Measurement Techniques for Low-Concentration Tritium Radiation in Water: Review and Prospects
Abstract
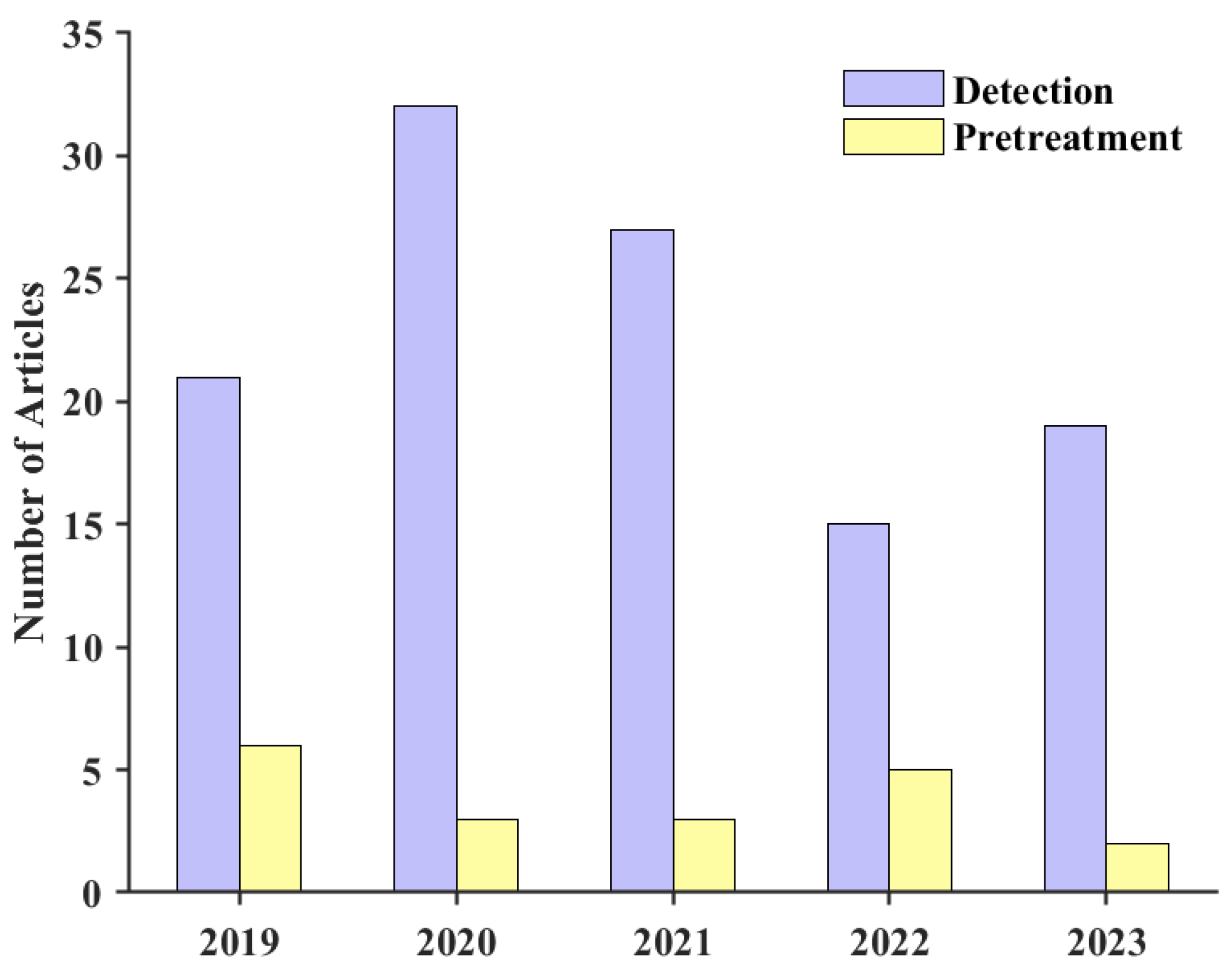
:1. Introduction
| Parameter Name | Parameter Meaning | Reference |
|---|---|---|
| MDA | Minimum detectable activity (Bq/L) | [12] |
| T1 | Total counting time (in minutes) | |
| B | Background count rate (in minutes) | |
| Sample volume (in liters) | ||
| Counting efficiency | ||
| βH/D | Separation coefficients (H and D) | |
| βH/T | Separation coefficients (H and T) | |
| kB | Boltzmann constant | [13] |
| T0 | Thermodynamic temperature | |
| ΔZPEHD | Zero-point vibration energy difference (H and D) | |
| ΔZPEHT | Zero-point vibration energy difference (H and T) | |
| Figure of merit | [14] |
2. Performance Parameter
3. Overview of Low-Concentration Tritium Technology
3.1. Tritium Pretreatment Techniques
3.1.1. Electrolytic Method
- Alkaline Electrolysis (AE)
- 2.
