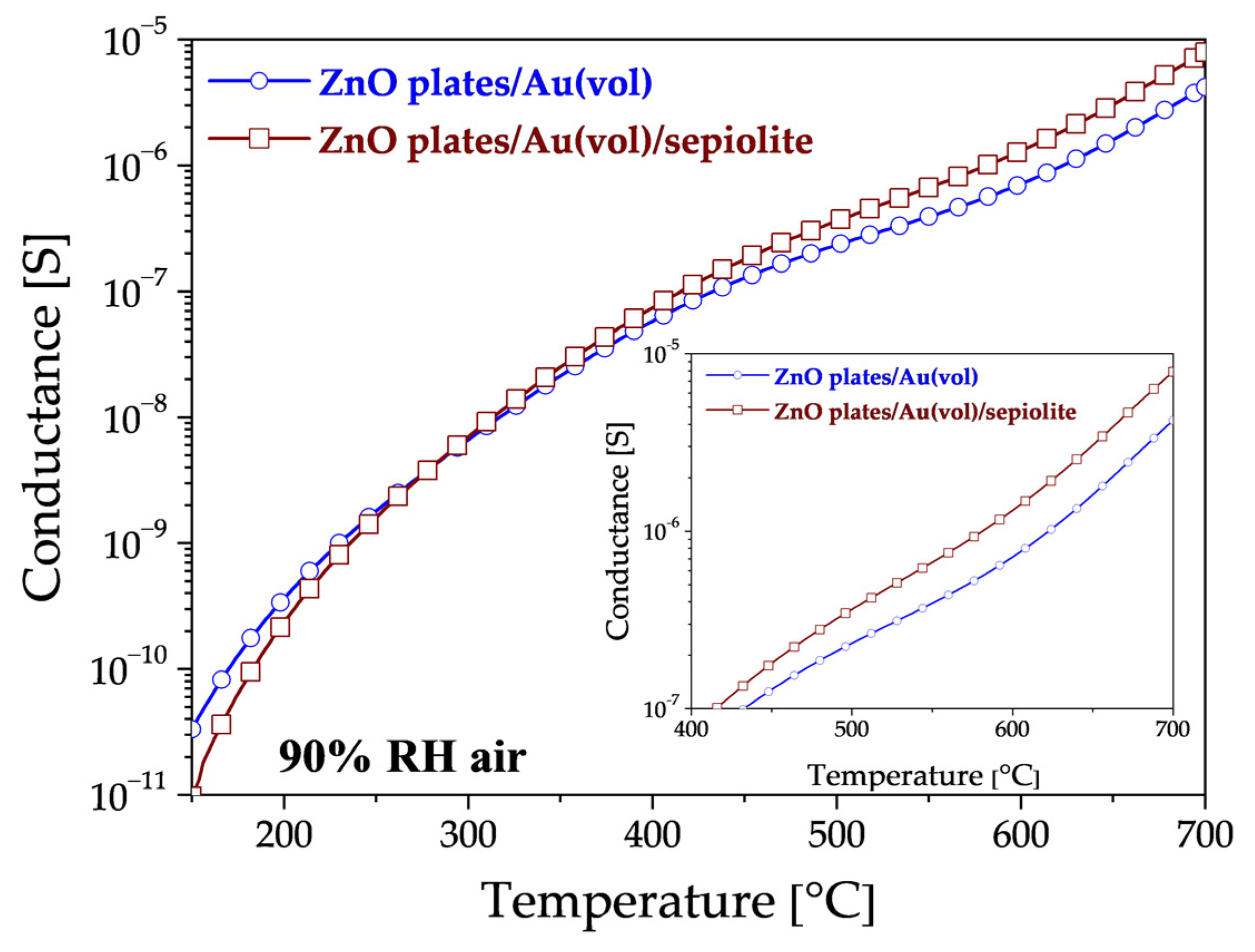
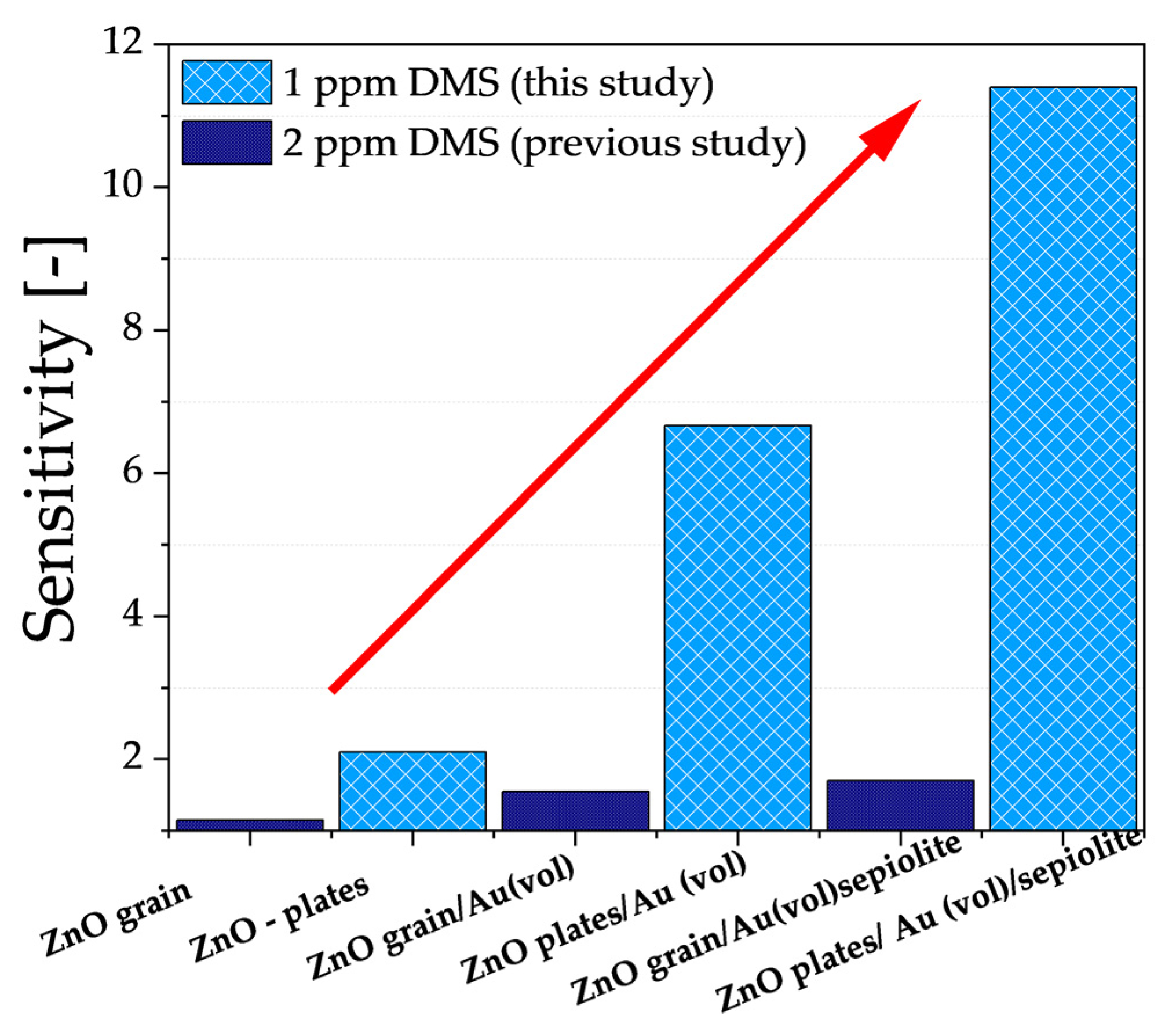
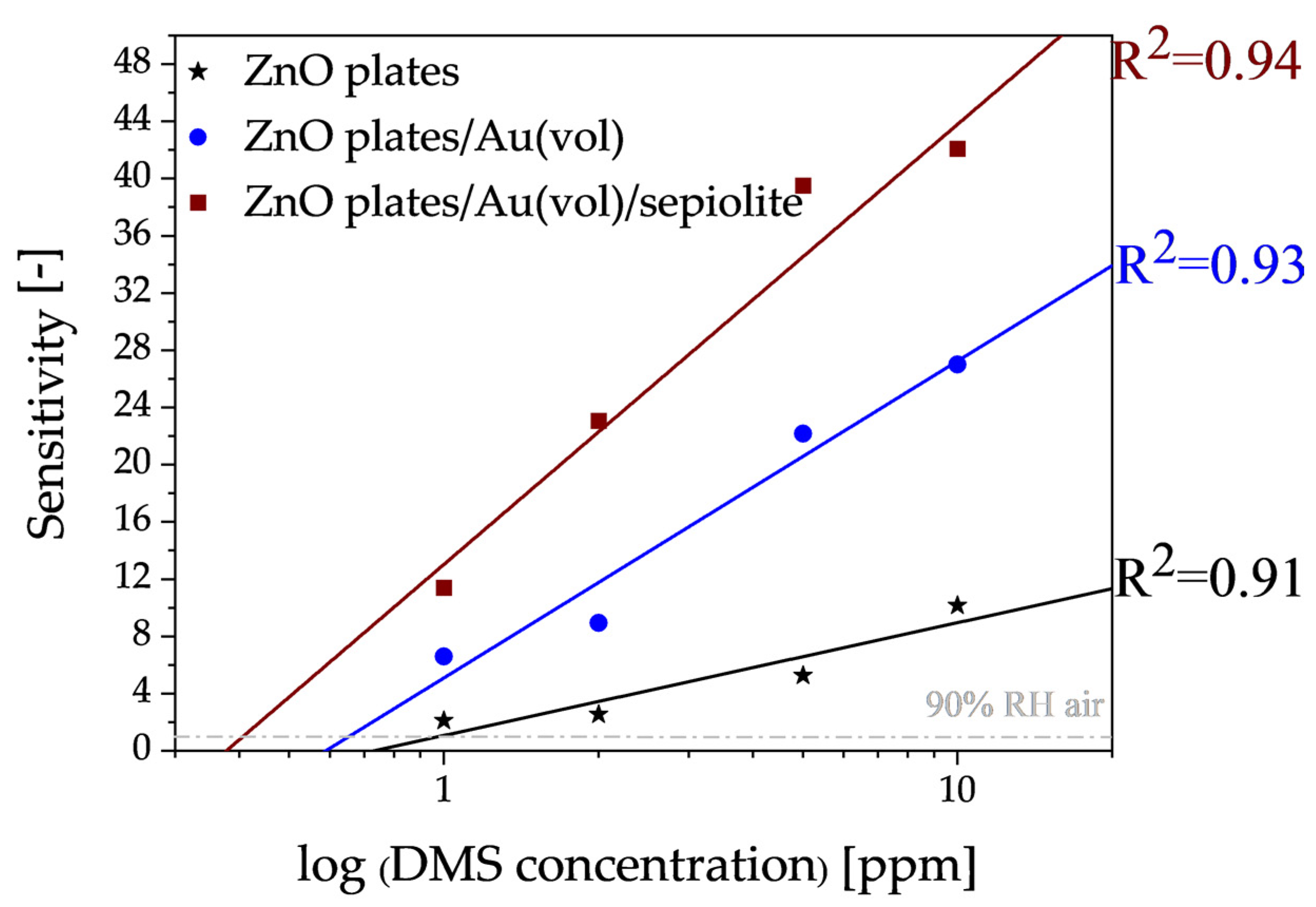
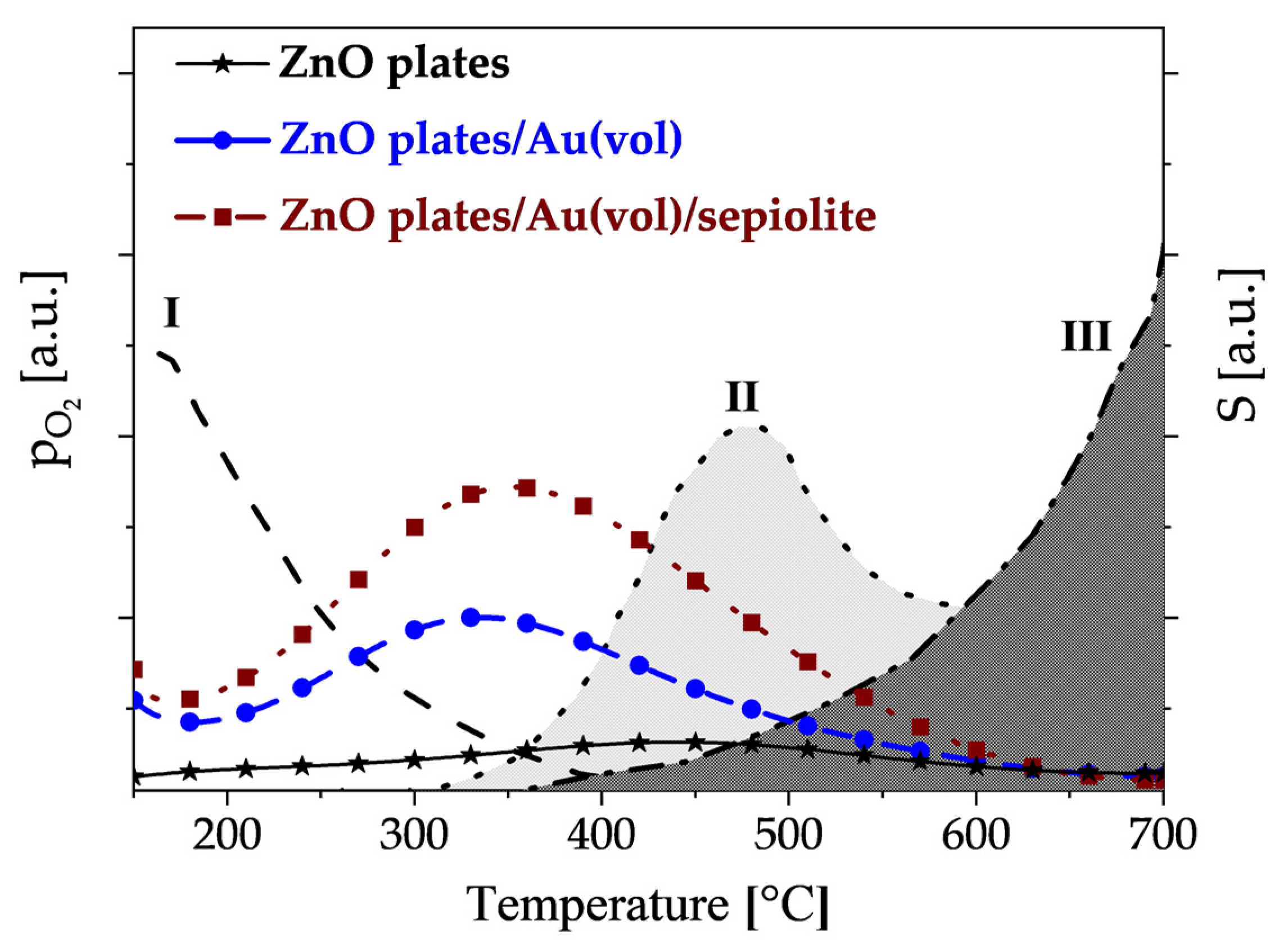
3.2. Electrical Characterisation
- A gas-sensitive layer in the form of hexagonal ZnO nano- and microplates (ZnO plates);
- A gas-sensitive layer in the form of hexagonal ZnO nano- and microplates doped with 0.75 wt.% gold nanoparticles (ZnO plates/Au(vol));
- A gas-sensitive layer in the form of hexagonal ZnO nano- and microplates doped with 0.75 wt.% gold nanoparticles, additionally coated with a sepiolite filter (ZnO plates/Au(vol)/sepiolite).
