Working Memory Workload When Making Complex Decisions: A Behavioral and EEG Study
Abstract
1. Introduction
1.1. Background
1.2. Related Studies
2. Method
2.1. Sample
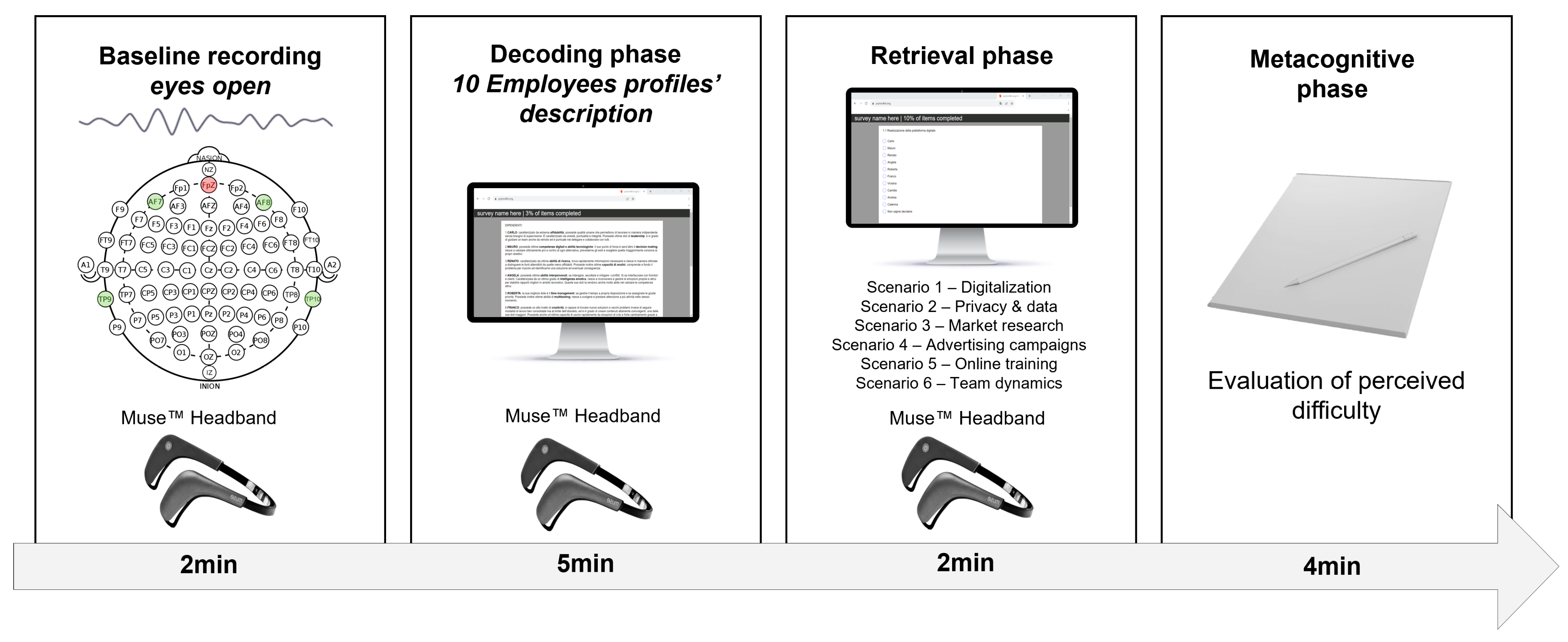
2.2. Procedure
2.3. Experimental Task
2.3.1. Encoding Phase
2.3.2. Retrieval Phase
2.3.3. Metacognitive Phase
2.4. Behavioural Data Acquisition and Behavioural Indices
2.4.1. Index of Efficiency in Complex Decision-Making (EffDecdec–i)
2.4.2. Index of Tolerance of Decisional Complexity (TolDecComdec–i)
2.4.3. Index of Metacognition of Difficulties (MetaDiffdec–i)
2.5. EEG Data Acquisition and Biosignal Analysis
2.6. Data Analysis
3. Results
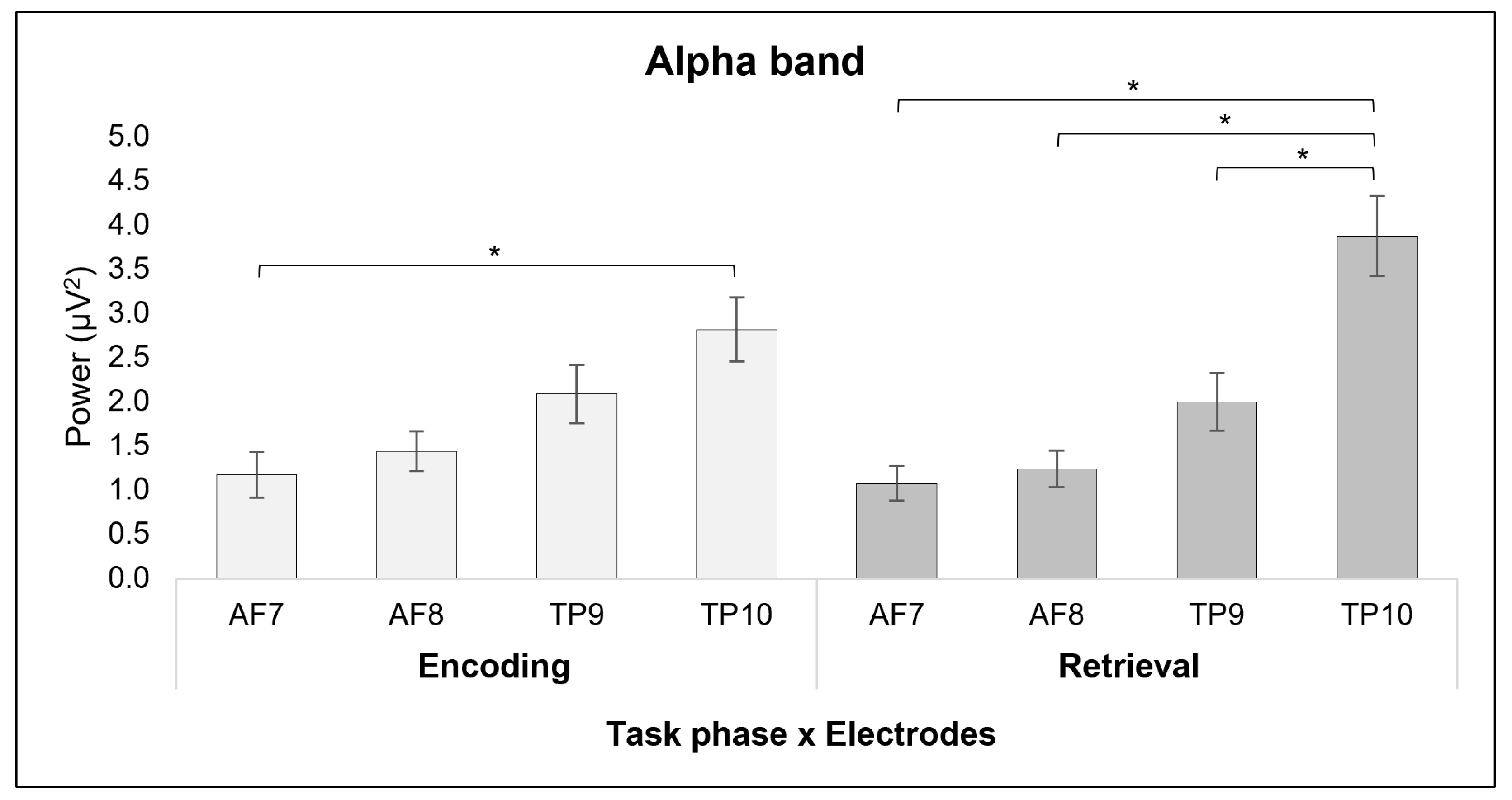
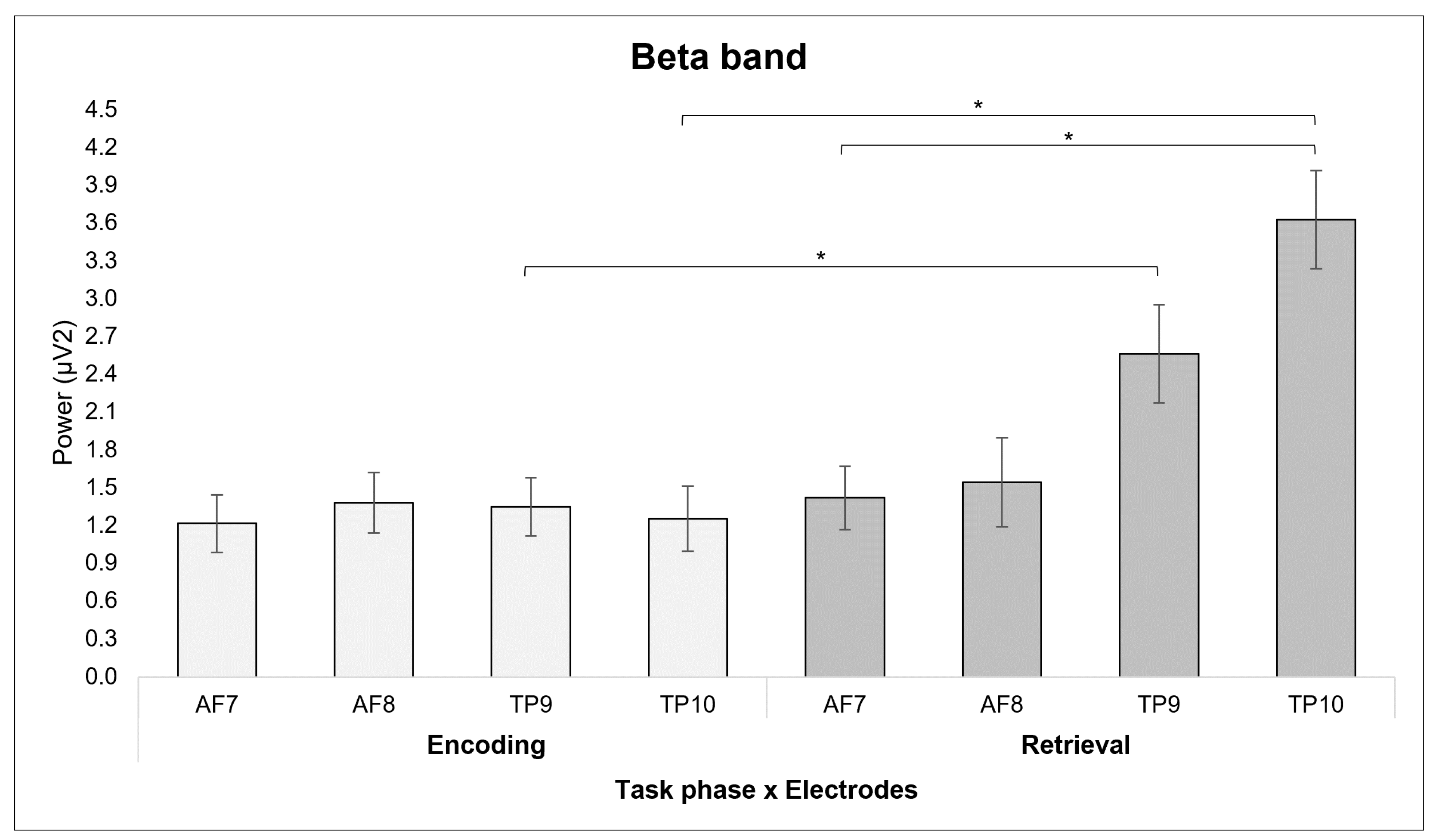
3.1. ANOVA Results
3.1.1. Alpha Band
3.1.2. Beta Band
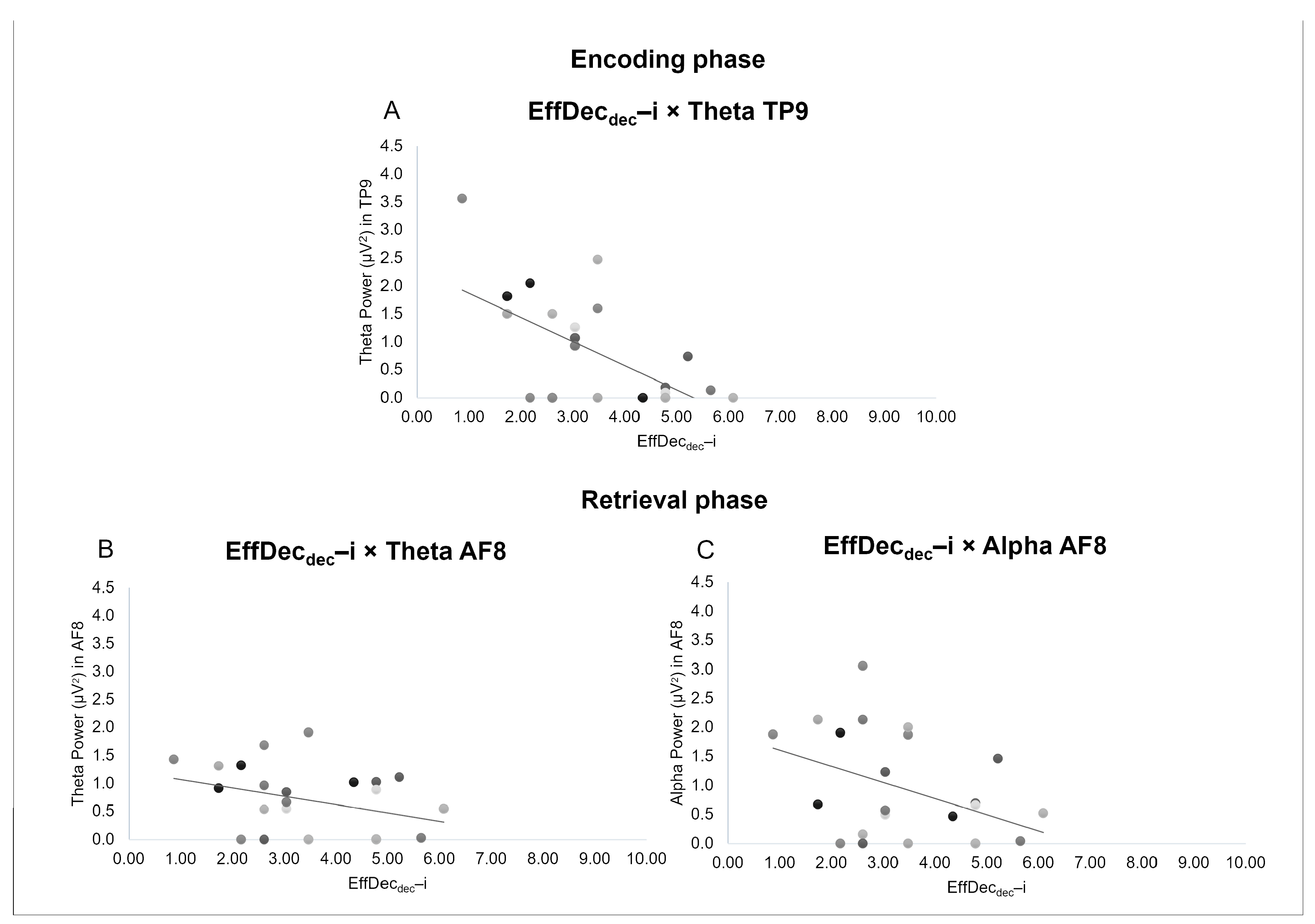
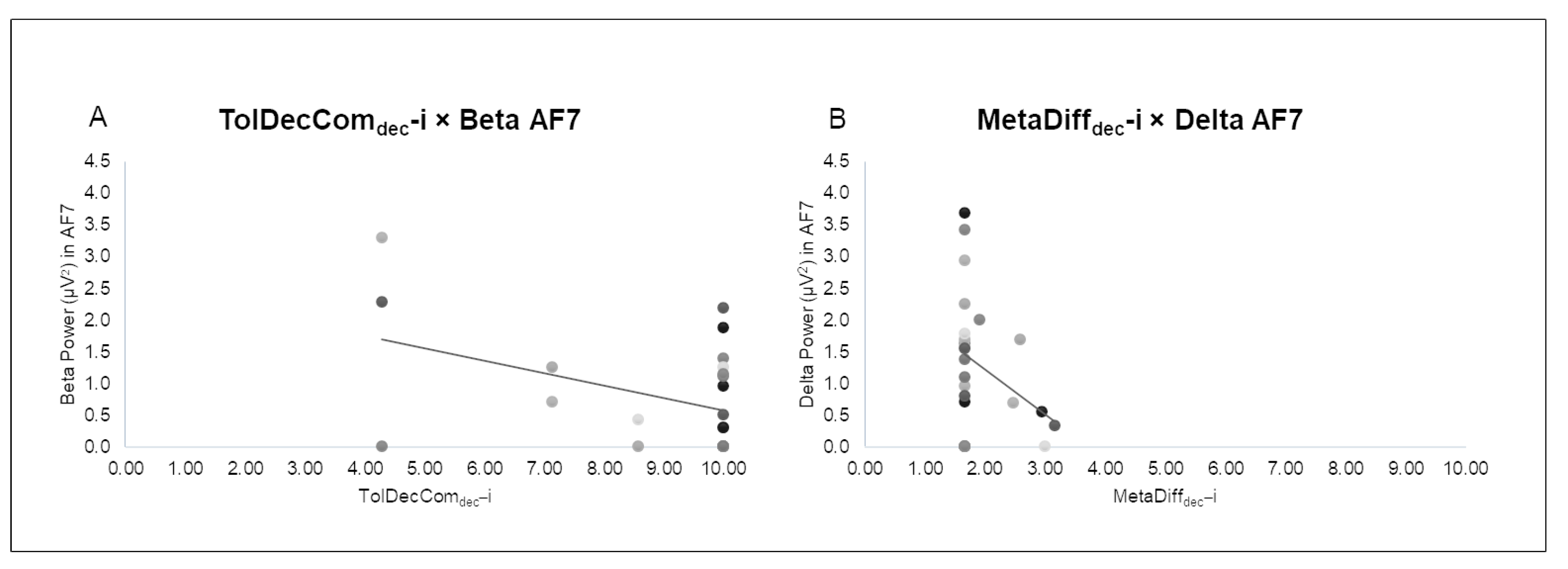
3.2. Correlational Analysis
4. Discussion
4.1. The Role of Alpha and Beta Bands in Encoding and Retrieval
4.2. Correlations between EEG Bands and Behavioral Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, R.; Bandura, A.; Bailey, T. Mechanisms Governing Organizational Performance in Complex Decision-Making Environments. Organ. Behav. Hum. Decis. Process. 1990, 46, 181–201. [Google Scholar] [CrossRef]
- Wood, R.E.; Bailey, T.C. Some Unanswered Questions about Goal Effects: A Recommended Change in Research Methods. Aust. J. Manag. 1985, 10, 61–73. [Google Scholar] [CrossRef]
- Samson, K.; Bhanugopan, R. Strategic Human Capital Analytics and Organisation Performance: The Mediating Effects of Managerial Decision-Making. J. Bus. Res. 2022, 144, 637–649. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L.; Crivelli, D. Neuro-Empowerment of Executive Functions in the Workplace: The Reason Why. Front. Psychol. 2020, 11, 1519. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.W.; Simons, J.S. Theories of Frontal Lobe Executive Function: Clinical Applications. In The Effectiveness of Rehabilitation for Cognitive Deficits; Oxford University Press: Oxford, UK, 2012; ISBN 9780191689420. [Google Scholar]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Laureiro-Martinez, D.; Brusoni, S.; Tata, A.; Zollo, M. The Manager’s Notepad: Working Memory, Exploration, and Performance. J. Manag. Stud. 2019, 56, 1655–1682. [Google Scholar] [CrossRef]
- Cowan, N. Working Memory Capacity: Classic Edition; Psychology Press: New York, NY, USA, 2016. [Google Scholar]
- March, J.G.; Simon, H.A. Organizations, 2nd ed.; Blackwell Business/Blackwell Publishers: Cambridge, MA, USA, 1993; ISBN 0-631-18631-X. [Google Scholar]