- Solid Polymer Electrolyte (SPE)
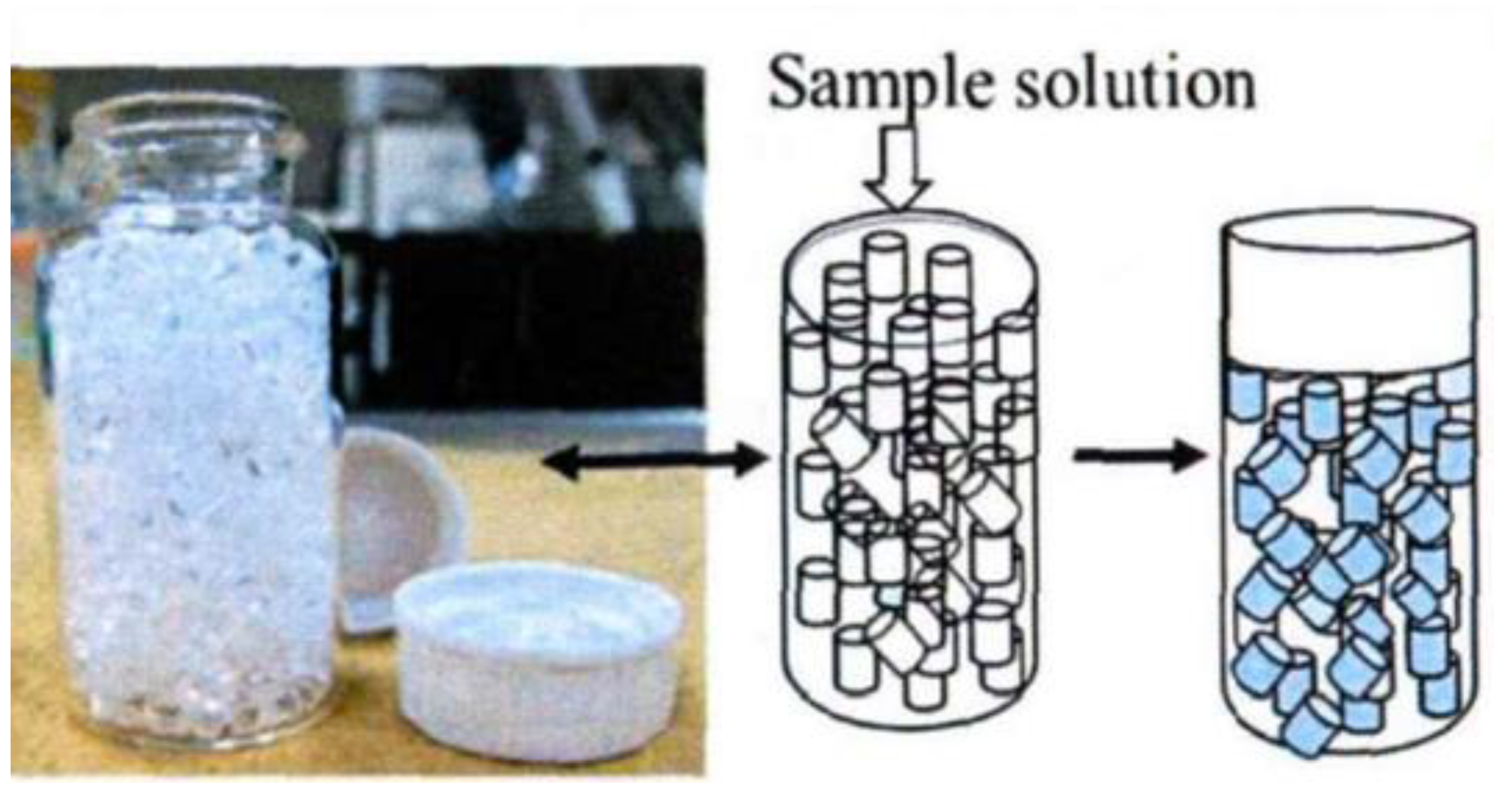
3.1.2. Reverse Osmosis (RO) Film
3.2. Tritium Detection Techniques
3.2.1. Liquid Scintillation Counting (LSC)
3.2.2. Plastic Scintillators (PSs)
3.2.3. Inorganic Scintillators (CaF2:Eu)
- Designing the optimal shape of scintillators for their application in conjunction with Monte Carlo simulation;
- Building and designing detectors to test the detection capability;
- Improving existing detectors, so as to reduce the MDA.
4. Discussion
- The time measurement gap in systematic MDA optimization studies
- 2.
- Monitoring system of organic radioactive waste generation is easily overlooked
- 3.
- A failure to distinguish non-tritiated nuclide interferences in environmental samples and fast solutions for machine learning
- (1)
- Tritium is not the sole radioactive element present. The contribution of other low-energy beta-emitting isotopes, such as 14C, to the total beta measurement results must be ascertained;
- (2)
- Interference from alpha-decaying nuclides, such as radon, is a real concern. The extent to which these nuclides affect the measurement outcomes remains to be determined;
- (3)
- The impact of background radiation on the measurement environment is also a significant factor that requires careful assessment.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Reaction Equations Related to Tritium Production and Transportation
Appendix A.2. Schematic Diagram of Tritium Transportation in Nature
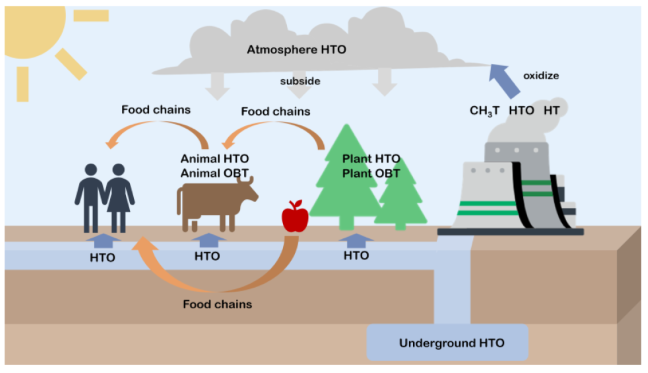
Appendix A.3. Tritium (Total Beta) Limits for Drinking Water by Different Organizations [71,72]
References
- Kang, H.; Min, S.; Seo, B.; Roh, C.; Hong, S.; Cheong, J.H. Low Energy Beta Emitter Measurement: A Review. Chemosensors 2020, 8, 106. [Google Scholar] [CrossRef]
- Okada, S.; Momoshima, N. Overview of tritium: Characteristics, sources, and problems. Health Phys. 1993, 65, 595–609. [Google Scholar] [CrossRef]
- Atwood, D.A. Radionuclides in the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Phillips, J.; Easterly, C. Sources of Tritium; Oak Ridge National Lab.(ORNL): Oak Ridge, TN, USA, 1980. [Google Scholar]
- Eyrolle, F.; Copard, Y.; Lepage, H.; Ducros, L.; Morereau, A.; Grosbois, C.; Cossonnet, C.; Gurriaran, R.; Booth, S.; Desmet, M. Evidence for tritium persistence as organically bound forms in river sediments since the past nuclear weapon tests. Sci. Rep. 2019, 9, 11487. [Google Scholar] [CrossRef] [PubMed]
- Delacroix, D.; Guerre, J.P.; Leblanc, P.; Hickman, C. Radionuclide and radiation protection data handbook 2002. Radiat. Prot. Dosim. 2002, 98, 1–168. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, H.; Toyoshima-Sasatani, M.; Nagashima, H.; Shimura, T.; Umata, T.; Tachibana, A. Tritium biology in Japan: A search for a new approach. Fusion Eng. Des. 2018, 128, 28–32. [Google Scholar] [CrossRef]
- Scott, B.R. Radiation toxicology, ionizing and nonionizing. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 29–43. [Google Scholar]
- Kherani, N.; Shmayda, W. Low-Level Tritium Research Facility for the University of Toronto Institute for Aerospace Studies; Canadian Fusion Fuels Technology Project: Toronto, ON, Canada, 1984. [Google Scholar]
- McCurry, J. Japan scraps mascot promoting Fukushima wastewater dump. 2021. Available online: https://www.theguardian.com/world/2021/apr/15/japan-scraps-mascot-promoting-fukushima-wastewater-dump (accessed on 22 July 2024).
- Liu, D.; Hoskin, M. Contemporary international Law:Regulating the upcoming fukushima radioactive wastewater discharge. Ocean. Coast. Manag. 2023, 234, 106452. [Google Scholar] [CrossRef]
- Currie, L.A. Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis–A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Millet, P.; Grigoriev, S. Water electrolysis technologies. In Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety; Newnes: Oxford, UK, 2013; Volume 19. [Google Scholar]
- Santos, D.M.; Sequeira, C.A.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Química Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Grubb, W. Batteries with solid ion exchange electrolytes: I. secondary cells employing metal electrodes. J. Electrochem. Soc. 1959, 106, 275. [Google Scholar] [CrossRef]