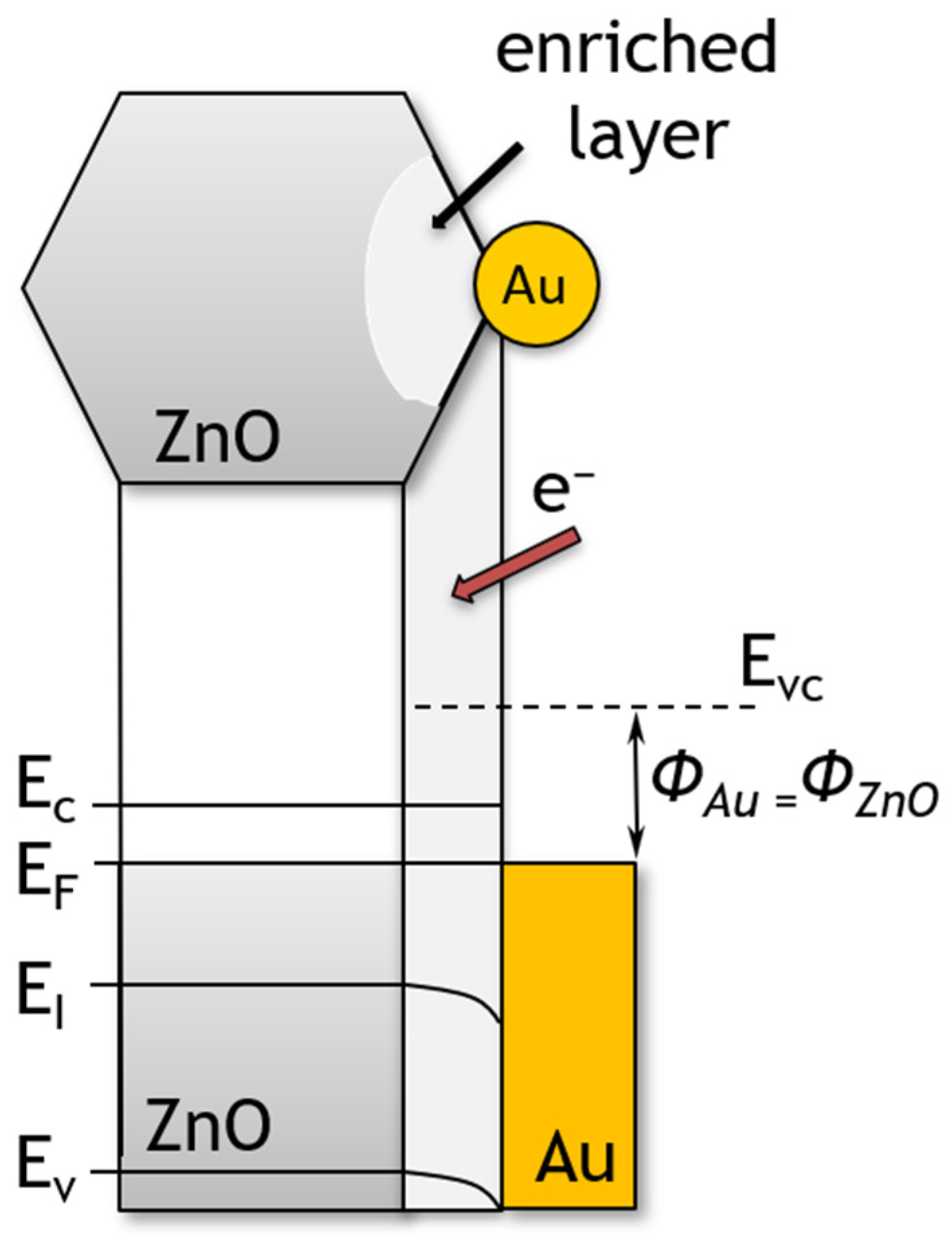
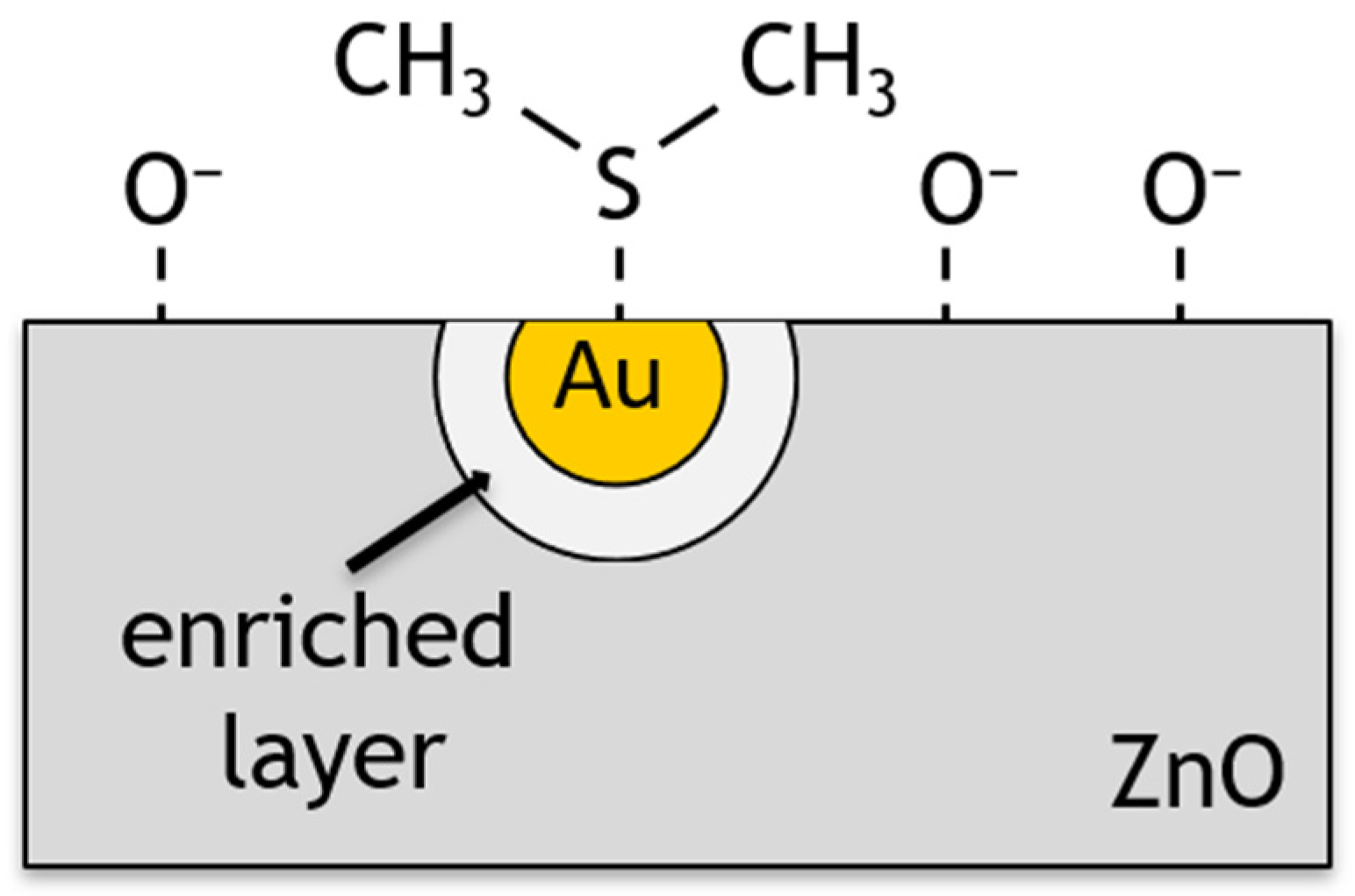
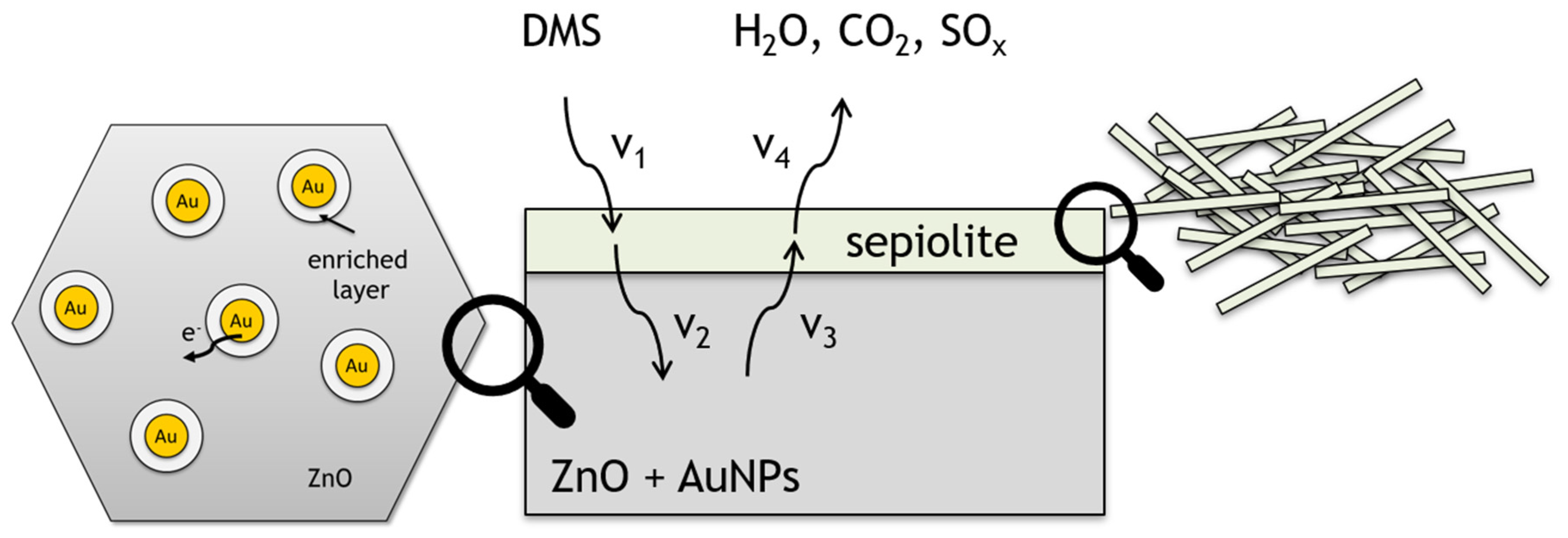
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matrai, P.A.; Keller, M.D. Dimethylsulfide in a large-scale coccolithophore bloom in the Gulf of Maine. Cont. Shelf Res. 1993, 13, 831–843. [Google Scholar] [CrossRef]
- Andreae, M.O.; Merlet, P. Emission of trace gases and aerosols from biomass burning. Glob. Biogeochem. Cycles 2001, 15, 955–966. [Google Scholar] [CrossRef]
- McGorrin, R.J. The significance of volatile sulfur compounds in food flavors. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 3–31. ISBN 9780841226166. [Google Scholar]
- Santos, J.P.; Lozano, J.; Aleixandre, M. Electronic noses applications in beer technology. In Brewing Technology; InTech: Vienna, Austria, 2017; ISBN 978-953-51-3342-1. [Google Scholar]
- Tangerman, A. Halitosis in medicine: A review. Int. Dent. J. 2002, 52, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Avincsal, M.O.; Altundag, A.; Ulusoy, S.; Dinc, M.E.; Dalgic, A.; Topak, M. Halitosis associated volatile sulphur compound levels in patients with laryngopharyngeal reflux. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1515–1520. [Google Scholar] [CrossRef]
- Bicak, D.A. A current approach to halitosis and oral malodor—A mini review. Open Dent. J. 2018, 12, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ding, Q.; Shi, F.; Han, Q.; Li, C.; Dong, B.; Xu, L.; Wang, L.; Seung Kim, J. Bio-sniffers for biomarkers of oral diseases in exhaled breath: State of art and future trends. Coord. Chem. Rev. 2024, 501, 215574. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Zhou, Y.; Wang, J.; You, R. Research progress of electronic nose technology in exhaled breath disease analysis. Microsyst. Nanoeng. 2023, 9, 129. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for enhanced detection of acetone as a potential tool for noninvasive diabetes monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef]
- Lewis, A.C.; Bartle, K.D.; Rattner, L. High-speed isothermal analysis of atmospheric isoprene and DMS using on-line two-dimensional gas chromatography. Environ. Sci. Technol. 1997, 31, 3209–3217. [Google Scholar] [CrossRef]
- Persson, C.; Leck, C. Determination of reduced sulfur compounds in the atmosphere using a cotton scrubber for oxidant removal and gas chromatography with flame photometric detection. Anal. Chem. 1994, 66, 983–987. [Google Scholar] [CrossRef]
