Industry 4.0-Compliant Occupational Chronic Obstructive Pulmonary Disease Prevention: Literature Review and Future Directions
Abstract
1. Introduction
2. COPD
2.1. COPD Diagnosis
2.2. COPD Indicators and Monitoring
- Activities of Daily Living (ADL): COPD is typically accompanied by decreased ADL [23], which makes ADL assessment one of the best COPD evaluation methods. Furthermore, it is vital for COPD patients to increase their ADLs [24]. A study implemented real-time activity classification by placing sensors on the forearm, thigh, and sternum [25]. Wearable technologies such as smart vests and t-shirts were developed to reduce the number of sensors required, and cloud-connected platforms were designed for remote monitoring and interactions [26,27].
- Volatile Organic Compounds (VOCs): VOCs, such as isoprene and hexadecane, are typical kinds of the COPD biomarkers in exhaled breath [28,29]. A portable spectrometer has been proposed for chemical analysis [30]. However, there are contrary opinions on using VOC profiles for COPD diagnosis. Research shows that VOC profiles could identify patients with COPD accurately [31,32,33], while it was also observed by some studies that VOC profiles cannot distinguish smokers, including former smokers, from COPD patients [34,35].
- Blood Lactate Level: The blood lactate level is another COPD biomarker [36]. It has been reported that people with COPD tend to have a higher blood lactate level than their healthy counterparts while doing the same activities at the same intensity [36]. As a result, lactic acid has been proposed and used as a biomarker of COPD severity [37]. Several novel approaches using flexible electronics were developed to measure the lactic acid through human tears, saliva, and sweat [38,39,40]. However, in tests, it was found that these electronics were not very comfortable to wear, and their practicality needs further discussion [41].
- Saliva: Dysphagia is regarded as one of the high-risk phenotypes for the prediction of COPD exacerbation by some studies [42]. Research has found, as a less invasive way to screen dysphagia, that a repetitive saliva swallowing test cut-off value of 5 could be a strong predictor of COPD exacerbation [43]. Compared to bio-samples like blood and sputum, saliva is relatively easy to use, especially for home monitoring. A novel biosensor called COPD saliva-based point-of-care monitor has been designed to enable patients to undergo testing at home and identify exacerbation in time [44,45].
- Respiration: As aforementioned, the main symptoms of COPD are related to the patient’s respiratory condition. This follows from the medical explanation of why breathing will change is that sternomastoid muscles, which are accessory muscles, are used during the exacerbation period of COPD [46]. Research has explored the consistency and accuracy of breathing sounds at various airflow levels and predetermined bodily sites in individuals with COPD [47]. A conclusion was drawn that the most reliable interval of air flow is 0.4–0.6 L/s, and this applies to respiratory sound parameters at all anatomic locations [47]. Moreover, it is recommended to be considered in computerized auscultation for future use [47]. On the other hand, research shows that the respiration characteristics of COPD differ from other dyspnea diseases [48]. Furthermore, a study found the potential of computerized analyses of respiratory sounds in respiratory status monitoring for people suffering from COPD, and this study achieved “75.8% exacerbations were early detected with an average of 5 ± 1.9 days in advance at [sic] medical attention” [49].
- Cough and Sputum Production: Chronic cough and sputum production are not only very common in subjects with COPD, but have also been suggested as being predictive of disease progression, exacerbations, and hospitalizations [50]. Research on using chronic cough or phlegm to predict or identify COPD risk severity achieved some success [51,52]. It has been pointed out that each of them is an independent and statistically significant predictor of COPD [51]. Experiments successfully identified a subgroup of participants at a high COPD risk, irrespective of smoking habits. The study contends that the occurrence of a chronic cough or phlegm serves as an early indicator of COPD in a significant number of patients, regardless of their smoking status [51]. Sumner et al. considered cough only in a study, and a comparison was made of 68 randomly selected current and ex-smokers with COPD and smokers without COPD and healthy nonsmokers, using a custom-built cough sound recording device over 24 h. An outcome of this study was that objective cough monitoring is viable. Moreover, it offers a great prospect that cough monitoring can provide timely feedback for COPD interventions and also allows for adapting the new strategy in development [52].
2.3. Environmental Risks
3. Industry 4.0-Compliant Management
3.1. IoT and Data Analysis
- Adherence to and staying current with the latest regulations or directives;
- Protection of workers by identifying workplace hazards and near misses;
- Monitoring of training activities of employees on important EHS policies and procedures;
- Improvement of employee exposure information.