- Saito, M.; Takata, S. Automatic stop type SPE tritium enrichment apparatus. Radioisotopes 1996, 45, 483–490. [Google Scholar] [CrossRef]
- Suntarapai, P.; Isogai, K.; Sato, K. The Analysis of Tritium in Natural Water by Electrolysis Enrichment using solid Polymer Electrolyte. 1998. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:30041187 (accessed on 22 July 2024).
- Ivanchuk, O.; Goryanina, V.; Rozenkevich, M. Isotopic effects of hydrogen during the decomposition of water in electrolysis with a solid polymer electrolyte. At. Energy 2000, 89, 745–749. [Google Scholar] [CrossRef]
- Bellanger, G.; Commission, F.A.E. Enrichment of slightly concentrated tritiated water by electrolysis using a palladium cathode coated on ionic solid polymer membrane—Design and results. Fusion Eng. Des. 2007, 82, 395–405. [Google Scholar] [CrossRef]
- Soreefan, A.M.; DeVol, T.A. Proportional counting of tritium gas generated by polymer electrolyte membrane (PEM) electrolysis. J. Radioanal. Nucl. Chem. 2009, 282, 517–521. [Google Scholar] [CrossRef]
- Lozada-Hidalgo, M.; Zhang, S.; Hu, S.; Esfandiar, A.; Grigorieva, I.; Geim, A. Scalable and efficient separation of hydrogen isotopes using graphene-based electrochemical pumping. Nat. Commun. 2017, 8, 15215. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Zabolockis, R.; Sondars, M.; Vaivars, G.; Reinholds, I.; Gostilo, V.; Malgin, V.; Kizilov, A.; Lescinskis, A.; Felsharuk, A.; Avotina, L. Graphene-based electrochemical system for tritium enrichment. Nucl. Fusion 2024, 64, 026022. [Google Scholar] [CrossRef]
- Prihasto, N.; Liu, Q.-F.; Kim, S.-H. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316. [Google Scholar] [CrossRef]
- Cornelissen, E.; Harmsen, D.; Blankert, B.; Wessels, L.; Van der Meer, W. Effect of minimal pre-treatment on reverse osmosis using surface water as a source. Desalination 2021, 509, 115056. [Google Scholar] [CrossRef]
- Koganezawa, T.; Iida, T.; Ogata, Y.; Tsuji, N.; Kakiuchi, M.; Satake, H.; Yamanishi, H.; Sakuma, Y. Development of a simplified treatment for measuring tritium concentration in the environmental water. Removal of dissolved ions by reverse osmosis membrane for electrolysis enrichment. Radioisotopes 2004, 53, 277–285. [Google Scholar] [CrossRef]
- Li, G.; Liang, M.; He, S.; Liu, J.; Pang, X.; Zeng, Z. RO film-based pretreatment method for tritium determination by LSC. Appl. Radiat. Isot. 2020, 166, 109343. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, D. Applications of Liquid Scintillation Counting; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Paramentier, J.; Ten Haaf, F. Developments in liquid scintillation counting since 1963. Int. J. Appl. Radiat. Isot. 1969, 20, 305–334. [Google Scholar] [CrossRef]
- Crook, M.; Johnson, P. Liquid Scintillation Counting: Incorporating whole-body counting and radioimmunoassay. In Proceedings of the Symposium on Scintillation Counting/Organised by the Radiochemical Methods Group (Analytical Division, The Chemical Society), Bath, UK, 13–16 September 1977; Crook, M.A., Ed.; P. Johnson: Heyden, UK, 1978; Volume 5. [Google Scholar]
- Ansari, M.A.; Mohokar, H.; Deodhar, A.; Jacob, N.; Sinha, U. Distribution of environmental tritium in rivers, groundwater, mine water and precipitation in Goa, India. J. Environ. Radioact. 2018, 189, 120–126. [Google Scholar] [CrossRef]
- Eyrolle, F.; Ducros, L.; Le Dizès, S.; Beaugelin-Seiller, K.; Charmasson, S.; Boyer, P.; Cossonnet, C. An updated review on tritium in the environment. J. Environ. Radioact. 2018, 181, 128–137. [Google Scholar] [CrossRef]
- Mahlangu, S.; Lorentz, S.; Diamond, R.; Dippenaar, M. Surface water-groundwater interaction using tritium and stable water isotopes: A case study of Middelburg, South Africa. J. Afr. Earth Sci. 2020, 171, 103886. [Google Scholar] [CrossRef]
- Knoll, G.F. Radiation Detection and Measurement; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lin, F.; Yu, T.; Yu, W.; Ni, J.; Lin, L. Electrolytic enrichment method for tritium determination in the Arctic Ocean using liquid scintillation counter. Acta Oceanol. Sin. 2020, 39, 73–77. [Google Scholar] [CrossRef]