- Baddeley, A. Working Memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory: Looking Back and Looking Forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef]
- Curtis, C.E.; Lee, D. Beyond Working Memory: The Role of Persistent Activity in Decision Making. Trends Cogn. Sci. 2010, 14, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Daneman, M.; Carpenter, P.A. Individual Differences in Working Memory and Reading. J. Verbal Learn. Verbal Behav. 1980, 19, 450–466. [Google Scholar] [CrossRef]
- Kirchner, W.K. Age Differences in Short-Term Retention of Rapidly Changing Information. J. Exp. Psychol. 1958, 55, 352. [Google Scholar] [CrossRef]
- Balconi, M. Neuromanagement: People and Organisations; Balconi, M., Ed.; LED Edizioni Universitarie: Milan, Italy, 2021; ISBN 9788879169523. [Google Scholar]
- Venturella, I.; Crivelli, D. Neuromanagement e Comunicazione. Ric. Psicol. 2017, 3, 295–311. [Google Scholar] [CrossRef]
- Camerer, C.; Loewenstein, G.; Prelec, D. Neuroeconomics: How Neuroscience Can Inform Economics. J. Econ. Lit. 2005, 43, 9–64. [Google Scholar] [CrossRef]
- Bleichner, M.G.; Debener, S. Concealed, Unobtrusive Ear-Centered EEG Acquisition: Ceegrids for Transparent EEG. Front. Hum. Neurosci. 2017, 11, 163. [Google Scholar] [CrossRef]
- Balconi, M.; Acconito, C.; Rovelli, K.; Angioletti, L. Influence of and Resistance to Nudge Decision-Making in Professionals. Sustainability 2023, 15, 14509. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L.; Acconito, C. Self-Awareness of Goals Task (SAGT) and Planning Skills: The Neuroscience of Decision Making. Brain Sci. 2023, 13, 1163. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Cassioli, F. “We Will Be in Touch”. A Neuroscientific Assessment of Remote vs. Face-to-Face Job Interviews via EEG Hyperscanning. Soc. Neurosci. 2022, 17, 209–224. [Google Scholar] [CrossRef]
- Knyazev, G.G. Motivation, Emotion, and Their Inhibitory Control Mirrored in Brain Oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sekuler, R. α-Oscillations and the Fidelity of Visual Memory. J. Vis. 2010, 10, 715. [Google Scholar] [CrossRef]
- Sadato, N.; Nakamura, S.; Oohashi, T.; Nishina, E.; Fuwamoto, Y.; Waki, A.; Yonekura, Y. Neural Networks for Generation and Suppression of Alpha Rhythm: A PET Study. Neuroreport 1998, 9, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG Alpha and Theta Oscillations Reflect Cognitive and Memory Performance: A Review and Analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG Alpha Oscillations: The Inhibition-Timing Hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Pizzagalli, D.A. Electroencephalography and High-Density Electrophysiological Source Localization. In Handbook of Psychophysiology; Cacioppo, J.T., Tassinary, L.G., Berntson, G.G., Eds.; Cambridge University Press: Cambridge, UK, 2007; Volume 3, pp. 56–84. [Google Scholar]