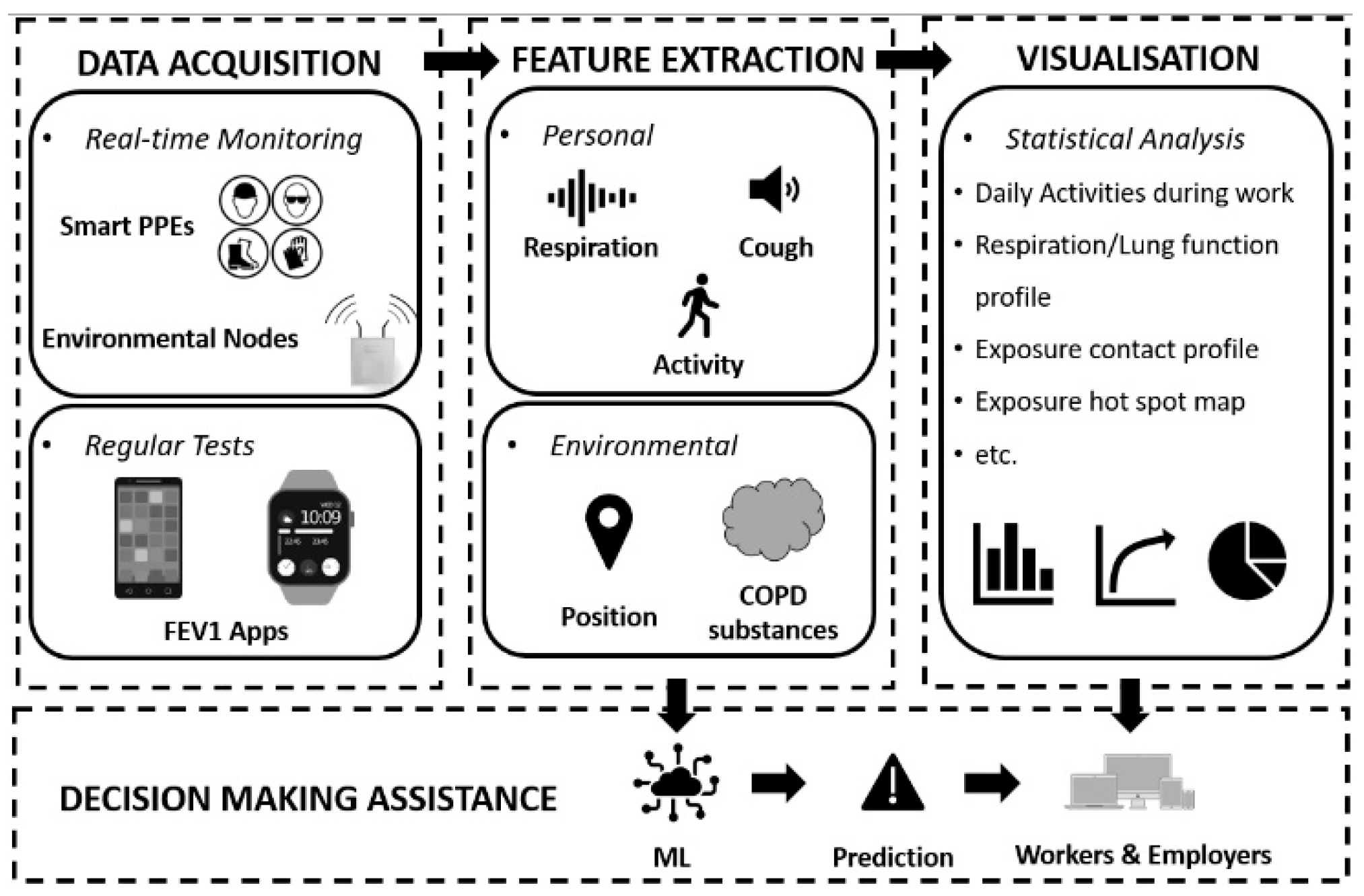
- Remote Health Condition Monitoring: Applying IoT for medical purposes, such as using wearable sensors for wireless monitoring, is a trending topic. A design of the Internet of Medical Things (IoMT) for remote respiratory rate monitoring of COPD patients was proposed to improve doctor–patient communication [67]. It used message queue telemetry transport protocol and could send clinical alarms according to configurable thresholds. Additionally, a smart vest was designed for breathing monitoring during the rest period, with embedded capacitive sensors [67]. Similarly, to make the vest more comfortable to wear, inkjet-printed sensor technology was used [68]. It is stretchable and wearable, and the design achieved high measurement accuracy at different postures and among different patients. In the research of these two smart vests, the only parameter considered was the breath rate of the wearer, and the measurements were supposed to be taken during the rest period. For the same purpose of remote respiration monitoring, much more physiological parameters were covered in the design of a smart mask proposed by Tipparaju et al. [69]. In this work, principle component analysis (PCA) was applied to analyze the respiration pattern of each participant, but neither the pathology basis nor how to use it for a particular disease was considered [69].
- Working Environment Control: To the best knowledge of the authors, universal examples of Industry 4.0-compliant environmental COPD risk control do not exist, probably due to the large variety of COPD substances. However, research on occupational exposure management IoT systems has been reported [70,71]. A project aimed at sustainable health management presented an IoT-based indoor environment monitoring system tracking O3 concentrations near photocopy machines [70]. The developed sensing node contains a Bluetooth module and a semiconductor O3 sensor, apart from which, the developed IoT system also includes gateway nodes and processing nodes. It was claimed that the design can be expanded to cover larger areas and more pollutants such as hydrocarbons and different-size particles [70]. Similarly, Fathallah et al. conducted work on occupational exposure estimation and proposed a real-time occupational exposure monitoring model [71]. It successfully quantified indoor worker exposure to formaldehyde and CO2 in real time using multi-pollutant sensor nodes and an indoor positioning system [71].
- ML-Assisted Assessment and Prediction: The introduction of ML to assist diagnosis is a new trend. Zarrin and Wenger developed an Artificial Neural Network (ANN) model for pattern recognition for COPD diagnosis [72]. In this study, eight fundamental parameters were considered: the viscosity of saliva samples, the ambient temperature, patient smoking background, cytokine level, pathogen load, mucin combinations, gender, and age. Moreover, the output was set to four different kinds of disease statuses: healthy, low probability, high probability, and COPD-diseased. After comparing to the actual states, an accuracy rate of 112 out of 200 was achieved [72]. Attempts of COPD readmission prediction have also been made. COPD patients were required to use accelerometer-based wrist-worn wearable devices during daily living and readmission risks for 30 days, and were predicted based on their physical activity, including the activity index and regularity index, and quality of activity [73]. The results from 16 COPD patients showed a sensitivity of 63% and a positive prediction rate of 37.78%, which can be considered a significant improvement in comparison to other clinical assessments [73].