- Feng, B.; Chen, B.; Zhao, C.; He, L.; Tang, F.; Zhuo, W. Application of a liquid scintillation system with 100-ml counting vials for environmental tritium determination: Procedure optimization, performance test, and uncertainty analysis. J. Environ. Radioact. 2020, 225, 106427. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Zhao, G.; Chen, C.; Zhuo, W. Measurement and sampling techniques verification for an online gaseous tritium monitoring method. J. Instrum. 2022, 17, P09032. [Google Scholar] [CrossRef]
- Sigg, R.; McCarty, J.; Livingston, R.; Sanders, M. Real-time aqueous tritium monitor using liquid scintillation counting. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1994, 353, 494–498. [Google Scholar] [CrossRef]
- Hofstetter, K. Continuous aqueous tritium monitoring. Fusion Technol. 1995, 28, 1527–1531. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Xu, Y.; Wu, H.; Xu, X.; Lu, P.; Zhang, X.; Zhao, X.; Xia, M.; Tang, J.; et al. All-Inorganic Glass Scintillators: Scintillation Mechanism, Materials, and Applications. Laser Photonics Rev. 2023, 17, 2300006. [Google Scholar] [CrossRef]
- Koshimizu, M. Recent progress of organic scintillators. Jpn. J. Appl. Phys. 2023, 62, 010503. [Google Scholar] [CrossRef]
- Erchinger, J.L.; Aalseth, C.E.; Bernacki, B.E.; Douglas, M.; Fuller, E.S.; Keillor, M.E.; Morley, S.M.; Mullen, C.A.; Orrell, J.L.; Panisko, M.E. Development of a low background liquid scintillation counter for a shallow underground laboratory. Appl. Radiat. Isot. 2015, 105, 209–218. [Google Scholar] [CrossRef]
- Coma, A.; Bagán, H.; Tarancón, A. Preparation and evaluation of crosslinked plastic scintillation microspheres (CPSm). Polym. Test. 2024, 137, 108514. [Google Scholar] [CrossRef]
- Bhakta, S.; Raman, A.; Ajish, J.K. Development of plastic scintillator with better performance than the commercial counterpart (UPS-923A) and effect of fluorophore composition on light output. Appl. Radiat. Isot. 2024, 212, 111453. [Google Scholar] [CrossRef]
- Serraïma-López, D.; Guillén, M.; Bagán, H.; Tarancón, A. Scintillation and structural properties of copolymers and mixtures of styrene, 9-vinylcarbazole and 4-vinylbenzyl chloride based plastic scintillators. Appl. Radiat. Isot. 2024, 211, 111409. [Google Scholar] [CrossRef]
- Furuta, E.; Ohyama, R.-I.; Yokota, S.; Nakajo, T.; Yamada, Y.; Kawano, T.; Uda, T.; Watanabe, Y. Measurement of tritium with high efficiency by using liquid scintillation counter with plastic scintillator. Appl. Radiat. Isot. 2014, 93, 13–17. [Google Scholar] [CrossRef]
- Furuta, E.; Kato, Y.; Fujisawa, S. Measurement of tritium with plastic scintillators in large vials of a low background liquid scintillation counter: An organic waste-less method. J. Radioanal. Nucl. Chem. 2017, 314, 701–708. [Google Scholar] [CrossRef]
- Furuta, E.; Iwasaki, N.; Kato, Y.; Tomozoe, Y. A new tritiated water measurement method with plastic scintillator pellets. Isot. Environ. Health Stud. 2016, 52, 560–566. [Google Scholar] [CrossRef]
- Azevedo, C.D.R.; Baeza, A.; Brás, M.; Cámara, T.; Cerna, C.; Chauveau, E.; Gil, J.M.; Corbacho, J.A.; Delgado, V.; Díaz, J.; et al. TRITIUM—A Real-Time Tritium Monitor System for Water Quality Surveillance. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), Sydney, NSW, Australia, 10–17 November 2018; pp. 1–6. [Google Scholar]
- Azevedo, C.D.R.; Baeza, A.; Chauveau, E.; Corbacho, J.A.; Díaz, J.; Domange, J.; Marquet, C.; Martínez-Roig, M.; Piquemal, F.; Roldán, C.; et al. Design, setup and routine operation of a water treatment system for the monitoring of low activities of tritium in water. Nucl. Eng. Technol. 2023, 55, 2349–2355. [Google Scholar] [CrossRef]
- Janda, J.; Pipal, V. The use of plastic scintillation microspheres in flow scintillation analysis. Appl. Radiat. Isot. 2022, 190, 110475. [Google Scholar] [CrossRef]
- Sanada, Y.; Abe, T.; Sasaki, M.; Kanno, M.; Yamada, T.; Nakasone, T.; Miyazaki, N.; Oshikiri, K.; Watabe, H. Basic study on tritium monitor using plastic scintillator for treated water discharge at Fukushima Daiichi Nuclear Power plant. J. Nucl. Sci. Technol. 2023, 61, 693–702. [Google Scholar] [CrossRef]
