Feasibility Study on the Use of NO2 and PM2.5 Sensors for Exposure Assessment and Indoor Source Apportionment at Fixed Locations
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Materials
2.3. Data Collection
2.4. Data Analysis
2.4.1. Overview
2.4.2. Sensor Data Quality Assurance
2.4.3. Activity Specific I/O Ratio
2.4.4. Source Apportionment
2.4.5. Symptomatology
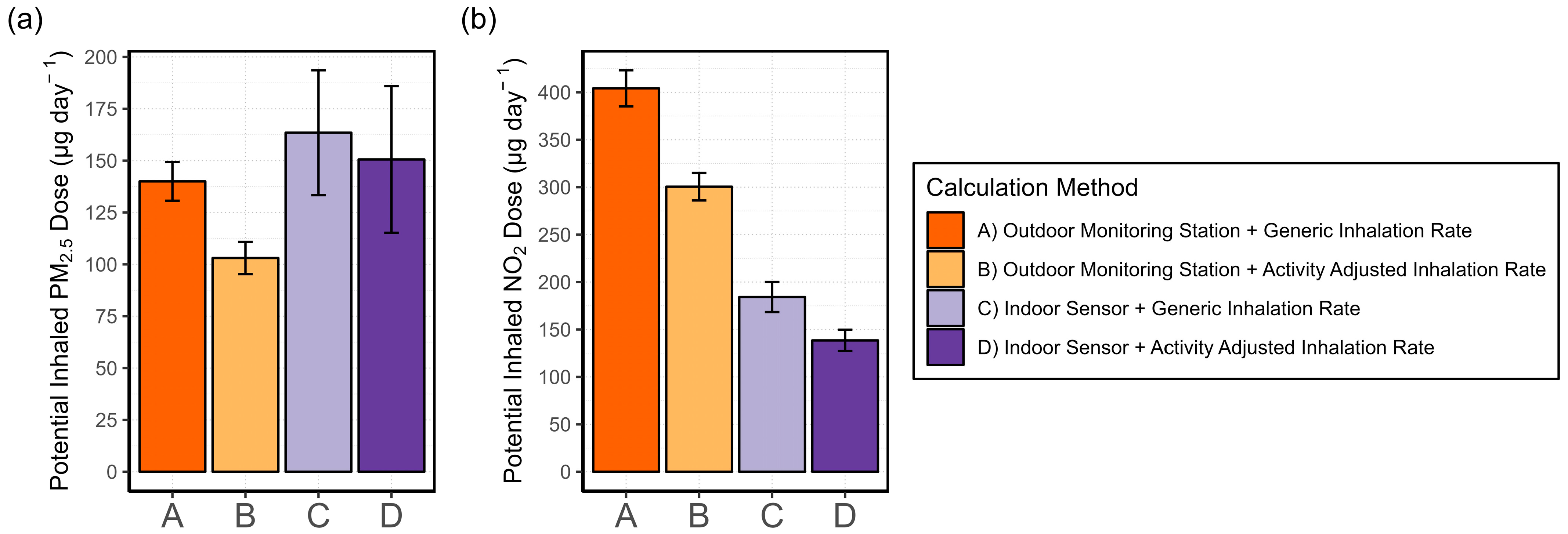
2.4.6. Exposure Assessment
- (A)
- Generic IR + outdoor monitoring station data
- (B)
- Activity-adjusted IR + outdoor monitoring station data
- (C)