3.2. Industry 4.0-Compliant Prevention Based on Underpinning OHS and Medical Management Approaches
- Elimination: physical removal of the hazard;
- Substitution: replacement of the hazard;
- Engineering Controls: isolation of people from the hazard;
- Administrative Controls: change the way people work;
- Personal Protective Equipment (PPE): protects the worker.
- The redefinition of medicine as an informative science;
- The interconnected domains composing complex diseases;
- The emerging technologies allowing for different approaches to understand and access patient data;
- New and powerful analytical systems.
4. Discussion
4.1. Identification of Opportunities and Challenges
- Health Condition Detection: current research on how digital technologies such as IoT and artificial intelligence can specially support the treatment of occupational-related COPD, rather than OHS management, more related to protection against and prevention of COPD. Systematic reviews reported that digital health interventions (DHIs) for COPD show some uptake problems, like low compliance rates and lack of personalization [77,78]. Some remote monitoring systems also present restricted utilization to specific times during the day [77]. Moreover, measurements should also be more adjustable to the requirements of the target population [78].
- Protection: active protection is one of the new trends in OHS 4.0; digital technologies like smart PPE and WSNs can provide more sources and types of data to support further analysis. However, COPD risk factors found in workplaces usually vary. Therefore, monitoring systems with fixed alarm values of one or two substances are not effective enough. It should be noted that some exposure exceeding critical values could be easily avoided by combining environmental monitoring systems with primary real-time intervention control, such as connecting traditional protection equipment like LEV systems and environmental sensors to cyber–physical systems (CPSs), allowing for reducing the exposure level in the workplace and maintaining it under WEL in real time. Regarding personal protection, Adjiski et al. devised smart underground mining PPE by introducing sensors and wireless communication modules into safety wear [75].
- Assessment: the integration of traditional assessment methods and ML algorithms can improve accuracy and help optimizing management. COPD risk assessment in workplaces needs to be more personalized and dynamic. The lack of personalization of current approaches, which use the same standard for different workers at different ages and different jobs can result in misdiagnoses. With the idea of new conceptual OHS management, digital technologies such as data fusion (e.g., sound and temperature) and ML show high potential for assessment assistance and decision optimization. For example, without motion working state recognition, health condition monitoring could be meaningless. Moreover, unlike other industrial diseases, such as HAVS and MSDs, there are no “ergonomic tools” for COPD, nor well-developed analysis and assessment standards. In addition, it is hard to diagnose or predict COPD as it is a “chronic” disease, influenced by several factors, including various substances and lifestyle habits like exercise and smoking.
4.2. Future Trends and a Vision of Implementation
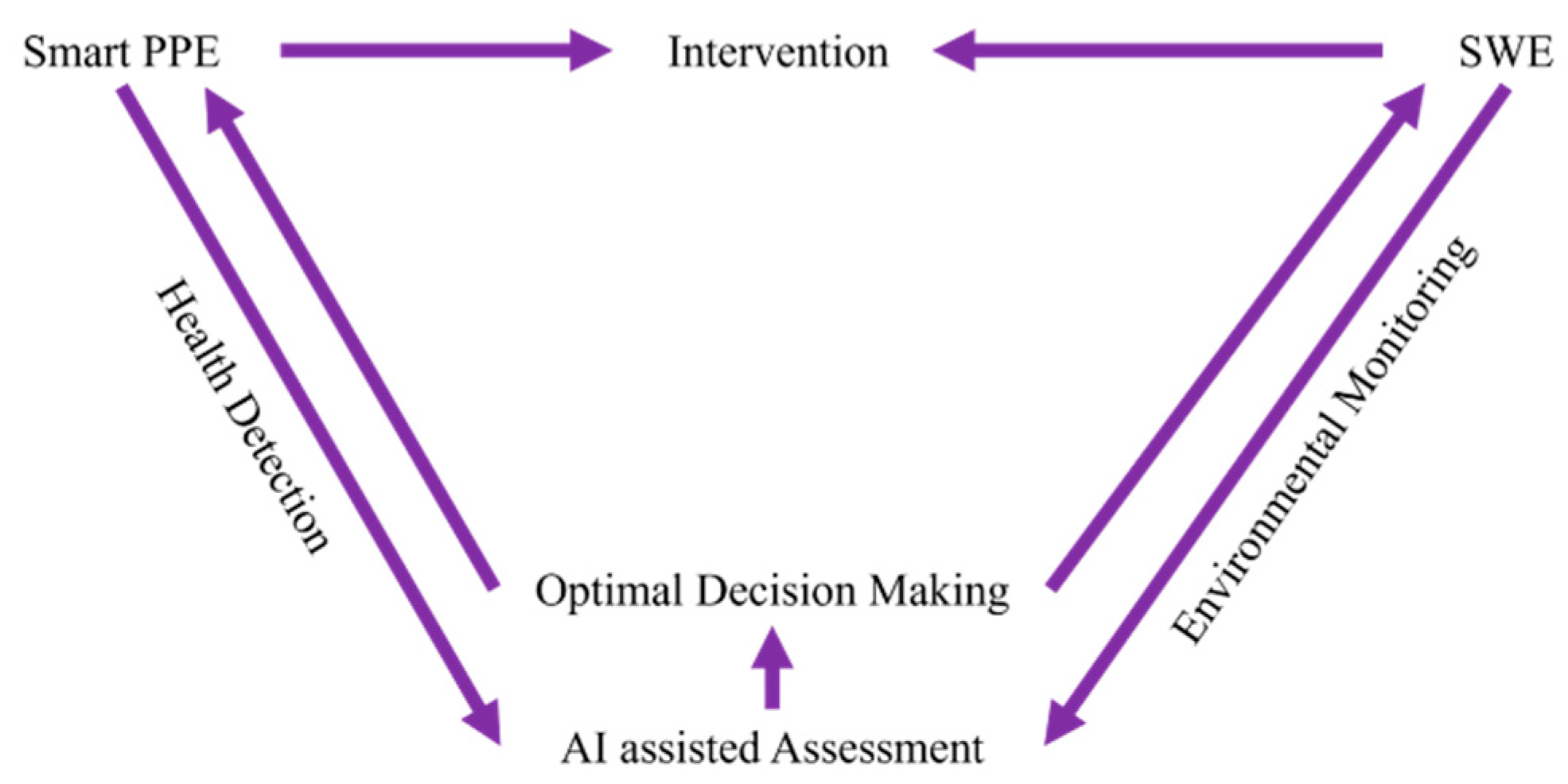
- Real-Time Monitoring: Continuous health condition monitoring is necessary to analyze the influences of COPD risk factors on workers. Motion monitoring is also needed for working state recognition, while real-time environmental monitoring helps identify the COPD risk factors and workers’ positions.