- Lindinger, W.; Jordan, A. Proton-transfer-reaction mass spectrometry (PTR–MS): On-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 1998, 27, 347. [Google Scholar] [CrossRef]
- Blomquist, B.W.; Huebert, B.J.; Fairall, C.W.; Faloona, I.C. Determining the sea-air flux of dimethylsulfide by eddy correlation using mass spectrometry. Atmos. Meas. Tech. 2010, 3, 1–20. [Google Scholar] [CrossRef]
- Li, J.; Luo, G.; Du, Z.; Ma, Y. Hollow waveguide enhanced dimethyl sulfide sensor based on a 3.3 μm interband cascade laser. Sens. Actuators B Chem. 2018, 255, 3550–3557. [Google Scholar] [CrossRef]
- Iyadomi, S.; Ezoe, K.; Ohira, S.-I.; Toda, K. Monitoring variations of dimethyl sulfide and dimethylsulfoniopropionate in seawater and the atmosphere based on sequential vapor generation and ion molecule reaction mass spectrometry. Environ. Sci. Process. Impacts 2016, 18, 464–472. [Google Scholar] [CrossRef]
- Saito, H.; Hashimoto, Y.; Minamide, T.; Kon, T.; Toma, K.; Arakawa, T.; Mitsubayashi, K. Fiber optic biosniffer (biochemical gas sensor) for gaseous dimethyl sulfide. Sens. Mater. 2016, 28, 1295–1301. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Cheng, Y.; Yang, X.; Yu, K.; Li, J.; Du, X.; Tang, X.; Jiang, Y. Detection of volatile organosulfur compounds by hydrogen-bond acidic polymer: A combined experimental and theoretical study. Mater. Lett. 2019, 237, 282–285. [Google Scholar] [CrossRef]
- Kumar, P.; Mohanty, S.K.; Guruswamy, S.; Smith, Y.R.; Misra, M. Detection of food decay products using functionalized one-dimensional titania nanotubular arrays. IEEE Sens. Lett. 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Gao, L.; Duan, G. Co3O4 nanosheet-built hollow spheres containing ultrafine neck-connected grains templated by PS@Co-LDH and their ppb-level gas-sensing performance. Sens. Actuators B Chem. 2018, 261, 553–565. [Google Scholar] [CrossRef]
- Suchorska-Woźniak, P.; Nawrot, W.; Rac, O.; Fiedot, M.; Teterycz, H. Improving the sensitivity of the ZnO gas sensor to dimethyl sulfide. IOP Conf. Ser. Mater. Sci. Eng. 2016, 104, 012030. [Google Scholar] [CrossRef]
- Maeda, I.; Yamashiro, H.; Yoshioka, D.; Onodera, M.; Ueda, S.; Kawase, M.; Miyasaka, H.; Yagi, K. Colorimetric dimethyl sulfide sensor using Rhodovulum sulfidophilum cells based on intrinsic pigment conversion by CrtA. Appl. Microbiol. Biotechnol. 2006, 70, 397–402. [Google Scholar] [CrossRef]