- Engel, A.K.; Fries, P. Beta-Band Oscillations-Signalling the Status Quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A. Beta Activity: A Carrier for Visual Attention. Acta Neurobiol. Exp. 2000, 60, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kang, E.; Kang, H.; Kim, J.S.; Jensen, O.; Chung, C.K.; Lee, D.S.; Lee, D.S. Cross-Frequency Power Correlations Reveal the Right Superior Temporal Gyrus as a Hub Region During Working Memory Maintenance. Brain Connect. 2011, 1, 460–472. [Google Scholar] [CrossRef]
- Gola, M.; Magnuski, M.; Szumska, I.; Wróbel, A. EEG Beta Band Activity Is Related to Attention and Attentional Deficits in the Visual Performance of Elderly Subjects. Int. J. Psychophysiol. 2013, 89, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Medendorp, W.P.; Kramer, G.F.I.; Jensen, O.; Oostenveld, R.; Schoffelen, J.M.; Fries, P. Oscillatory Activity in Human Parietal and Occipital Cortex Shows Hemispheric Lateralization and Memory Effects in a Delayed Double-Step Saccade Task. Cereb. Cortex 2007, 17, 2364–2374. [Google Scholar] [CrossRef]
- Khader, P.H.; Jost, K.; Ranganath, C.; Rösler, F. Theta and Alpha Oscillations during Working-Memory Maintenance Predict Successful Long-Term Memory Encoding. Neurosci. Lett. 2010, 468, 339–343. [Google Scholar] [CrossRef]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control Mechanisms in Working Memory: A Possible Function of EEG Theta Oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. EEG Delta Oscillations as a Correlate of Basic Homeostatic and Motivational Processes. Neurosci. Biobehav. Rev. 2012, 36, 677–695. [Google Scholar] [CrossRef]
- Hobson, J.A.; Pace-Schott, E.F. The Cognitive Neuroscience of Sleep: Neuronal Systems, Consciousness and Learning. Nat. Rev. Neurosci. 2002, 3, 679–693. [Google Scholar] [CrossRef]
- Balconi, M.; Venturella, I.; Fronda, G.; Vanutelli, M.E. Leader-Employee Emotional “Interpersonal Tuning”. An EEG Coherence Study. Soc. Neurosci. 2020, 15, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G.; Savostyanov, A.N.; Bocharov, A.V.; Dorosheva, E.A.; Tamozhnikov, S.S.; Saprigyn, A.E. Oscillatory Correlates of Moral Decision-Making: Effect of Personality. Soc. Neurosci. 