- Dynamic Exposure Assessment: Data fusion technology can combine the information acquired from the wearable sensors and environmental sensors, using it to establish what happened where, when, and to whom. Exposure assessments like exposure profiles and hot points should be carried out considering job types and layout.
- Effective and Targeted Intervention: Intervention must be performed in a personalized way. Instead of pre-setting values, ML algorithms can help producing effective and targeted intervention standards based on different health conditions and jobs. For example, intervention including alarm, LEV control, and smoke cessation suggestions could be delivered through CPS in the workplace. This way, the lung function prediction of each worker and COPD risk assessment of a workplace could be performed without long-time observations.
5. Conclusions
- Heath condition detection methods from industrial perspective are needed for the purpose of occupational protection. Moreover, for different target populations, measurements should be adjusted for a better uptake.
- Traditional hazard assessments rely on manual periodic checks, which are both time-consuming and expensive, and lead to less accurate results. Sensor-based hazard monitoring is supposed to deal with a wide range of hazards. Dynamic WELs, i.e., exposure thresholds varying with time, should be calculated and derived to drive active protection or real-time intervention control introduced by CPS.
- COPD is a chronic disease with complex causes and varies from person to person. Compared to other diseases, it is difficult to convert current COPD diagnosis criteria into computer algorithms. A personalized diagnosis taking an individual’s physical states and circumstances into account is vital for accurate conclusion in decision making.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Prevention, Diagnosis and Management of COPD. Available online: https://goldcopd.org/archived-reports/ (accessed on 2 July 2023).
- HSE. COPD Causes-Occupations and Substances. Available online: https://www.hse.gov.uk/copd/causes.htm (accessed on 2 July 2023).
- Adisesh, A.; Waters-Banker, C. Causes, diagnosis, and progression of COPD following workplace exposure to vapours, gases, dust and fumes. Methods 2021, 2, Q6. [Google Scholar]
- Soumagne, T.; Caillaud, D.; Degano, B.; Dalphin, J.C. Similarities and differences between occupational COPD and COPD after smoking {BPCO professionnelles et BPCO post-tabagique: Similarités et différences}. Rev. Mal. Respir. 2017, 34, 607–617. [Google Scholar] [CrossRef]
- Bala, S.; Tabaku, A. Chronic obstructive pulmonary disease in iron-steel and ferrochrome industry workers. Cent. Eur. J. Public. Health 2010, 18, 93–98. [Google Scholar] [CrossRef]
- Dement, J.M.; Welch, L.; Ringen, K.; Bingham, E.; Quinn, P. Airways obstruction among older construction and trade workers at Department of Energy nuclear sites. Am. J. Ind. Med. 2010, 53, 224–240. [Google Scholar] [CrossRef]
- Melville, A.M.; Pless-Mulloli, T.; Afolabi, O.A.; Stenton, S.C. COPD prevalence and its association with occupational exposures in a general population. Eur. Respir. J. 2010, 36, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, S.; Rushton, L.; Sadhra, S.; Fishwick, D. Estimating the Burden of Occupational Chronic Obstructive Disease due to occupation in Great Britain. Occup. Environ. Med. 2017, 74, A114. [Google Scholar] [CrossRef]
- Department of Health (UK). Consultation on a Strategy for Services for Chronic Obstructive Pulmonary Disease (COPD) in England. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213840/dh_113279.pdf (accessed on 4 July 2023).
- Forum of International Respiratory Societies (FIRS). The Global Impact of Respiratory Disease-Second Edition. Eur. Resp. Soci. 2017. Available online: https://static.physoc.org/app/uploads/2019/04/22192917/The_Global_Impact_of_Respiratory_Disease.pdf (accessed on 30 May 2021).
- Guarascio, A.J.; Ray, S.M.; Finch, C.K.; Self, T.H. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon. Outcomes Res. 2013, 5, 235–245. [Google Scholar] [CrossRef]
- Fletcher, M.J.; Upton, J.; Taylor-Fishwick, J.; Buist, S.A.; Jenkins, C.; Hutton, J.; Barnes, N.; Van Der Molen, T.V.; Walsh, J.W.; Jones, P.; et al. COPD uncovered: An international survey on the impact of chronic obstructive pulmonary disease [COPD] on a working age population. BMC Public Health 2011, 11, 612. [Google Scholar] [CrossRef]
- Foo, J.; Landis, S.H.; Maskell, J.; Oh, Y.M.; van der Molen, T.; Han, M.K.; Mannino, D.M.; Ichinose, M.; Punekar, Y. Continuing to Confront COPD International Patient Survey: Economic Impact of COPD in 12 Countries. PLoS ONE 2016, 11, e0152618. [Google Scholar] [CrossRef]
- HSE. Work-related Chronic Obstructive Pulmonary Disease (COPD) Statistics in Great Britain 2023. Available online: https://www.hse.gov.uk/statistics/assets/docs/copd.pdf (accessed on 22 November 2023).