- Jang, J.S.; Lim, Y.W.; Kim, D.H.; Lee, D.; Koo, W.T.; Lee, H.; Bae, B.S.; Kim, I.D. Glass-Fabric Reinforced Ag Nanowire/Siloxane Composite Heater Substrate: Sub-10 nm Metal@Metal Oxide Nanosheet for Sensitive Flexible Sensing Platform. Small 2018, 14, 1802260. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, Z.; Yuan, L.; Ma, Y.; Wang, X.; Han, R.; Meng, S. Measurement of atmospheric dimethyl sulfide with a distributed feedback interband cascade laser. Sensors 2018, 18, 3216. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing technologies for detection of acetone in human breath for diabetes diagnosis and monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath sensors for health monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef]
- Ashori, E.; Nazari, F.; Illas, F. Adsorption of H2S on carbonaceous materials of different dimensionality. Int. J. Hydrog. Energy 2014, 39, 6610–6619. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for breath testing: From nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef]
- Krawczyk, M.; Korbutowicz, R.; Szukiewicz, R.; Suchorska-Woźniak, P.; Kuchowicz, M.; Teterycz, H. P-type inversion at the surface of β-Ga2O3 epitaxial layer modified with Au nanoparticles. Sensors 2022, 22, 932. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Suchorska-Woźniak, P.; Rac, O.; Klimkiewicz, R.; Fiedot, M.; Teterycz, H. Dehydrogenation properties of ZnO and the impact of gold nanoparticles on the process. Appl. Catal. A Gen. 2016, 514, 135–145. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Galstyan, V.; Poli, N.; Comini, E. Highly sensitive and selective H2S chemical sensor based on ZnO nanomaterial. Appl. Sci. 2019, 9, 1167. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured gas sensors for medical and health applications: Low to high dimensional materials. Biosensors 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Suchorska-Woźniak, P.; Rac, O.; Fiedot, M.; Teterycz, H. The impact of sepiolite on sensor parameters during the detection of low concentrations of alcohols. Sensors 2016, 16, 1881. [Google Scholar] [CrossRef] [PubMed]
- Licznerski, B.W.; Nitsch, K.; Teterycz, H.; Sobański, T.; Wiśniewski, K. Characterisation of electrical parameters for multilayer SnO2 gas sensors. Sens. Actuators B Chem. 2004, 103, 69–75. [Google Scholar] [CrossRef]