2016, 11, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, J.A.; Zaveri, H.P.; Goncharova, I.I.; Distasio, M.M.; Papademetris, X.; Spencer, S.S.; Spencer, D.D.; Constable, R.T. Effects of Working Memory Load on Oscillatory Power in Human Intracranial EEG. Cereb. Cortex 2008, 18, 1843–1855. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; McEvoy, L.; Yu, D. High-Resolution EEG Mapping of Cortical Activation Related to Working Memory: Effects of Task Difficulty, Type of Processing, and Practice. Cereb. Cortex 1979, 7, 374–385. [Google Scholar] [CrossRef]
- Michels, L.; Bucher, K.; Lüchinger, R.; Klaver, P.; Martin, E.; Jeanmonod, D.; Brandeis, D. Simultaneous EEG-FMRI during a Working Memory Task: Modulations in Low and High Frequency Bands. PLoS ONE 2010, 5, e10298. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG-Alpha Rhythms and Memory Processes. Int. J. Psychophysiol. 1997, 26, 319–340. [Google Scholar] [CrossRef]
- Kardan, O.; Adam, K.C.S.; Mance, I.; Churchill, N.W.; Vogel, E.K.; Berman, M.G. Distinguishing Cognitive Effort and Working Memory Load Using Scale-Invariance and Alpha Suppression in EEG. Neuroimage 2020, 211, 116622. [Google Scholar] [CrossRef]
- Deiber, M.-P.; Missonnier, P.; Bertrand, O.; Gold, G.; Fazio-Costa, L.; Ibañez, V.; Giannakopoulos, P. Distinction between Perceptual and Attentional Processing in Working Memory Tasks: A Study of Phase-Locked and Induced Oscillatory Brain Dynamics. J. Cogn. Neurosci. 2007, 19, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Bočková, M.; Chládek, J.; Jurák, P.; Halámek, J.; Rektor, I. Executive Functions Processed in the Frontal and Lateral Temporal Cortices: Intracerebral Study. Clin. Neurophysiol. 2007, 118, 2625–2636. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.M.; Èki, L.S.; Koivisto, M.; Saarela, C.; Èggqvist, A.H.; Laine, M.; Èma, H.; Èinen, È. The Effects of Memory Load on Event-Related EEG Desynchronization and Synchronization. Clin. Neurophysiol. 2000, 111, 2071–2078. [Google Scholar] [CrossRef]
- Pesonen, M.; Hämäläinen, H.; Krause, C.M. Brain Oscillatory 4-30 Hz Responses during a Visual n-Back Memory Task with Varying Memory Load. Brain Res. 2007, 1138, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Brevers, D.; Cleeremans, A.; Bechara, A.; Greisen, M.; Kornreich, C.; Verbanck, P.; Noël, X. Impaired Self-Awareness in Pathological Gamblers. J. Gambl. Stud. 2013, 29, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M. Introduction: Communication as an Intentionalization Process Intentions and Communication: Cognitive Strategies, Metacognition and Social Cognition. In Neuropsychology of Communication; Springer: Milan, Italy, 2010; pp. 159–175. [Google Scholar]
