Pd Nanoparticles Loaded on Cu Nanoplate Sensor for Ultrasensitive Detection of Dopamine
Abstract
:1. Introduction
2. Experimental Method
2.1. Reagents
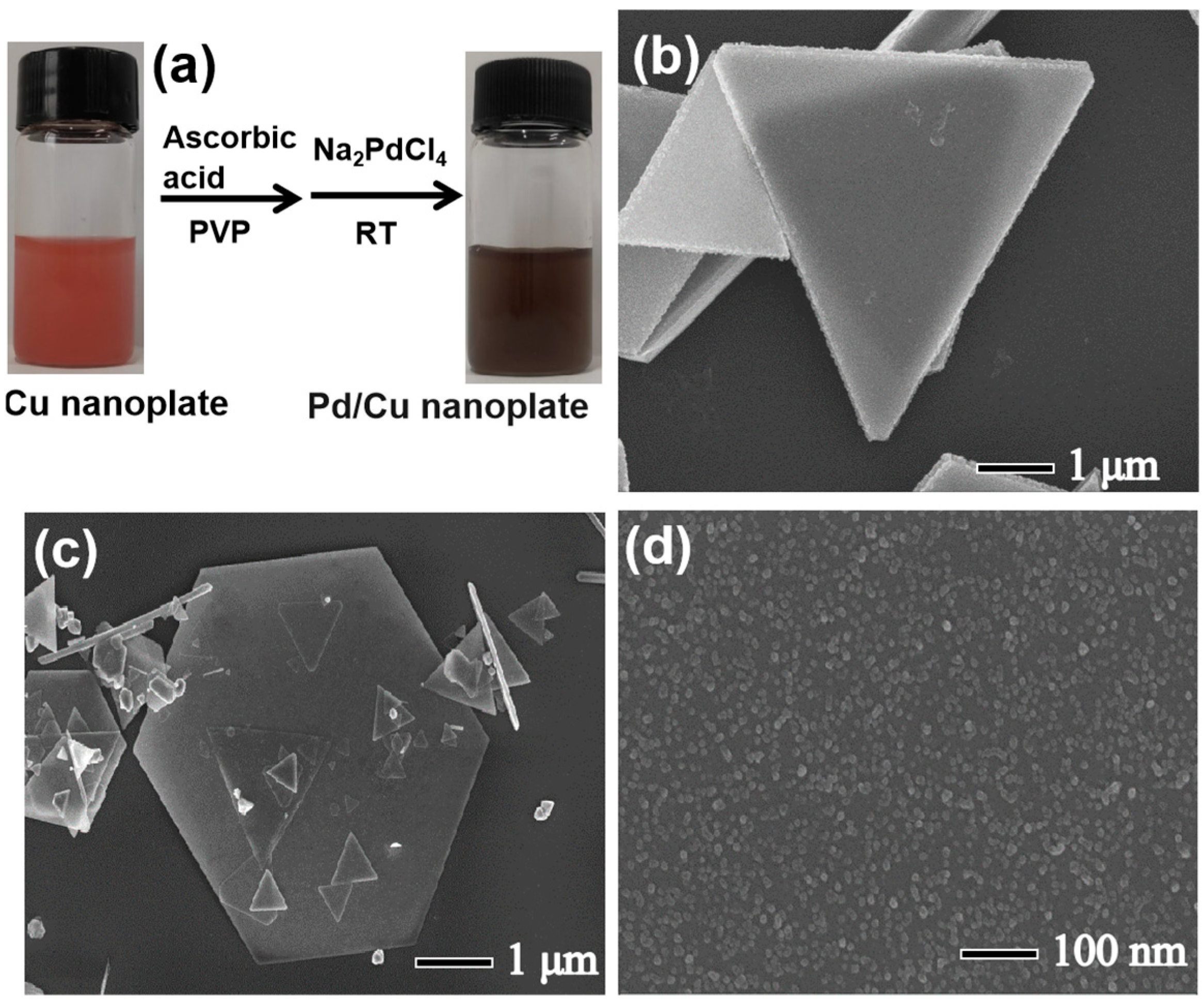
2.2. Synthesis of Pd/Cu NPTs Nanoplates
2.3. Electrochemical Detection of DA
2.4. Material Characterization
3. Results and Discussion
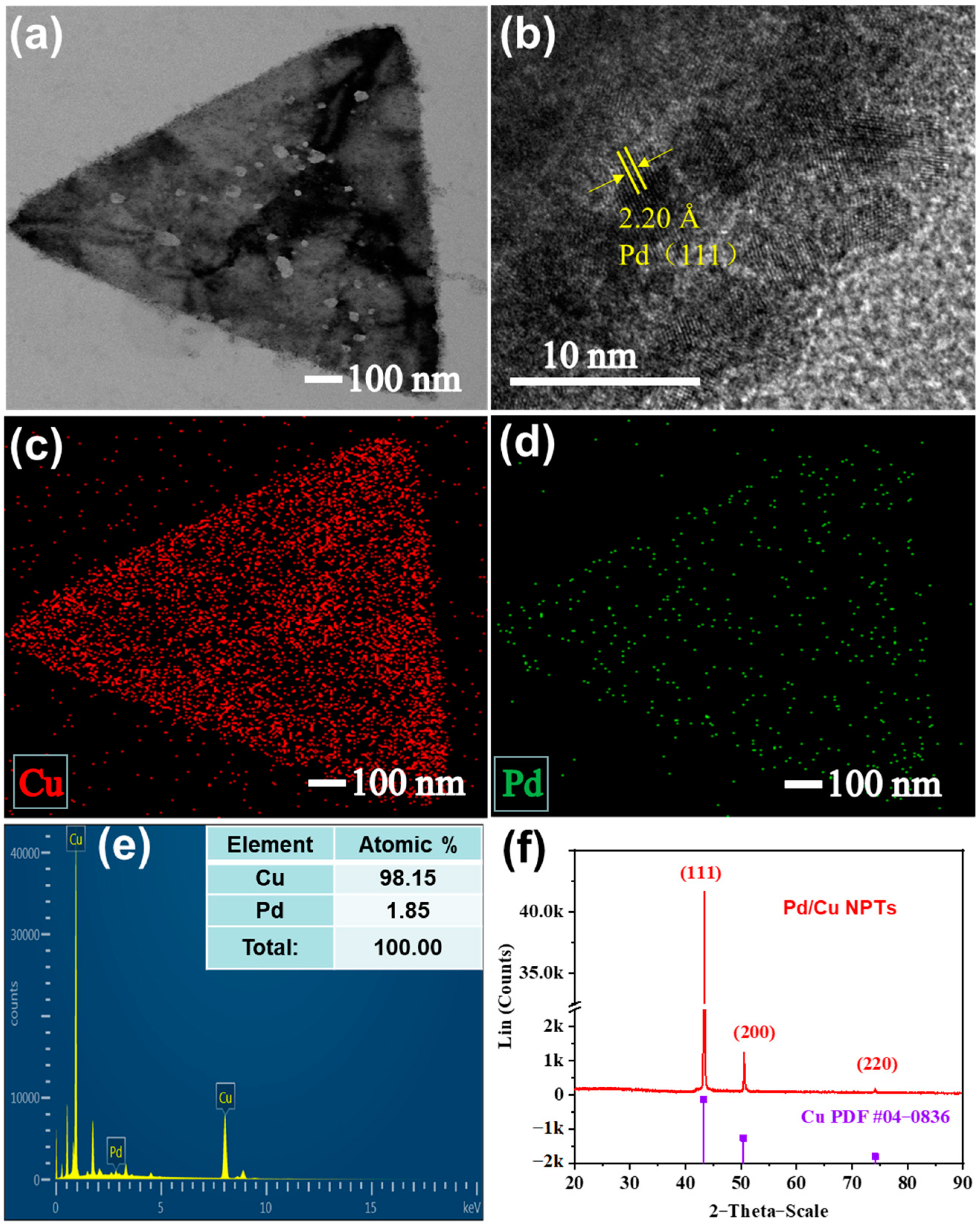
3.1. Preparation and Characterization of Pd/Cu NPTs
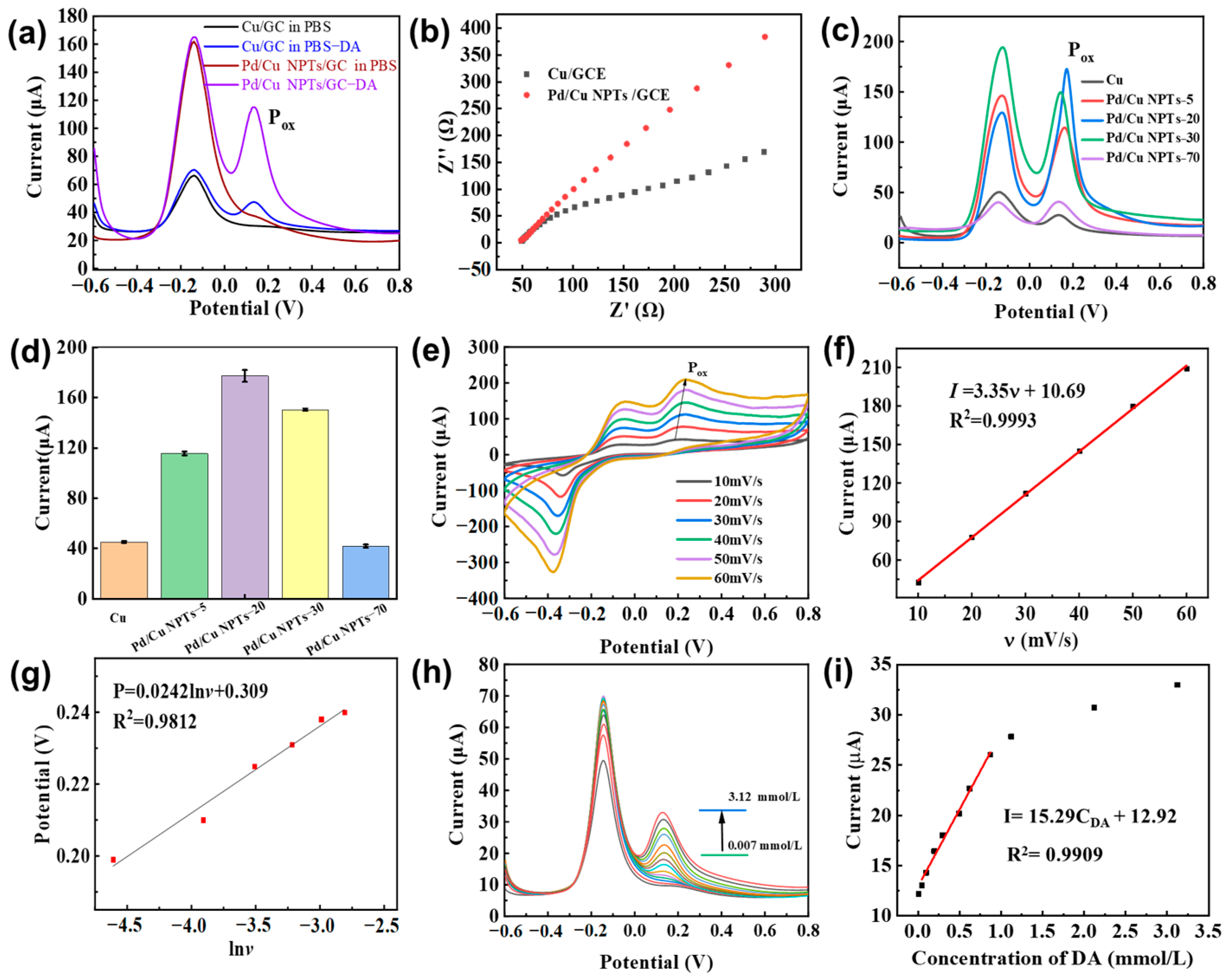
3.2. Electrochemical Sensing Properties of DA on Pd/Cu NPTs/GCE Electrode
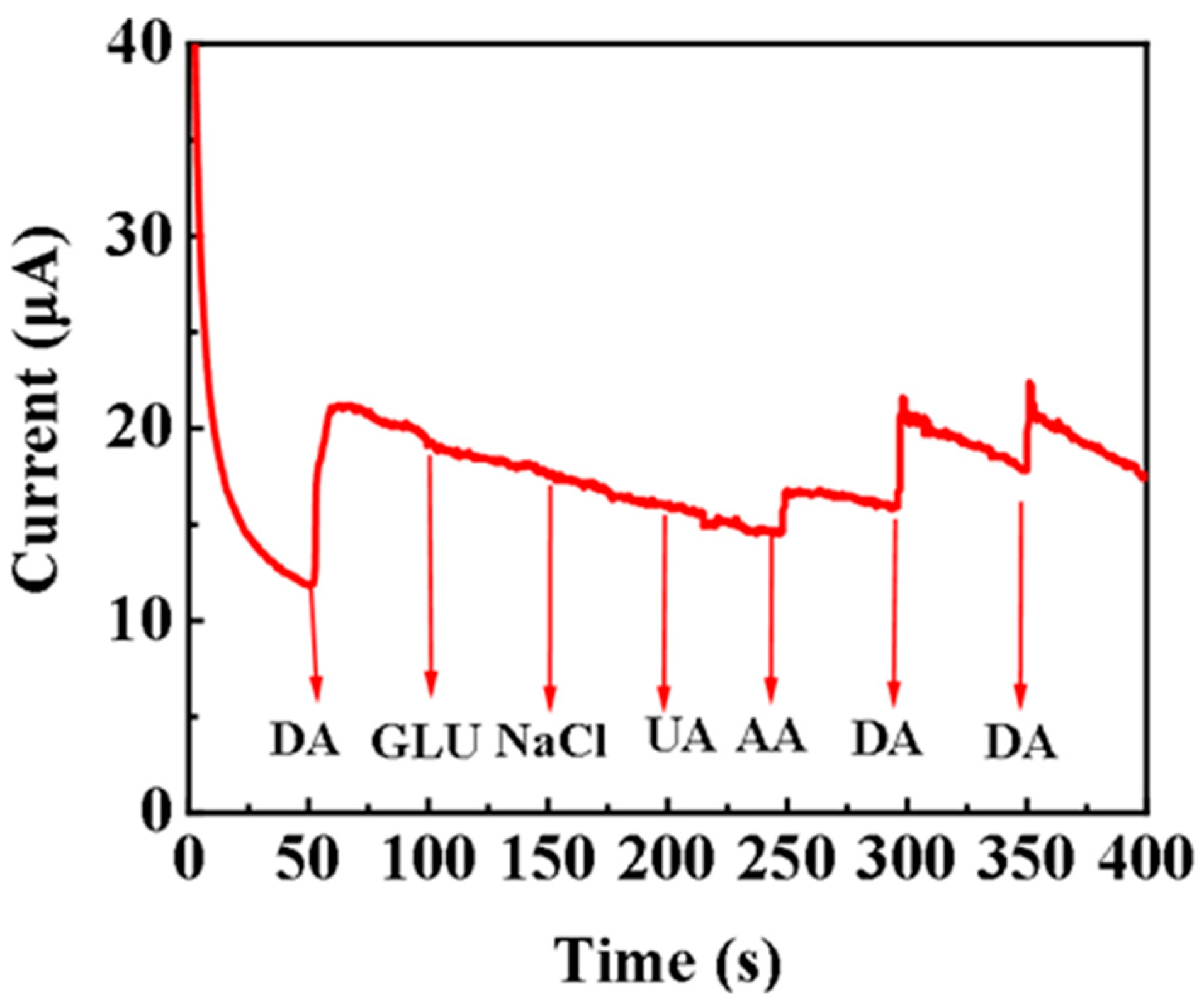
3.3. Anti-Interference Ability of Pd/Cu NPTs/GC Electrode
3.4. Repeatability and Stability of Pd/Cu NPT Electrode
3.5. Detection in Human Serum Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karim, A.; Yasser, M.; Ahmad, A.; Natsir, H.; Wahid Wahab, A.; Fauziah, S.; Taba, P.; Pratama, I.; Rosalin; Rajab, A.; et al. A review: Progress and trend advantage of dopamine electrochemical sensor. J. Electroanal. Chem. 2024, 959, 118157. [Google Scholar] [CrossRef]
- Burns, G.; Ali, M.Y.; Howlader, M.M.R. Advanced functional materials for electrochemical dopamine sensors. TrAC Trends Anal. Chem. 2023, 169, 117367. [Google Scholar] [CrossRef]
- Cogal, S. A review of poly (3,4-ethylenedioxythiophene) and its composites-based electrochemical sensors for dopamine detection. Polym.-Plast. Technol. Mater. 2021, 60, 345–357. [Google Scholar] [CrossRef]
- Siddeeg, S.M. Electrochemical detection of neurotransmitter dopamine. Int. J. Electrochem. Sci. 2020, 15, 599–612. [Google Scholar] [CrossRef]