- Generic IR + indoor AQSS data
- (D)
- Activity-adjusted IR + indoor AQSS data
3. Results
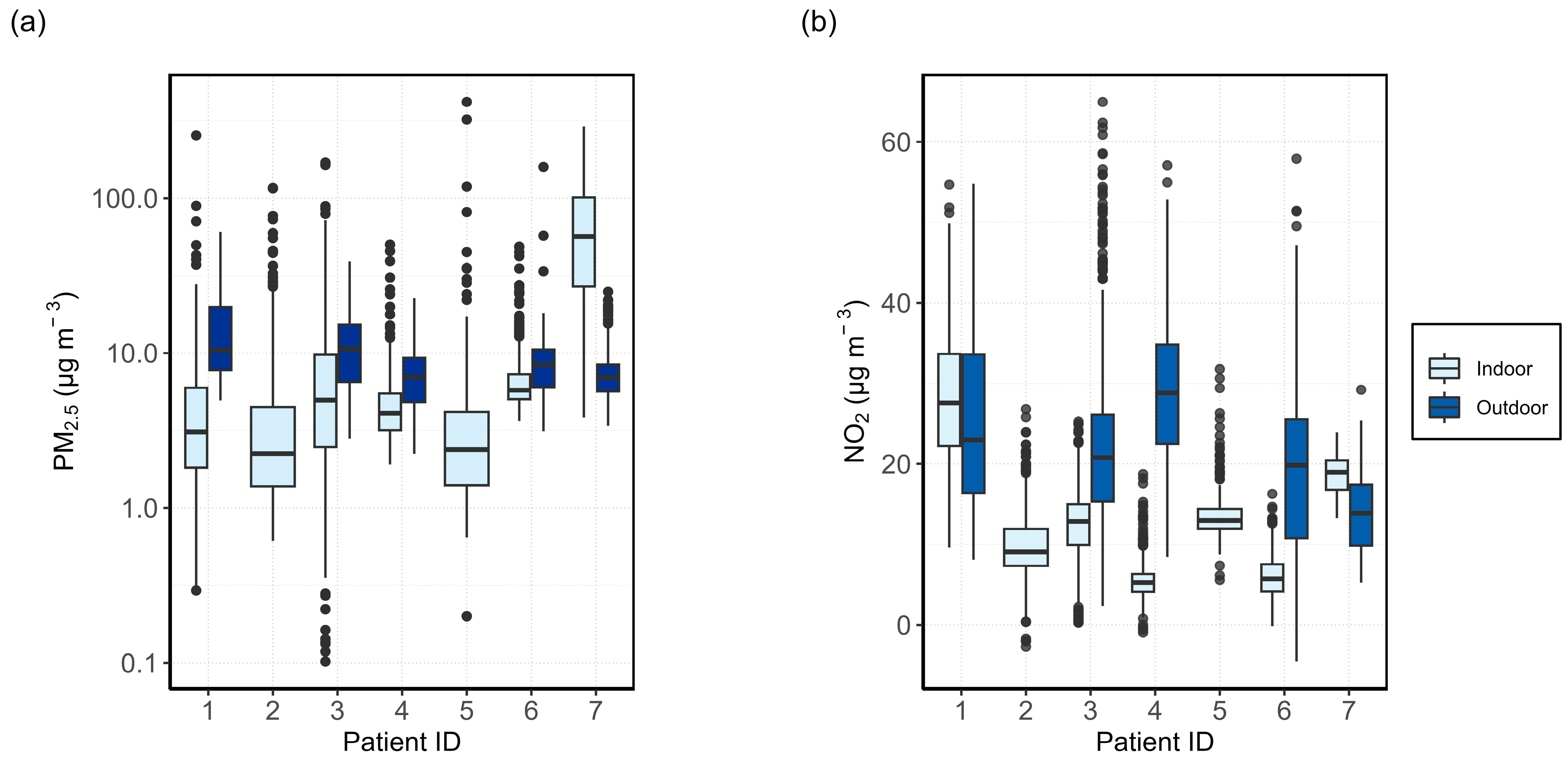
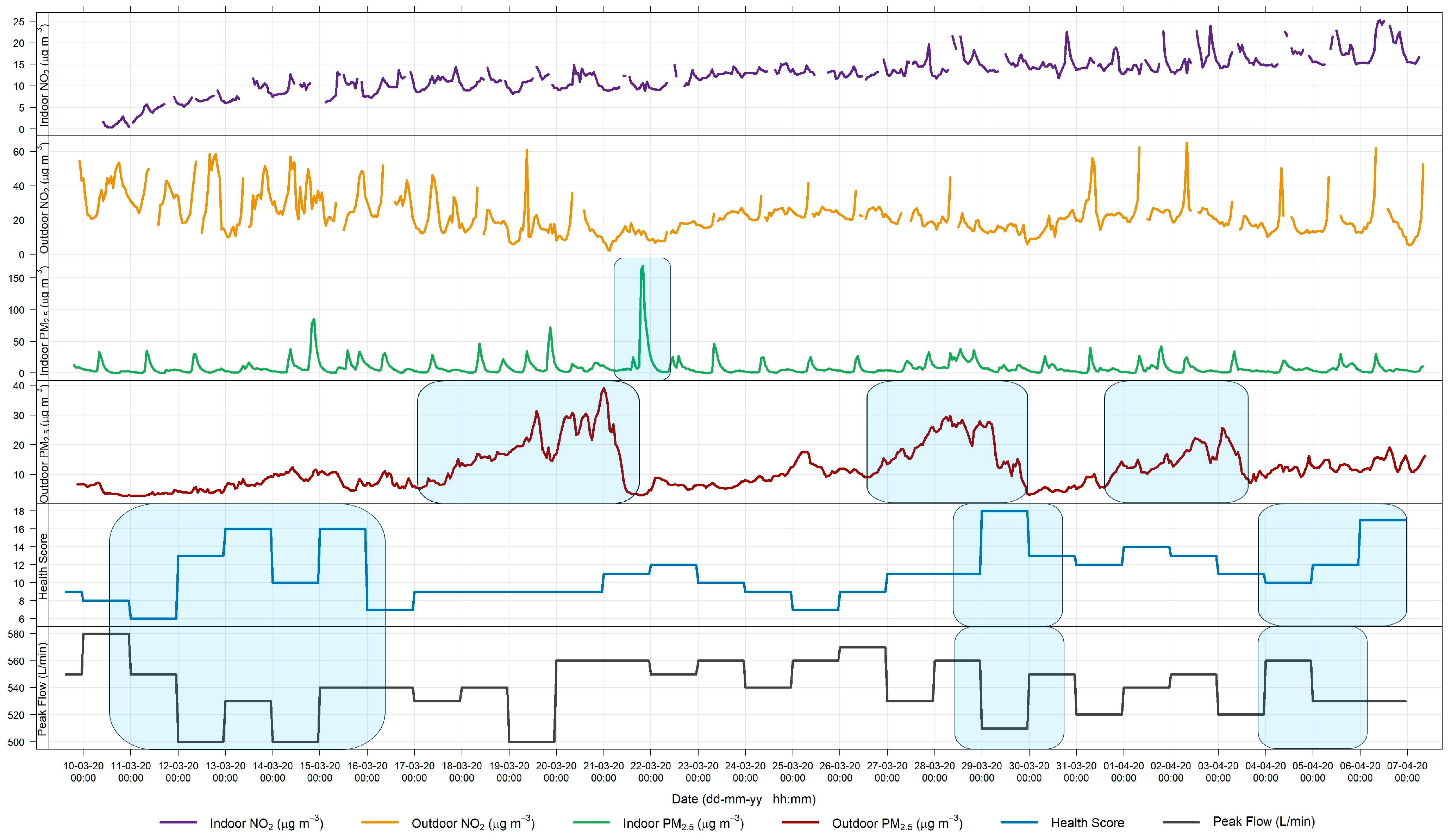