- Cona, G.; Chiossi, F.; Di Tomasso, S.; Pellegrino, G.; Piccione, F.; Bisiacchi, P.; Arcara, G. Theta and Alpha Oscillations as Signatures of Internal and External Attention to Delayed Intentions: A Magnetoencephalography (MEG) Study. Neuroimage 2020, 205, 116295. [Google Scholar] [CrossRef]
- Cabeza, R.; Nyberg, L. Neural Bases of Learning and Memory: Functional Neuroimaging Evidence; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; Volume 13. [Google Scholar]
- Nee, D.E.; Brown, J.W.; Askren, M.K.; Berman, M.G.; Demiralp, E.; Krawitz, A.; Jonides, J. A Meta-Analysis of Executive Components of Working Memory. Cereb. Cortex 2013, 23, 264–282. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-Band Oscillations, Attention, and Controlled Access to Stored Information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Klimesch, W. Event-Related Desynchronization during Motor Behavior and Visual Information Processing. Electroencephalogr. Clin. Neurophysiol. Suppl. 1991, 42, 58–65. [Google Scholar]
- Proskovec, A.L.; Heinrichs-Graham, E.; Wilson, T.W. Load Modulates the Alpha and Beta Oscillatory Dynamics Serving Verbal Working Memory. Neuroimage 2019, 184, 256–265. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, X. Modulation of Alpha and Beta Oscillations during an N-Back Task with Varying Temporal Memory Load. Front. Psychol. 2016, 6, 2031. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Caldiroli, C. Semantic Violation Effect on Object-Related Action Comprehension. N400-like Event-Related Potentials for Unusual and Incorrect Use. Neuroscience 2011, 197, 191–199. [Google Scholar] [CrossRef]
- Grunwald, M.; Weiss, T.; Mueller, S.; Rall, L. EEG Changes Caused by Spontaneous Facial Self-Touch May Represent Emotion Regulating Processes and Working Memory Maintenance. Brain Res. 2014, 1557, 111–126. [Google Scholar] [CrossRef]
- Stoet, G. PsyToolkit: A Software Package for Programming Psychological Experiments Using Linux. Behav. Res. Methods 2010, 42, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Stoet, G. PsyToolkit: A Novel Web-Based Method for Running Online Questionnaires and Reaction-Time Experiments. Teach. Psychol. 2017, 44, 24–31. [Google Scholar] [CrossRef]
- Ratti, E.; Waninger, S.; Berka, C.; Ruffini, G.; Verma, A. Comparison of Medical and Consumer Wireless EEG Systems for Use in Clinical Trials. Front. Hum. Neurosci. 2017, 11, 266829. [Google Scholar] [CrossRef]