- Suhito, I.R.; Angeline, N.; Kim, T.H. Nanomaterial-modified hybrid platforms for precise electrochemical detection of dopamine. BioChip J. 2019, 13, 20–29. [Google Scholar] [CrossRef]
- Aghanavesi, S.; Memedi, M.; Dougherty, M.; Nyholm, D.; Westin, J. Verification of a method for measuring Parkinson’s disease related temporal irregularity in spiral drawings. Sensors 2017, 17, 2341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sheng, M.; Jiang, X.; Wu, G.; Gao, F. Simultaneous determination of dopamine, serotonin and ascorbic acid at a glassy carbon electrode modified with carbon-spheres. Sensors 2013, 13, 14029–14040. [Google Scholar] [CrossRef]
- Ali, S.R.; Parajuli, R.R.; Balogun, Y.; Ma, Y.; He, H. A nonoxidative electrochemical sensor based on a self-doped polyaniline/carbon nanotube composite for sensitive and selective detection of the neurotransmitter dopamine: A Review. Sensors 2008, 8, 8423–8452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, S.; Huang, J.; Ye, F. Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis. Talanta 2011, 85, 2650–2654. [Google Scholar] [CrossRef]
- Yang, D.; Ran, J.; Yi, H.; Feng, P.; Liu, B. A homogeneous colorimetric strategy based on rose-like CuS@prussian blue/Pt for detection of dopamine. Sensors 2023, 23, 9029. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Ying, Y.; Wu, J. Detection of dopamine based on aptamer-modified graphene microelectrode. Sensors 2024, 24, 2934. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Yuan, F.; Fereja, T.H.; Wang, C.; Lou, B.; Li, J.; Xu, G. Chemiluminescence of lucigenin/riboflavin and its application for selective and sensitive dopamine detection. Anal. Chem. 2019, 91, 2135–2139. [Google Scholar] [CrossRef]