- Vilaseca, M.; Coronas, J.; Cirera, A.; Cornet, A.; Morante, J.R.; Santamaria, J. Gas detection with SnO2 sensors modified by zeolite films. Sens. Actuators B Chem. 2007, 124, 99–110. [Google Scholar] [CrossRef]
- Alkan, M.; Benlikaya, R. Poly(vinyl alcohol) nanocomposites with sepiolite and heat-treated sepiolites. J. Appl. Polym. Sci. 2009, 112, 3764–3774. [Google Scholar] [CrossRef]
- Reynolds, R.C. Interstratified clay minerals. In Crystal Structures of Clay Minerals and Their X-ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society: London, UK, 1984; pp. 249–303. [Google Scholar]
- Barnes, I.; Hjorth, J.; Mihalopoulos, N. Dimethyl sulfide and dimethyl sulfoxide and their oxidation in the atmosphere. Chem. Rev. 2006, 106, 940–975. [Google Scholar] [CrossRef]
- Hagemark, K.I. Defect structure of Zn-doped ZnO. J. Solid State Chem. 1976, 16, 293–299. [Google Scholar] [CrossRef]
- Sukkar, M.H.; Johnson, K.H.; Tuller, H.L. Defects and electronic structure of interfaces in ZnO: Cluster molecular orbital calculations. Mater. Sci. Eng. B 1990, 6, 49–59. [Google Scholar] [CrossRef]
- Göpel, W. Reactions of oxygen with ZnO–101̄0-surfaces. J. Vac. Sci. Technol. 1978, 15, 1298–1310. [Google Scholar] [CrossRef]
- Iden, H.; de Faria, R.A.D.; Heneine, L.G.D.; Matencio, T.; Messaddeq, Y. A novel impedimetric sensor for trace level detection of dimethyl sulfide (DMS). J. Mater. Sci. Mater. Electron. 2020, 31, 10398–10407. [Google Scholar] [CrossRef]
- Martinelli, G.; Carotta, M.C. Influence of additives on the sensing properties of screen-printed SnO2 gas sensors. Sens. Actuators B Chem. 1993, 16, 363–366. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Shen, T.; Zhao, Z.; Feng, H.; Cui, F. One-step synthesis of noble metal/oxide nanocomposites with tunable size of noble metal particles and their size-dependent catalytic activity. RSC Adv. 2014, 4, 30624–30629. [Google Scholar] [CrossRef]
- Kapse, V.D.; Ghosh, S.A.; Chaudhari, G.N.; Raghuwanshi, F.C.; Gulwade, D.D. H2S sensing properties of La-doped nanocrystalline In2O3. Vacuum 2008, 83, 346–352. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Gulina, L.B.; Cho, B.K. The influence of gold nanoparticles on the conductivity response of SnO2-based thin film gas sensors. Appl. Surf. Sci. 2015, 353, 793–803. [Google Scholar] [CrossRef]