- Proskovec, A.L.; Wiesman, A.I.; Heinrichs-Graham, E.; Wilson, T.W. Beta Oscillatory Dynamics in the Prefrontal and Superior Temporal Cortices Predict Spatial Working Memory Performance. Sci. Rep. 2018, 8, 8488. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef]
- Murta, T.; Leite, M.; Carmichael, D.W.; Figueiredo, P.; Lemieux, L. Electrophysiological Correlates of the BOLD Signal for EEG-Informed FMRI. Hum. Brain Mapp. 2015, 36, 391–414. [Google Scholar] [CrossRef]
- Scheeringa, R.; Petersson, K.M.; Oostenveld, R.; Norris, D.G.; Hagoort, P.; Bastiaansen, M.C.M. Trial-by-Trial Coupling between EEG and BOLD Identifies Networks Related to Alpha and Theta EEG Power Increases during Working Memory Maintenance. Neuroimage 2009, 44, 1224–1238. [Google Scholar] [CrossRef]
- Scheeringa, R.; Fries, P.; Petersson, K.M.; Oostenveld, R.; Grothe, I.; Norris, D.G.; Hagoort, P.; Bastiaansen, M.C.M. Neuronal Dynamics Underlying High- and Low-Frequency EEG Oscillations Contribute Independently to the Human BOLD Signal. Neuron 2011, 69, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Fegen, D.; Buchsbaum, B.R.; D’Esposito, M. The Effect of Rehearsal Rate and Memory Load on Verbal Working Memory. Neuroimage 2015, 105, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Jonides, J. Working Memory: A View from Neuroimaging. Cogn. Psychol. 1997, 33, 5–42. [Google Scholar] [CrossRef]
- Baddeley, A.; Logie, R.; Bressi, S.; Sala, S.D.; Spinnler, H. Dementia and Working Memory. Q. J. Exp. Psychol. Sect. A 1986, 38, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, S. Working Memory in the Prefrontal Cortex. Brain Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Schabus, M.; Doppelmayr, M. Fronto-Parietal EEG Coherence in Theta and Upper Alpha Reflect Central Executive Functions of Working Memory. Int. J. Psychophysiol. 2005, 57, 97–103. [Google Scholar] [CrossRef]
- Manza, P.; Hau, C.L.V.; Leung, H.C. Alpha Power Gates Relevant Information during Working Memory Updating. J. Neurosci. 2014, 34, 5998–6002. [Google Scholar] [CrossRef]
- Román-López, T.V.; Caballero-Sánchez, U.; Cisneros-Luna, S.; Franco-Rodríguez, J.A.; Méndez-Díaz, M.; Prospéro-García, O.; Ruiz-Contreras, A.E. Brain Electrical Activity from Encoding to Retrieval While Maintaining and Manipulating Information in Working Memory. Memory 2019, 27, 1063–1078. [Google Scholar] [CrossRef]
- Zanto, T.P.; Gazzaley, A. Neural Suppression of Irrelevant Information Underlies Optimal Working Memory Performance. J. Neurosci. 2009, 29, 3059–3066. [Google Scholar] [CrossRef]