- Soni, R.; Palit, K.; Soni, M.; Kumar, R.; Sharma, S.K. Highly sensitive electrochemical sensing of neurotransmitter dopamine from scalable UV irradiation-based nitrogen-doped reduced graphene oxide-modified electrode. Bull. Mater. Sci. 2020, 43, 175. [Google Scholar] [CrossRef]
- Feng, S.; Yu, L.; Yan, M.; Ye, J.; Huang, J.; Yang, X. Holey nitrogen-doped graphene aerogel for simultaneously electrochemical determination of ascorbic acid, dopamine and uric acid. Talanta 2021, 224, 121851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ding, D.; Wang, J.; Lin, X.; Diao, G. Three-dimensional nitrogen-doped graphene-based metal-free electrochemical sensors for simultaneous determination of ascorbic acid, dopamine, uric acid, and acetaminophen. Analyst 2021, 146, 964–970. [Google Scholar] [CrossRef]
- Choo, S.; Kang, E.S.; Song, I.; Lee, D.; Choi, J.W.; Kim, T.H. Electrochemical detection of dopamine using 3D porous graphene oxide/gold nanoparticle composites. Sensors 2017, 17, 861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, M.; Chen, X.; Li, Y.; Wang, J. Electrochemical co-reduction synthesis of AuPt bimetallic nanoparticles-graphene nanocomposites for selective detection of dopamine in the presence of ascorbic acid and uric acid. Sensors 2015, 15, 16614–16631. [Google Scholar] [CrossRef]
- Tang, Z.M.; Zhang, L.; Tang, S.J.; Li, J.P.; Xu, J.X.; Li, N.; Xu, L.J.; Du, J.J. Synthesis of Co3O4 nanoplates by thermal decomposition for the colorimetric detection of dopamine. Nanomaterials 2022, 12, 2990. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, J.; Wang, Y.; Qi, W.; Su, R.; He, Z. Self-sssembly of ferrocene-phenylalanine@graphene oxide hybrid hydrogels for dopamine detection. ChemPlusChem 2020, 85, 2341–2348. [Google Scholar] [CrossRef]