- Golse, N.; Joly, F.; Combari, P.; Lewin, M.; Nicolas, Q.; Audebert, C.; Samuel, D.; Allard, M.A.; Cunha, A.S.; Castaing, D.; et al. Predicting the risk of post-hepatectomy portal hypertension using a digital twin: A clinical proof of concept. J. Hepatol. 2021, 74, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Low, J.X.; Wei, Y.; Chow, J.; Ali, I.F. ActSen-AI-Enabled Real-Time IoT-Based Ergonomic Risk Assessment System. In Proceedings of the 2019 IEEE International Congress on Internet of Things (ICIOT), Milan, Italy, 8–13 July 2019; pp. 76–78. [Google Scholar]
- Aiello, G.; Giallanza, A.; Giovino, I. Safety optimized shift-scheduling system based on wireless vibration monitoring for mechanical harvesting operations. Chem. Eng. Trans. 2017, 58, 349–354. [Google Scholar] [CrossRef]
- NIH. What Is COPD? Available online: https://www.nhlbi.nih.gov/health/copd (accessed on 24 February 2024).
- NHS. Chronic Obstructive Pulmonary Disease (COPD)-Symptoms. Available online: https://www.nhs.uk/conditions/chronic-obstructive-pulmonary-disease-copd/symptoms/ (accessed on 20 September 2022).
- NHS. Spirometry. Available online: https://www.nhs.uk/conditions/spirometry/ (accessed on 8 June 2022).
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Leidy, N.K. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2019, 34, 648–654. [Google Scholar] [CrossRef]
- Peruzza, S.; Sergi, G.; Vianello, A.; Pisent, C.; Tiozzo, F.; Manzan, A.; Coin, A.; Inelmen, E.M.; Enzi, G. Chronic obstructive pulmonary disease (COPD) in elderly subjects: Impact on functional status and quality of life. Respir. Med. 2013, 97, 612–617. [Google Scholar] [CrossRef]
- Monjazebi, F.; Dalvandi, A.; Ebadi, A.; Khankeh, H.R.; Rahgozar, M.; Richter, J. Functional status assessment of COPD based on ability to perform daily living activities: A systematic review of paper and pencil instruments. Glob. J. Health Sci. 2015, 8, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.R.; Patel, S.; Toffola, L.D.; Bonato, P. Long-term monitoring of COPD using wearable sensors. In Proceedings of the 2nd Conference on Wireless Health, San Diego, CA, USA, 10–13 October 2011; pp. 1–2. [Google Scholar] [CrossRef]
- Bellos, C.C.; Papadopoulos, A.; Rosso, R.; Fotiadis, D.I. Identification of COPD patients’ health status using an intelligent system in the CHRONIOUS wearable platform. IEEE J. Biomed. Health Inform. 2014, 18, 731–738. [Google Scholar] [CrossRef]
- Chouvarda, I.; Philip, N.Y.; Natsiavas, P.; Kilintzis, V.; Sobnath, D.; Kayyali, R.; Henriques, J.; Paiva, R.P.; Raptopoulos, A.; Chetelat, O.; et al. WELCOME—Innovative integrated care platform using wearable sensing and smart cloud computing for COPD patients with comorbidities. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3180–3183. [Google Scholar]
- Van Berkel, J.J.B.N.; Dallinga, J.W.; Möller, G.M.; Godschalk, R.W.L.; Moonen, E.J.; Wouters, E.F.M.; Van Schooten, F.J. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir. Med. 2010, 104, 557–563. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma and COPD. J. Clin. Med. 2021, 10, 32. [Google Scholar] [CrossRef]
- Hendricks, P.I.; Dalgleish, J.K.; Shelley, J.T.; Kirleis, M.A.; McNicholas, M.T.; Li, L.; Chen, T.C.; Chen, C.H.; Duncan, J.S.; Boudreau, F.; et al. Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: Concept, instrumentation development, and performance. Anal. Chem. 2014, 86, 2900–2908. [Google Scholar] [CrossRef]
- Basanta, M.; Jarvis, R.M.; Xu, Y.; Blackburn, G.; Tal-Singer, R.; Woodcock, A.; Singh, D.; Goodacre, R.; Thomas, C.P.; Fowler, S.J. Non-invasive metabolomic analysis of breath using differential mobility spectrometry in patients with chronic obstructive pulmonary disease and healthy smokers. Analyst 2010, 135, 315–320. [Google Scholar] [CrossRef]
- Hauschild, A.C.; Baumbach, J.I.; Baumbach, J. Integrated statistical learning of metabolic ion mobility spectrometry profiles for pulmonary disease identification. Genet. Mol. Res. 2012, 11, 2733–2744. [Google Scholar] [CrossRef]
- Phillips, C.O.; Syed, Y.; Parthaláin, N.M.; Zwiggelaar, R.; Claypole, T.C.; Lewis, K.E. Machine learning methods on exhaled volatile organic compounds for distinguishing COPD patients from healthy controls. J. Breath. Res. 2012, 6, 036003. [Google Scholar] [CrossRef]
- Cristescu, S.M.; Gietema, H.A.; Blanchet, L.; Kruitwagen, C.L.J.J.; Munnik, P.; Van Klaveren, R.J.; Lammers, J.W.J.; Buydens, L.; Harren, F.J.M.; Zanen, P. Screening for emphysema via exhaled volatile organic compounds. J. Breath. Res. 2011, 5, 046009. [Google Scholar] [CrossRef]
- Fens, N.; Zwinderman, A.H.; Schee, M.P.; Nijs, S.B.; Dijkers, E.; Roldaan, A.C.; Cheung, D.; Bel, E.H.; Sterk, P.J. Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 1076–1082. [Google Scholar] [CrossRef]
- Engelen, M.P.; Casaburi, R.; Rucker, R.; Carithers, E. Contribution of the respiratory muscles to the lactic acidosis of heavy exercise in COPD. Chest 1995, 108, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Maekura, R.; Hiraga, T.; Miki, K.; Kitada, S.; Yosimura, K.; Miki, M.; Tateishi, Y. Difference in the physiological response to exercise in patients with distinct severity of COPD pathology. Respir. Care 2014, 59, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Ingebrigtsen, T.S.; Marott, J.L.; Dahl, M.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013, 309, 2353–2361. [Google Scholar] [CrossRef]
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef]
- Bergeron, M.F. Heat cramps: Fluid and electrolyte challenges during tennis in the heat. J. Sci. Med. Sport. 2003, 6, 19–27. [Google Scholar] [CrossRef]
- Implantable & Wearable Medical Devices for Chronic Obstructive Pulmonary Disease. Available online: https://www.nihr.ac.uk/documents/implantable-and-wearable-medical-devices-for-chronic-obstructive-pulmonary-disease/11943 (accessed on 3 June 2022).