- Kim, H. Neural Activity during Working Memory Encoding, Maintenance, and Retrieval: A Network-Based Model and Meta-Analysis. Hum. Brain Mapp. 2019, 40, 4912–4933. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A Dual-Networks Architecture of Top-down Control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, E.; Niedermeyer, E.; Lopes da Silva, F. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1993. [Google Scholar]
- Vincent, J.L.; Kahn, I.; Snyder, A.Z.; Raichle, M.E.; Buckner, R.L. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J. Neurophysiol. 2008, 100, 3328–3342. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J. Frontal Theta as a Mechanism for Cognitive Control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M.; Bergner, S.; Könen, T.; Fink, A.; Neubauer, A.C. EEG Alpha Synchronization Is Related to Top-down Processing in Convergent and Divergent Thinking. Neuropsychologia 2011, 49, 3505–3511. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S.; Weekes, N.Y.; Wang, T.H. The Effect of a Naturalistic Stressor on Frontal EEG Asymmetry, Stress, and Health. Biol. Psychol. 2007, 75, 239–247. [Google Scholar] [CrossRef]
- Tran, Y.; Thuraisingham, R.A.; Wijesuriya, N.; Nguyen, H.T.; Craig, A. Detecting Neural Changes during Stress and Fatigue Effectively: A Comparison of Spectral Analysis and Sample Entropy. In Proceedings of the 2007 3rd International IEEE/EMBS Conference on Neural Engineering, Kohala Coast, HI, USA, 2–5 May 2007; pp. 350–353. [Google Scholar] [CrossRef]
- Harmony, T. The Functional Significance of Delta Oscillations in Cognitive Processing. Front. Integr. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef]
- Zarjam, P.; Epps, J.; Chen, F. Characterizing Working Memory Load Using EEG Delta Activity. In Proceedings of the 19th European Signal Processing Conference (EUSIPCO 2011), Barcelona, Spain, 29 August–2 September 2011. [Google Scholar]
- Ochab, J.K.; Wątorek, M.; Ceglarek, A.; Fafrowicz, M.; Lewandowska, K.; Marek, T.; Sikora-Wachowicz, B.; Oświęcimka, P. Task-Dependent Fractal Patterns of Information Processing in Working Memory. Sci. Rep. 2022, 12, 17866. [Google Scholar] [CrossRef]
- Cannard, C.; Wahbeh, H.; Delorme, A. Validating the Wearable MUSE Headset for EEG Spectral Analysis and Frontal Alpha Asymmetry. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Houston, TX, USA, 9–12 December 2021; pp. 3603–3610. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, H. Reliability of MUSE 2 and Tobii Pro Nano at Capturing Mobile Application Users’ Real-Time Cognitive Workload Changes. Front. Neurosci. 2022, 16, 1011475. [Google Scholar] [CrossRef]
| Profile | Description |