- Baloach, Q.; Nafady, A.; Tahira, A.; Sherazi, S.T.H.; Shaikh, T.; Arain, M.; Willander, M.; Ibupoto, Z.H. An amperometric sensitive dopamine biosensor based on novel copper oxide nanostructures. Microsyst. Technol. 2017, 23, 1229–1235. [Google Scholar] [CrossRef]
- Zhu, Q.; Bao, J.; Huo, D.; Yang, M.; Hou, C.; Guo, J.; Chen, M.; Fa, H.; Luo, X.; Ma, Y. 3D Graphene hydrogel—Gold nanoparticles nanocomposite modified glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2017, 238, 1316–1323. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Liu, B.; Zhang, H. Hydrogen peroxide and dopamine sensors based on electrodeposition of reduced graphene oxide/silver nanoparticles. Sensors 2024, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, C.; Raju, C.V.; Muthukumaran, P.; Wilson, J.; Ravi, G. Au-Pd bimetallic nanoparticles anchored on α-Fe2O3nonenzymatic hybrid nanoelectrocatalyst for simultaneous electrochemical detection of dopamine and uric acid in the presence of ascorbic acid. J. Mater. Chem. B 2016, 4, 2561–2569. [Google Scholar] [CrossRef]
- Wang, X.; Shao, M.; Liu, L. Rhombic polyaniline microsheets constituted of nanoparticles: Synthesis and application to the detection of dopamine. Chin. J. Chem. 2010, 28, 471–474. [Google Scholar] [CrossRef]
- Ulubay, Ş.; Dursun, Z. Cu nanoparticles incorporated polypyrrole modified GCE for sensitive simultaneous determination of dopamine and uric acid. Talanta 2010, 80, 1461–1466. [Google Scholar] [CrossRef]