- Sanada, Y.; Oshikiri, K.; Kanno, M.; Abe, T. Development of a practical tritiated water monitor to supervise the discharge of treated water from Fukushima Daiichi Nuclear Power Plant. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2024, 1062, 169208. [Google Scholar] [CrossRef]
- Plettner, C.; Pausch, G.; Scherwinski, F.; Herbach, C.-M.; Lentering, R.; Kong, Y.; Roemer, K.; Grodzicka, M.; Szcześniak, T.; Iwanowska, J. CaF2 (Eu): An “old” scintillator revisited. J. Instrum. 2013, 8, P06010. [Google Scholar] [CrossRef]
- Kawano, T.; Ohashi, H.; Hamada, Y.; Jamsranjav, E. Comparative testing of various flow-cell detectors fabricated using CaF2 solid scintillator. Fusion Sci. Technol. 2015, 67, 404–407. [Google Scholar] [CrossRef]
- Vasil’ev, A.N.; Gektin, A.V. Multiscale approach to estimation of scintillation characteristics. IEEE Trans. Nucl. Sci. 2013, 61, 235–245. [Google Scholar] [CrossRef]
- Kawano, T.; Ohashi, H. Development and improvement of a water flow tritium monitoring system utilizing a CaF 2 flow cell detector. Radioisotopes 2020, 69, 171–175. [Google Scholar] [CrossRef]
- Alton, T.L. In-Situ Monitoring of Groundwater Radionuclides with Emphasis on Tritium Detection; Lancaster University: Lancaster, UK, 2019. [Google Scholar]
- Wu, J.; Chen, Z.; Chen, Z.; Li, J.; Wang, H. The use of optical fibers as passageways for the scintillation light produced in CaF2 (Eu) powder in water for low-level tritiated water measurement. J. Instrum. 2019, 14, P06015. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Z.; Wu, G.; Chen, Z.; Wang, H. The substitutability of liquid scintillation cocktail for CaF2 (Eu) powder in low-level tritiated water measurement. J. Instrum. 2019, 14, P01026. [Google Scholar] [CrossRef]
- Song, K.; Chen, Z.; Chen, Z.; Huang, P.; Yang, Y.; Lai, C. Design of a solid scintillation counter for tritium water measurement based on Ca2F(Eu) sheet using Monte Carlo simulation. Fusion Eng. Des. 2021, 170, 112701. [Google Scholar] [CrossRef]
- Varlam, C.; Stefanescu, I.; Duliu, O.G.; Faurescu, I.; Popescu, I. Applying direct liquid scintillation counting to low level tritium measurement. Appl. Radiat. Isot. 2009, 67, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Akata, N.; Okada, K.; Kuwata, H.; Kheamsiri, K.; Hosoda, M.; Tazoe, H.; Yasuhara, R.; Sugihara, S.; Yamada, R.; Tanaka, M. Tritium Concentration in Natural Spring Water Collected at Hirosaki, Japan. Plasma Fusion Res. 2023, 18, 2405030. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, H.; Chen, N.; Wang, Y.; Zhang, M.; Hou, X. Determination of tritium in large volume of seawater using electrolytic enrichment and LSC and its application for the East China Sea water. J. Radioanal. Nucl. Chem. 2023, 332, 981–987. [Google Scholar] [CrossRef]
- Joung, S.; Kim, Y.; Kim, J.; Park, J.; Jang, M.; Lee, J.; Kim, C.-J.; Lee, M.S.; Lim, J.-M. Simultaneous quantitative analysis of 3H and 14C radionuclides in aqueous samples via artificial neural network with a liquid scintillation counter. Appl. Radiat. Isot. 2021, 170, 109593. [Google Scholar] [CrossRef]
- Dragović, S. Artificial neural network modeling in environmental radioactivity studies—A review. Sci. Total Environ. 2022, 847, 157526. [Google Scholar] [CrossRef]
- The Canadian Nuclear Safety Commission (CNSC). Tritium in Drinking Water. Available online: http://www.nuclearsafety.gc.ca/eng/resources/health/tritium/tritium-in-drinking-water.cfm (accessed on 20 August 2009).
- Alton, T.T.; Monk, S.D.; Cheneler, D. Beta particle energy spectra shift due to self-attenuation effects in environmental sources. Nucl. Eng. Technol. 2017, 49, 1483–1488. [Google Scholar] [CrossRef]
- 7500-3H TRITIUM; Standard Methods For the Examination of Water and Wastewater. American Public Health Association: Washington, DC, USA, 2012.
- European Union. Council Directive 2013/51/Euratom of 22 October 2013 Laying down requirements for the protection of the health of the general public with regard to radioactive substances in water intended for human consumption. Off. J. Eur. Union 2013, L296, 12–21. [Google Scholar]
- GB 5749-2022; Standards for Drinking Water Quality. National Bureau for Disease Control and Prevention: Beijing, China, 2022.