|---|---|
| Viviana | Viviana has excellent teamwork skills. She has the ability to work excellently in a team regardless of hierarchical position. She is characterized by enthusiasm and often proposes good initiatives. She is able to create an optimal work environment for production purposes. |
| Mauro | Mauro has excellent digital and technological skills. His strong point is undoubtedly the decision-making process: he is able to excellently evaluate the pros and cons of each alternative, predict the outcomes and choose the one best suited to his objectives. |
| Caterina | Angela has excellent interpersonal skills, knows how to interact, listen, and mitigate conflicts. Characterized by an excellent degree of emotional intelligence, she is able to recognize and manage her own and other people’s emotions to establish better relationships in the workplace. These qualities also make her very qualified in evaluating the skills of others. She is extremely creative and innovative in creating digital, social and educational content. |
| Scenario | Description | Work Assignment |
|---|---|---|
| 1—Digitalization | Your company is launching a new project that aims at digitalizing the hiring interviews. In fact, it is planned to include a phase of simulations and computerized tests within the interviews. To carry out this project, it will be necessary to complete two steps: to design a digital platform and design the most appropriate and creative tests for the evaluation to be included in the platform. Which of your employees would you assign to the following work assignments? | 1a. Design of the digital platform1b. Design of creative content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balconi, M.; Rovelli, K.; Angioletti, L.; Allegretta, R.A. Working Memory Workload When Making Complex Decisions: A Behavioral and EEG Study. Sensors 2024, 24, 5754. https://doi.org/10.3390/s24175754
Balconi M, Rovelli K, Angioletti L, Allegretta RA. Working Memory Workload When Making Complex Decisions: A Behavioral and EEG Study. Sensors. 2024; 24(17):5754. https://doi.org/10.3390/s24175754
Chicago/Turabian StyleBalconi, Michela, Katia Rovelli, Laura Angioletti, and Roberta A. Allegretta. 2024. "Working Memory Workload When Making Complex Decisions: A Behavioral and EEG Study" Sensors 24, no. 17: 5754. https://doi.org/10.3390/s24175754
APA StyleBalconi, M., Rovelli, K., Angioletti, L., & Allegretta, R. A. (2024). Working Memory Workload When Making Complex Decisions: A Behavioral and EEG Study. Sensors, 24(17), 5754. https://doi.org/10.3390/s24175754