- Xu, L.; Tang, S.; Zhang, L.; Du, J.; Xu, J.; Li, N.; Tang, Z. Preparation of copper nanoplates in aqueous phase and electrochemical detection of dopamine. Life 2022, 12, 999. [Google Scholar] [CrossRef]
- Liu, A.; Li, M.; Wang, J.; Feng, F.; Zhang, Y.; Qiu, Z.; Chen, Y.; Meteku, B.E.; Wen, C.; Yan, Z.; et al. Ag@Au core/shell triangular nanoplates with dual enzyme-like properties for the colorimetric sensing of glucose. Chin. Chem. Lett. 2020, 31, 1133–1136. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Jiu, J.; Yang, Y.; Jing, J.; Suganuma, K.; Li, C. Large-scale and galvanic replacement free synthesis of Cu@Ag core–shell nanowires for flexible electronics. Inorg. Chem. 2019, 58, 3374–3381. [Google Scholar] [CrossRef]
- Li, S.; Cheng, D.; Qiu, X.; Cao, D. Synthesis of Cu@Pd core-shell nanowires with enhanced activity and stability for formic acid oxidation. Electrochim. Acta 2014, 143, 44–48. [Google Scholar] [CrossRef]
- Prasad, G.V.; Jang, S.J.; Sekhar, Y.C.; Reddy, T.M.; Sarma, L.S.; Kim, H.B.; Kim, T.H. Fine-tuning of Pd–CeO2/rGO nanocomposite: A facile synergetic strategy for effective electrochemical detection of dopamine in pharmaceutical and biological samples. J. Electroanal. Chem. 2023, 941, 117544. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Hong, B.D.; Lee, C.L. Non-enzymatic sensing of dopamine using a glassy carbon electrode modified with a nanocomposite consisting of palladium nanocubes supported on reduced graphene oxide in a nafion matrix. Microchim. Acta 2016, 183, 905–910. [Google Scholar] [CrossRef]