- Steidl, E.; Ribeiro, C.S.; Gonçalves, B.F.; Fernandes, N.; Antunes, V.; Mancopes, R. Relationship between dysphagia and exacerbations in chronic obstructive pulmonary disease: A literature review. Int. Arch. Otorhinolaryngol. 2015, 19, 74–79. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Tobino, K.; Sueyasu, T.; Nishizawa, S.; Ko, Y.; Yasuda, M.; Ide, H.; Tsuruno, K.; Miyajima, H. Repetitive saliva swallowing test predicts COPD exacerbation. Int. J. Chron. Obstruct Pulmon Dis. 2019, 14, 2777–2785. [Google Scholar] [CrossRef]
- Patel, N.; Belcher, J.; Thorpe, G.; Forsyth, N.R.; Spiteri, M.A. Measurement of C-reactive protein, procalcitonin and neutrophil elastase in saliva of COPD patients and healthy controls: Correlation to self-reported wellbeing parameters. Respir. Res. 2015, 16, 62. [Google Scholar] [CrossRef]
- Patel, N.; Jones, P.; Adamson, V.; Spiteri, M.; Kinmond, K. Chronic Obstructive Pulmonary Disease Patients’ Experiences of an Enhanced Self-Management Model of Care. Qual. Health Res. 2016, 26, 568–577. [Google Scholar] [CrossRef]
- Higginson, R. Respiratory assessment in critically ill patients: Airway and breathing. Br. J. Nurs. 2013, 18, 456–461. [Google Scholar] [CrossRef]
- Jácome, C.; Marques, A. Computerized respiratory sounds are a reliable marker in subjects with COPD. Respir. Care 2015, 60, 1264–1275. [Google Scholar] [CrossRef]
- Faisal, A.; Alghamdi, B.J.; Ciavaglia, C.E.; Elbehairy, A.F.; Webb, K.A.; Ora, J.; Neder, J.A.; O’Donnell, D.E. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am. J. Respir. Crit. Care Med. 2016, 193, 299–309. [Google Scholar] [CrossRef]
- Fernandez-Granero, M.A.; Sanchez-Morillo, D.; Leon-Jimenez, A. Computerized analysis of telemonitored respiratory sounds for predicting acute exacerbations of COPD. Sensors 2015, 15, 26978–26996. [Google Scholar] [CrossRef]
- Miravitlles, M. Cough and sputum production as risk factors for poor outcomes in patients with COPD. Respir. Med. 2011, 105, 1118–1128. [Google Scholar] [CrossRef]
- Di Marco, R.; Accordini, S.; Cerveri, I.; Corsico, A.; Antó, J.M.; Kunzli, N.; Janson, C.; Sunyer, J.; Jarvis, D.; Chinn, S.; et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am. J. Respir. Crit. Care Med. 2007, 175, 32–39. [Google Scholar] [CrossRef]
- Sumner, H.; Woodcock, A.; Kolsum, U.; Dockry, R.; Lazaar, A.L.; Singh, D.; Vestbo, J.; Smith, J.A. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 943–949. [Google Scholar] [CrossRef]
- Zuo, J.; Rameezdeen, R.; Hagger, M.; Zhou, Z.; Ding, Z. Dust pollution control on construction sites: Awareness and self-responsibility of managers. J. Clean. Prod. 2017, 166, 312–320. [Google Scholar] [CrossRef]
- Kurth, L.; Doney, B.; Weinmann, S. Occupational exposures and chronic obstructive pulmonary disease (COPD): Comparison of a COPD-specific job exposure matrix and expert-evaluated occupational exposures. Occup. Environ. Med. 2017, 74, 290–293. [Google Scholar] [CrossRef]
- Fishwick, D.; Naylor, S. COPD and the workplace. Is it really possible to detect early cases? Occup. Med. 2007, 57, 82–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saraei, M.; Heydarbeygi, E.; Mehrdad, R.; Rouryaghoub, G. Quality of spirometry tests in periodic examination of workers. Int. J. Occup. Hyg. 2018, 10, 75–79. [Google Scholar]
- Wang, B.H.; Cong, S.; Bao, H.L.; Feng, Y.J.; Fan, J.; Wang, N.; Fang, L.W.; Wang, L.H. Analysis on occupational exposure to dust and harmful gas and corresponding protection in adults aged 40 years and older in China, 2014. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 563–568. [Google Scholar] [CrossRef]
- Li, C.Z.; Zhao, Y.; Xu, X. Investigation of dust exposure and control practices in the construction industry: Implications for cleaner production. J. Clean. Prod. 2019, 227, 810–824. [Google Scholar] [CrossRef]
- Lebecki, K.; Małachowski, M.; Sołtysiak, T. Continuous dust monitoring in headings in underground coal mines. J. Sustain. Min. 2016, 15, 125–132. [Google Scholar] [CrossRef]