- Wei, M.; Li, C.F.; Deng, X.R.; Deng, H. Surface Work Function of Transparent Conductive ZnO Films. Energy Procedia 2012, 16, 76–80. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Chakravadhanula, V.S.K.; Hrkac, V.; Jebril, S.; Agarwal, D.C.; Mohapatra, S.; Avasthi, D.K.; Kienle, L.; Adelung, R. Crystal growth behaviour in Au-ZnO nanocomposite under different annealing environments and photoswitchability. J. Appl. Phys. 2012, 112, 820242. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, D.; Song, Z.; Shan, C.; Zhang, Z.; Li, B.; Shen, D. Gold nanoparticles modified ZnO nanorods with improved photocatalytic activity. J. Colloid Interface Sci. 2011, 363, 175–181. [Google Scholar] [CrossRef]
- Rivière, J.C. The work function of gold. Appl. Phys. Lett. 1966, 8, 172. [Google Scholar] [CrossRef]
- Alosfur, F.K.M.; Ridha, N.J. Synthesis and characterization of ZnO/SnO2 nanorods core–shell arrays for high performance gas sensors. Appl. Phys. A 2021, 127, 203. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Du, Z.; Yu, H.; Xiang, Y.; Li, Y.; Cai, Y.; Wang, T. Rapid and ultrahigh ethanol sensing based on Au-coated ZnO nanorods. Nanotechnology 2008, 19, 035501. [Google Scholar] [CrossRef] [PubMed]
- Ramgir, N.S.; Sharma, P.K.; Datta, N.; Kaur, M.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Room temperature H2S sensor based on Au modified ZnO nanowires. Sens. Actuators B Chem. 2013, 186, 718–726. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, J.-H.; Kim, S.-H.; Kim, S.S. Dual functional sensing mechanism in SnO2 –ZnO core–shell nanowires. ACS Appl. Mater. Interfaces 2014, 6, 8281–8287. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchorska-Woźniak, P.; Teterycz, H. ZnO Hexagonal Nano- and Microplates Modified with Nanomaterials as a Gas-Sensitive Material for DMS Detection—Extended Studies. Sensors 2024, 24, 5690. https://doi.org/10.3390/s24175690
Suchorska-Woźniak P, Teterycz H. ZnO Hexagonal Nano- and Microplates Modified with Nanomaterials as a Gas-Sensitive Material for DMS Detection—Extended Studies. Sensors. 2024; 24(17):5690. https://doi.org/10.3390/s24175690
Chicago/Turabian StyleSuchorska-Woźniak, Patrycja, and Helena Teterycz. 2024. "ZnO Hexagonal Nano- and Microplates Modified with Nanomaterials as a Gas-Sensitive Material for DMS Detection—Extended Studies" Sensors 24, no. 17: 5690. https://doi.org/10.3390/s24175690