- Hassan, S.S.; Hanna, D.H.; Medany, S.S. The double-edged sword of the amoxicillin antibiotic against prostate cancer in nano palladium form and its electrochemical detection of dopamine. Appl. Organomet. Chem. 2023, 37, e7026. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, C.; Veerakumar, P.; Chen, S.; Thirumalraj, B.; Liu, S. Facile and novel synthesis of palladium nanoparticles supported on a carbon aerogel for ultrasensitive electrochemical sensing of biomolecules. Nanoscale 2017, 9, 6486–6496. [Google Scholar] [CrossRef]
- Chiang, M.H.; Hong, B.D.; Wang, T.P.; Lin, Y.M.; Lee, C.L. Copper-induced synthesis of palladium/copper popcorn nanoparticles as sensors for differential pulse voltammetric determination of dopamine. Microchim. Acta 2019, 186, 718. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Lin, Y.; Li, J.; Yang, Y.; Zhao, P.; Fei, J.; Xie, Y. Ultrasensitive determination of natural flavonoid rutin using an electrochemical sensor based on metal-organic framework CAU-1/acidified carbon nanotubes composites. Molecules 2022, 27, 7761. [Google Scholar] [CrossRef]
- Wu, L.N.; Tan, Y.L.; Wang, L.; Sun, S.; Qu, Z.; Zhang, J.; Fan, Y. Dopamine sensor based on a hybrid material composed of cuprous oxide hollow microspheres and carbon black. Microchim. Acta 2015, 182, 1361–1369. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Lai, C.; Zhang, Y.; Deng, J.; Li, H.; Yao, S. A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@Au nanoparticles with graphene sheet. Biosens. Bioelectron. 2013, 48, 75–81. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.; Zhang, M.; Zhang, W.; Qi, J.; Sun, X.; Wang, L.; Li, J. N-doped Cu-MOFs for efficient electrochemical determination of dopamine and sulfanilamide. J. Hazard. Mater. 2020, 390, 122157. [Google Scholar] [CrossRef]