- Podgorski, D.; Majchrzycka, K.; Dąbrowska, A.; Gralewicz, G.; Okrasa, M. Towards a conceptual framework of OSH risk management in smart working environments based on smart PPE, ambient intelligence and the Internet of Things technologies. Int. J. Occup. Saf. Ergon. 2017, 23, 1–20. [Google Scholar] [CrossRef]
- Babrak, L.M.; Menetski, J.; Rebhan, M.; Nisato, G.; Zinggeler, M.; Brasier, N.; Baerenfaller, K.; Brenzikofer, T.; Baltzer, L.; Vogler, C.; et al. Traditional and digital biomarkers: Two worlds apart? Digit. Biomark. 2019, 3, 92–102. [Google Scholar] [CrossRef]
- Connected Health: How Digital Technology Is Transforming Health and Social Care. Available online: https://www2.deloitte.com/content/dam/Deloitte/uk/Documents/life-sciences-health-care/deloitte-uk-connected-health.pdf (accessed on 4 September 2023).
- Wearables in United Kingdom Market Overview 2023–2027. Available online: https://www.reportlinker.com/market-report/Consumer-Electronics/513215/Wearables (accessed on 1 August 2023).
- Wearable Technology Market Size, Share & Trends Analysis Report By Product (Head & Eyewear, Wristwear), by Application (Consumer Electronics, Healthcare), by Region (Asia Pacific, Europe), and Segment Forecasts, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/wearable-technology-market (accessed on 4 May 2024).
- What is EHS Software?|Intelex. Available online: https://www.intelex.com/ehs/ehs-software/ (accessed on 19 September 2023).
- What is EHS Software? The Complete Guide to EHS Software. Available online: http://www.perillon.com/what-is-ehs-software (accessed on 19 September 2023).
- Naranjo-Hernández, D.; Talaminos-Barroso, A.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rostan, G.; Cejudo-Ramos, P.; Márquez-Martín, E.; Ortega-Ruiz, F. Smart vest for respiratory rate monitoring of COPD patients based on non-contact capacitive sensing. Sensors 2018, 18, 2144. [Google Scholar] [CrossRef]
- Al-Halhouli, A.; Al-Ghussain, L.; Khallouf, O.; Rabadi, A.; Alawadi, J.; Liu, H.; Oweidat, K.A.; Chen, F.; Zheng, D. Clinical Evaluation of Respiratory Rate Measurements on COPD (Male) Patients Using Wearable Inkjet-Printed Sensor. Sensors 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Tipparaju, V.V.; Wang, D.; Yu, J.; Chen, F.; Tsow, F.; Forzani, E.; Tao, N.; Xian, X. Respiration pattern recognition by wearable mask device. Biosens. Bioelectron. 2020, 169, 112590. [Google Scholar] [CrossRef]
- Firdhous, M.F.M.; Sudantha, B.H.; Karunaratne, P.M. IoT enabled proactive indoor air quality monitoring system for sustainable health management. In Proceedings of the 2nd International Conference on Computing and Communications Technologies (ICCCT), Chennai, India, 23–24 February 2017; pp. 216–221. [Google Scholar] [CrossRef]
- Fathallah, H.; Lecuire, V.; Rondeau, E.; Calvé, S.L. Development of an IoT-based system for real time occupational exposure monitoring. In Proceedings of the The Tenth International Conference on Systems and Networks Communications, ICSNC 2015, Barcelone, Spain, 15–20 November 2015. [Google Scholar]
- Zarrin, P.S.; Wenger, C. Pattern recognition for COPD diagnostics using an artificial neural network and its potential integration on hardware-based neuromorphic platforms. In Proceedings of the Artificial Neural Networks and Machine Learning–ICANN 2019: Workshop and Special Sessions: 28th International Conference on Artificial Neural Networks, Munich, Germany, 17–19 September 2019; pp. 284–288. [Google Scholar]
- Lin, W.Y.; Verma, V.K.; Lee, M.Y.; Lin, H.C.; Lai, C.S. Prediction of 30-Day Readmission for COPD Patients Using Accelerometer-Based Activity Monitoring. Sensors 2019, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Hierarchy of Controls|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/topics/hierarchy/ (accessed on 13 January 2022).