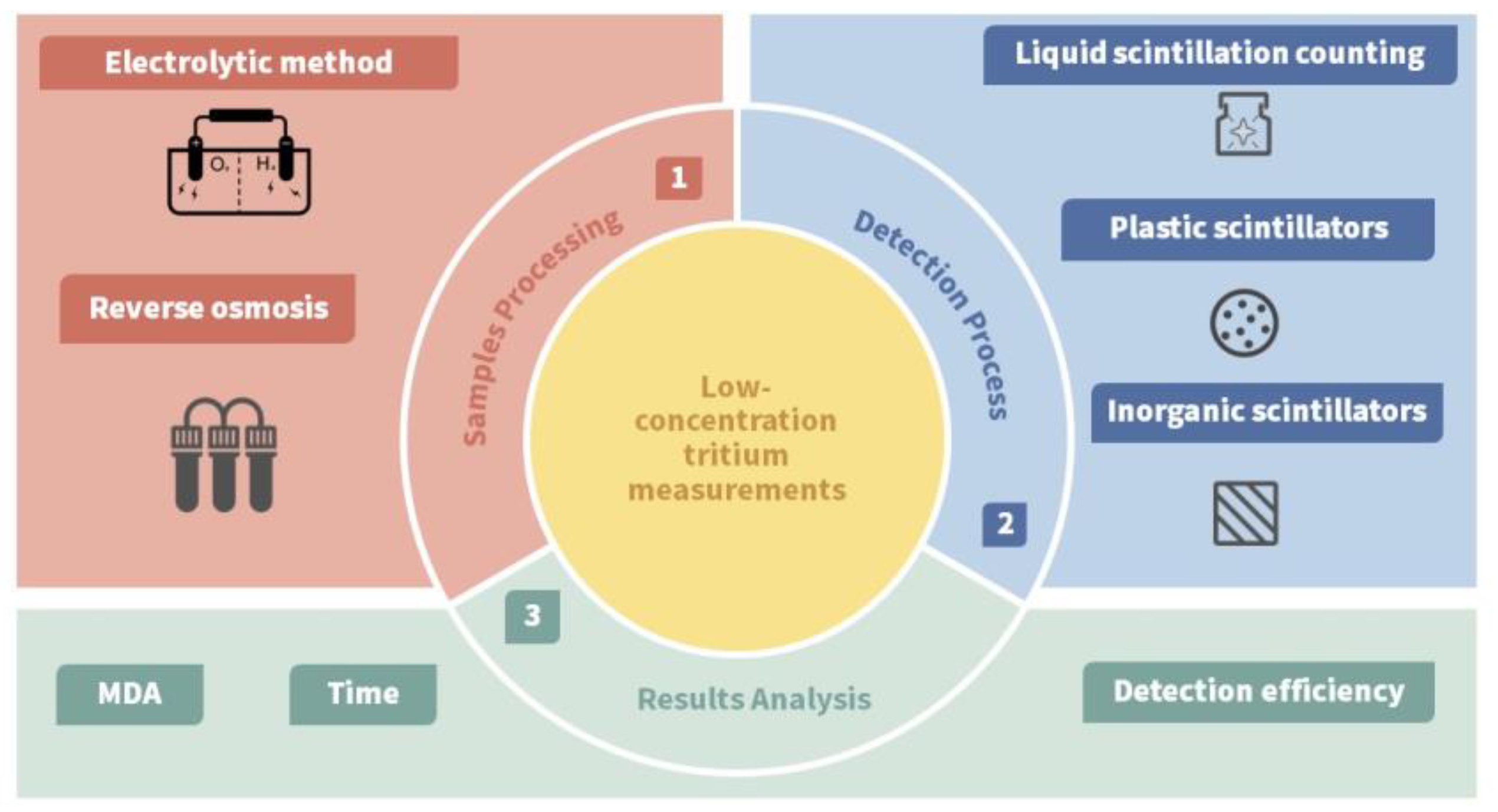




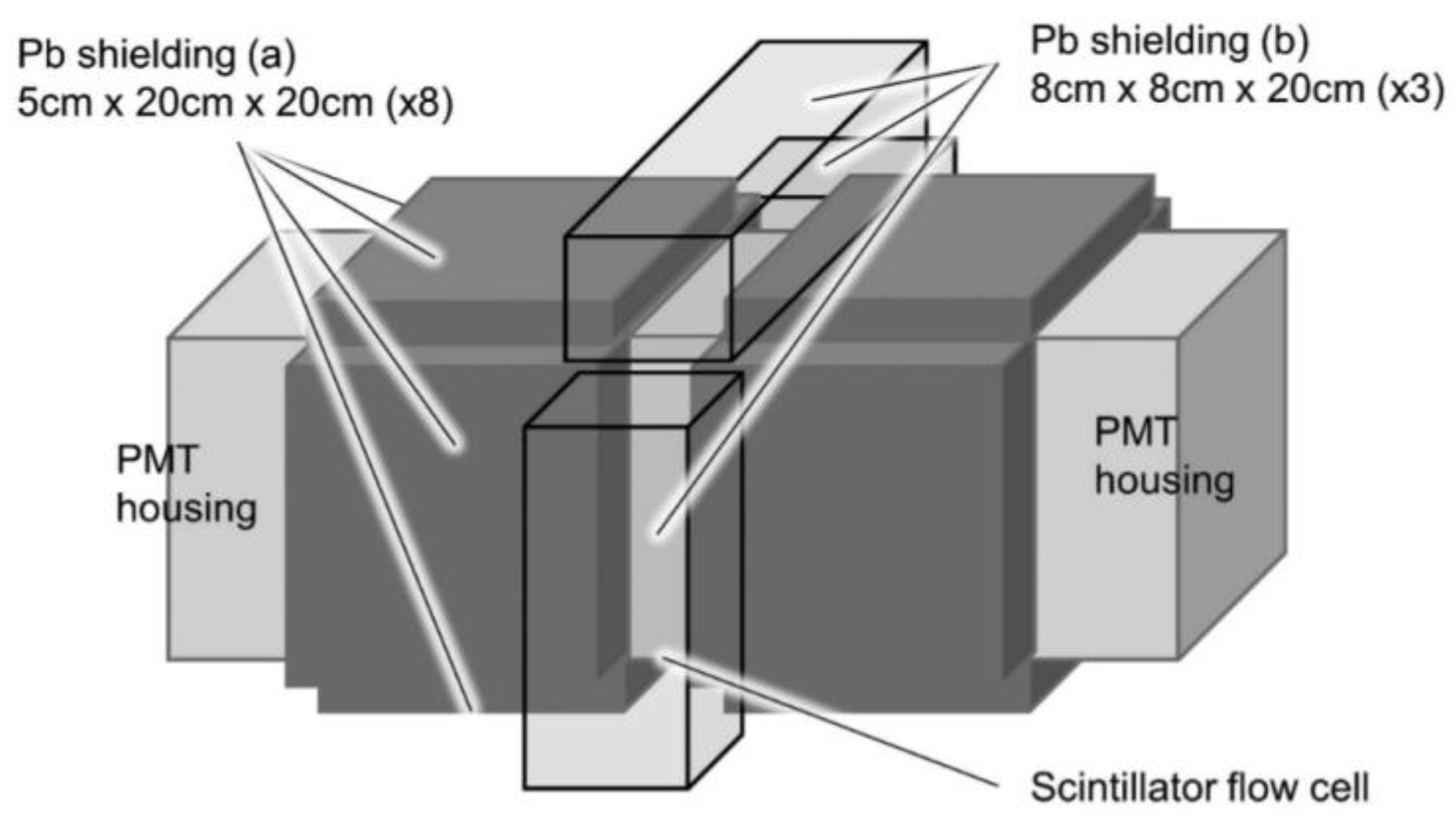

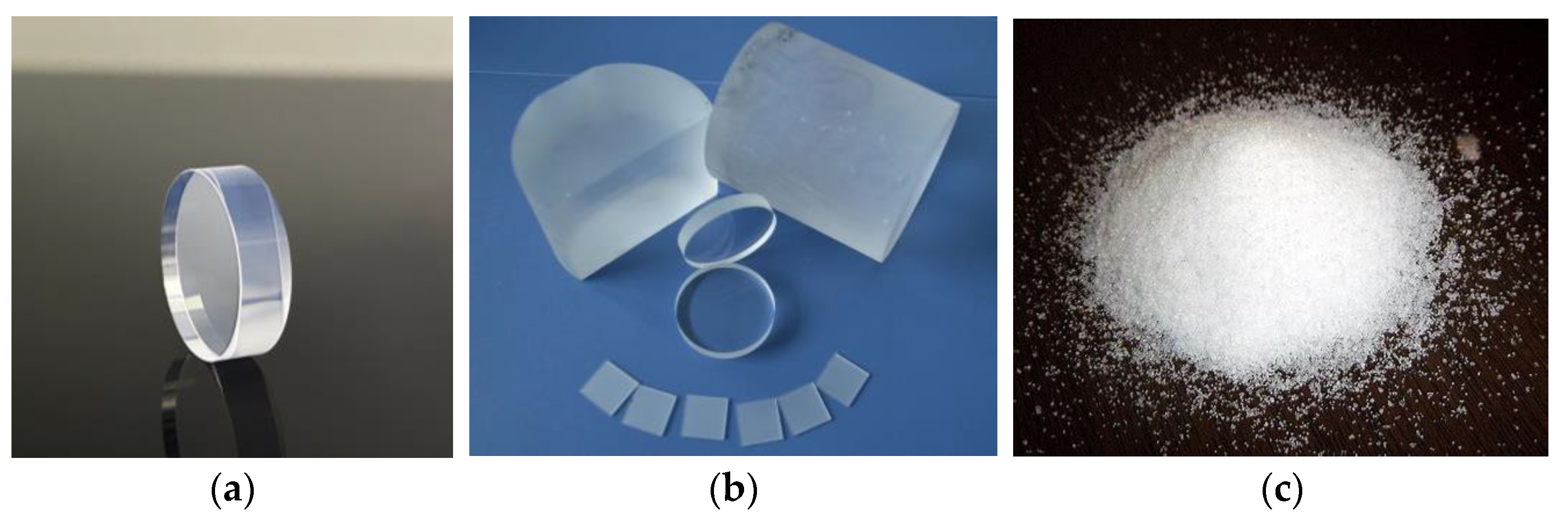
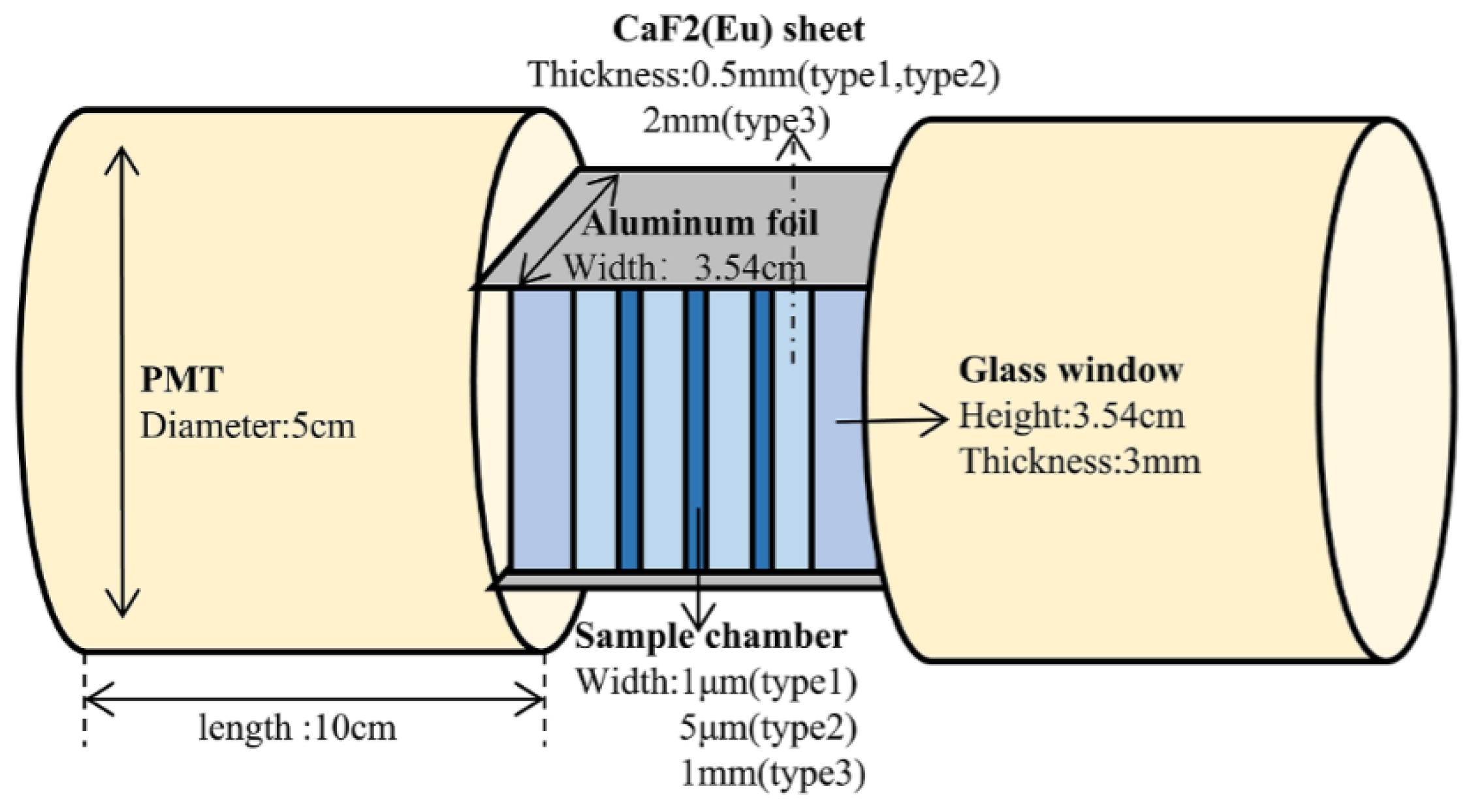
| Categorization | Type of Technology | Key Features |
|---|---|---|
| Pretreatment | Electrolytic method | Common method, easy to setup |
| Reverse osmosis (RO) film | Newly proposed method in recent years, with shorter processing time than electrolysis | |
| Detection | Liquid scintillation counting (LSC) | The most mature and best performing method for measuring low-energy radiation signals |
| Plastic scintillators (PSs) | Premature, no organic waste compared to LSC | |
| Inorganic scintillators (CaF2:Eu) | Premature, no organic waste compared to LSC |
| Pretreatment Technical Method | Effect | Estimated Processing Speed |
|---|---|---|
| Alkaline electrolysis | Optimal βH/T about 10, electrolytic efficiency 59~70% [31] | - |