- Rajamani, A.R.; Peter, S.C. Novel nanostructured Pt/CeO2@Cu2O carbon-based electrode to magnify the electrochemical detection of the neurotransmitter dopamine and analgesic paracetamol. ACS Appl. Nano Mater. 2018, 1, 5148–5157. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 74, 1130–1141. [Google Scholar] [CrossRef]
- Gajjala, R.K.R.; Naveen, B.; Kumar, P.S. Cu@Pd core–shell nanostructures on pencil graphite substrates as disposable electrochemical sensors for the detection of biological amines. ACS Appl. Nano Mater. 2021, 4, 5047–5057. [Google Scholar] [CrossRef]



| Electrodes | Method | Linear Detection Range | LOD | Refs |
|---|---|---|---|---|
| Graphene/GCE | DPV | 4–100 μM | 2.64 μM | [39] |
| Fe3O4@Au–Gr/GCE | DPV | 0.5–50 μM | 0.65 μM | [40] |
| N–Cu–MOF/GCE | DPV | 0.0005–1.78 mM | 0.15 nM | [41] |
| Pt/CeO2@Cu2O | CV | 0.5–100 μM | 0.079 μM | [42] |
| Au–Cu2O/rGO/GCE | DPV | 10–90 μM | 3.90 μM | [43] |
| Pd/Cu NPTs/GCE | DPV | 0.047–1.12 mM | 0.1045 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Zhang, X.; Xie, J.; Tang, Z.; Tang, S.; Xu, L.; Yang, P. Pd Nanoparticles Loaded on Cu Nanoplate Sensor for Ultrasensitive Detection of Dopamine. Sensors 2024, 24, 5702. https://doi.org/10.3390/s24175702
Tan H, Zhang X, Xie J, Tang Z, Tang S, Xu L, Yang P. Pd Nanoparticles Loaded on Cu Nanoplate Sensor for Ultrasensitive Detection of Dopamine. Sensors. 2024; 24(17):5702. https://doi.org/10.3390/s24175702
Chicago/Turabian StyleTan, Haihu, Xuan Zhang, Jinpu Xie, Zengmin Tang, Sijia Tang, Lijian Xu, and Pingping Yang. 2024. "Pd Nanoparticles Loaded on Cu Nanoplate Sensor for Ultrasensitive Detection of Dopamine" Sensors 24, no. 17: 5702. https://doi.org/10.3390/s24175702