- Adjiski, V.; Despodov, Z.; Mirakovski, D.; Serafimovski, D. System architecture to bring smart personal protective equipment wearables and sensors to transform safety at work in the underground mining industry. Rud.-Geološko-Naft. Zb. 2019, 34, 37–44. [Google Scholar] [CrossRef]
- Hood, L.; Friend, S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef]
- O’Connor, S.; Hanlon, P.; O’Donnell, C.A.; Garcia, S.; Glanville, J.; Mair, F.S. Understanding factors affecting patient and public engagement and recruitment to digital health interventions: A systematic review of qualitative studies. BMC Med. Inform. Decis. Mak. 2016, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Brooks, D.; Marques, A. Home telemonitoring in COPD: A systematic review of methodologies and patients’ adherence. Int. J. Med. Inform. 2014, 83, 249–263. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef]
- Nnaji, C.; Awolusi, I.; Park, J.W.; Albert, A. Wearable Sensing Devices: Towards the Development of a Personalized System for Construction Safety and Health Risk Mitigation. Sensors 2021, 21, 682. [Google Scholar] [CrossRef]
- Gimhae, G.N. Six human factors to acceptability of wearable computers. Int. J. Multimedia Ubiquitous Eng. 2013, 8, 103–114. [Google Scholar]
- Yaacoub, J.A.; Salman, O.; Noura, H.N.; Kaaniche, N.; Chehab, A.; Malli, M. Cyber-physical systems security: Limitations, issues and future trends. Microprocess. Microsyst. 2020, 77, 103201. [Google Scholar] [CrossRef]
- Kalirai, K.K. The Effects of Chronic Obstructive Pulmonary Disease on Work Related Outcomes. University of Birmingham, 2016. Available online: https://etheses.bham.ac.uk/id/eprint/6846/ (accessed on 19 November 2022).
- Shanmuganathan, V.; Suresh, A. LSTM-Markov based efficient anomaly detection algorithm for IoT environment. Appl. Soft Comput. 2023, 136, 110054. [Google Scholar] [CrossRef]
- Chouvarda, I.; Kilintzis, V.; Haris, K.; Kaimakamis, V.; Perantoni, E.; Maglaveras, N.; Mendes, L.; Lucio, C.; Teixeira, C.; Henriques, J.; et al. Combining pervasive technologies and Cloud Computing for COPD and comorbidities management. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare-Transforming Healthcare Through Innovations in Mobile and Wireless Technologies (MOBIHEALTH), Athens, Greece, 3–5 November 2014; pp. 352–356. [Google Scholar]
- Chung, H.; Jeong, C.; Luhach, A.K.; Nam, Y.; Lee, J. Remote pulmonary function test monitoring in Cloud platform via smartphone built-in microphone. Evol. Bioinform. Online 2019, 15, 1176934319888904. [Google Scholar] [CrossRef]
- Tavakol, E.; Azari, M.; Zendehdel, R.; Salehpour, S.; Khodakrim, S.; Nikoo, S.; Saranjam, B. Risk Evaluation of Construction Workers’ Exposure to Silica Dust and the Possible Lung Function Impairments. Tanaffos 2017, 16, 295–303. [Google Scholar]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiol. Meas. 2017, 40, 07TR01. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Han, M.K.; Allinson, J.P.; Barr, R.G.; Boucher, R.C.; Calverley, P.M.; Celli, B.R.; Christenson, S.A.; Crystal, R.G.; Fagerås, M.; et al. At the root: Defining and halting progression of early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Drugman, T.; Urbain, J.; Bauwens, N.; Chessini, R.; Valderrama, C.; Lebecque, P.; Dutoit, T. Objective study of sensor relevance for automatic cough detection. IEEE J. Biomed. Health Inf. 2013, 17, 699–707. [Google Scholar] [CrossRef][Green Version]
- Chang, Z.; Jia, K.; Han, T.; Wei, Y.M. Towards more reliable photovoltaic energy conversion systems: A weakly-supervised learning perspective on anomaly detection. Energy Convers. Manag. 2024, 316, 118845. [Google Scholar] [CrossRef]
| Occupation | Substance | |
|---|---|---|
| Agriculture | Brick making | Cadmium dust |
| Construction | Dock workers | Organic dusts |
| Textiles | Quarries | Grain and flour dust |
| Mining | Welders | Welding fumes |
| Stonemasonry | Cadmium | Cadmium fumes |
| Rubber | Plastics | Silica dust |
| Petroleum workers | Foundry workers | Mineral dust |
| Flour and grain workers in the food industry | ||
| Variable | Standard | Indication | Sensor |
|---|---|---|---|
| FEV1 | 15% or 500 mL decline in one year [55] | COPD alarm | Portable spirometer [79,85,86] |
| Respiration rate | 25 breaths per min (bpm) [85,88] | COPD exacerbation | Acoustic sensor [88] |
| Cough | 3 months per year for 2 years [89] | Chronic bronchitis | Microphone [90] |
| Activity | N/A | Working, walking, or sitting | Accelerometer |
| COPD substance | N/A | COPD substance concentration | Air composition analysis |
| Position | WELs | Workers’ positions | Wi-Fi, Bluetooth, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Bakker, O.J.; Bartolo, P.J. Industry 4.0-Compliant Occupational Chronic Obstructive Pulmonary Disease Prevention: Literature Review and Future Directions. Sensors 2024, 24, 5734. https://doi.org/10.3390/s24175734
Jiang Z, Bakker OJ, Bartolo PJ. Industry 4.0-Compliant Occupational Chronic Obstructive Pulmonary Disease Prevention: Literature Review and Future Directions. Sensors. 2024; 24(17):5734. https://doi.org/10.3390/s24175734
Chicago/Turabian StyleJiang, Zhihao, Otto Jan Bakker, and Paulo JDS Bartolo. 2024. "Industry 4.0-Compliant Occupational Chronic Obstructive Pulmonary Disease Prevention: Literature Review and Future Directions" Sensors 24, no. 17: 5734. https://doi.org/10.3390/s24175734
APA StyleJiang, Z., Bakker, O. J., & Bartolo, P. J. (2024). Industry 4.0-Compliant Occupational Chronic Obstructive Pulmonary Disease Prevention: Literature Review and Future Directions. Sensors, 24(17), 5734. https://doi.org/10.3390/s24175734