| Solid polymer electrolyte | Optimal βH/T about 20, electrolytic efficiency 65~82% [14] | 14 mL/h |
| Reverse osmosis (RO) film | Can handle water samples below 10 Bq/L | 36 mL/min |
| Material | BC408 | PMMA | PS (Polystyrene) | VMB (Vinyl Methyl Benzene) | LiME (Lithium Methacrylate) | EJ-309 (A Liquid Scintillator Used in LSC) |
|---|---|---|---|---|---|---|
| Relative light yield (100%) | 116.8079 | 85.20481 | 115.179 | 111.2911 | 108.153 | 90.813 |
| Detection efficiency | 0.41% | 0.41% | 0.41% | 0.40% | 0.40% | 95.9% |
| Technical Detection Method | Estimated MDA | Detection Time | MDA < EPA (740 Bq/L) | MDA < EC (100 Bq/L) | |
|---|---|---|---|---|---|
| Liquid scintillation counting (LSC) | 0.6 Bq/L [46] | 3 h | 102% | √ | √ |
| Plastic scintillator powder (flow cell with Pb shielding) | 593 Bq/L | 1 h | 5% | √ | - |
| Plastic scintillation fibers | 100 Bq/L | 1 h | 5% | √ | - |
| CaF2:Eu powder (flow cell) | 640 Bq/L | 1 h | 3% | √ | - |
| CaF2:Eu sheets | 2.95 Bq/mL | 1 h | 25% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Chen, L.; Xia, W.; Gong, J.; Chen, J.; Liang, C. Measurement Techniques for Low-Concentration Tritium Radiation in Water: Review and Prospects. Sensors 2024, 24, 5722. https://doi.org/10.3390/s24175722
Mao J, Chen L, Xia W, Gong J, Chen J, Liang C. Measurement Techniques for Low-Concentration Tritium Radiation in Water: Review and Prospects. Sensors. 2024; 24(17):5722. https://doi.org/10.3390/s24175722
Chicago/Turabian StyleMao, Junxiang, Ling Chen, Wenming Xia, Junjun Gong, Junjun Chen, and Chengqiang Liang. 2024. "Measurement Techniques for Low-Concentration Tritium Radiation in Water: Review and Prospects" Sensors 24, no. 17: 5722. https://doi.org/10.3390/s24175722