3.1. Relationship between Indoor and Outdoor Air Quality
3.1.1. General Comparison
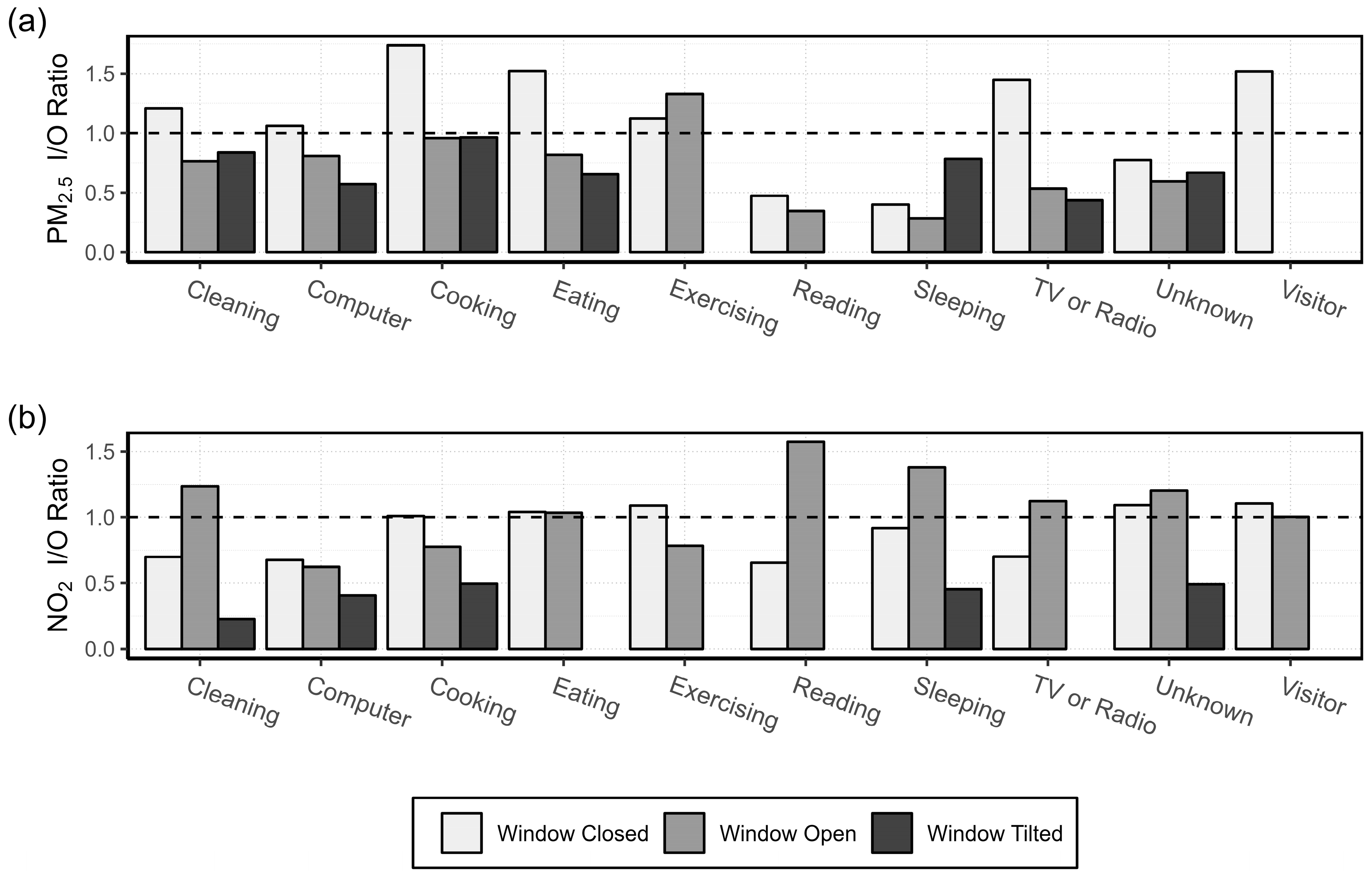
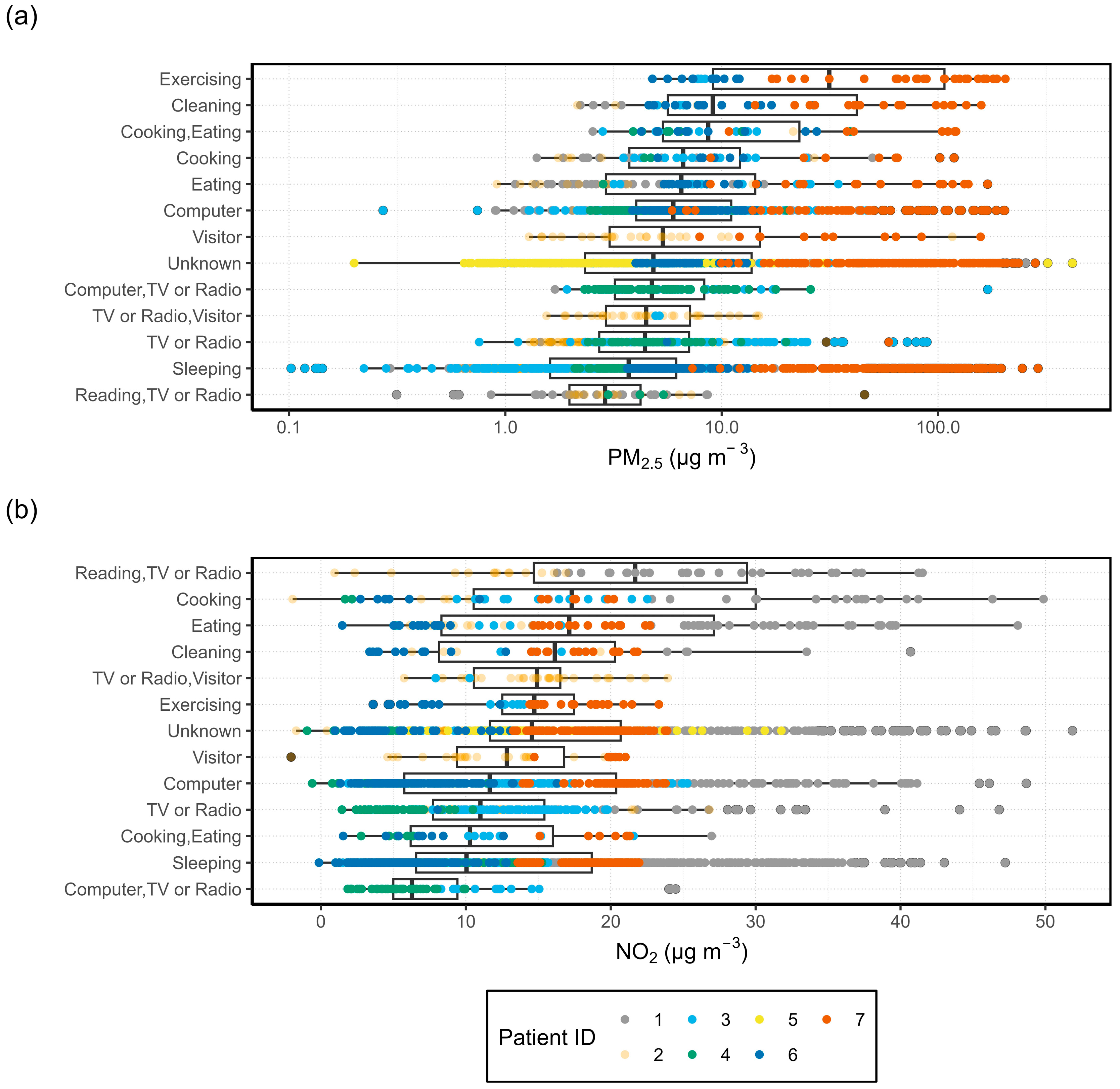
3.1.2. Activity Specific I/O Ratio
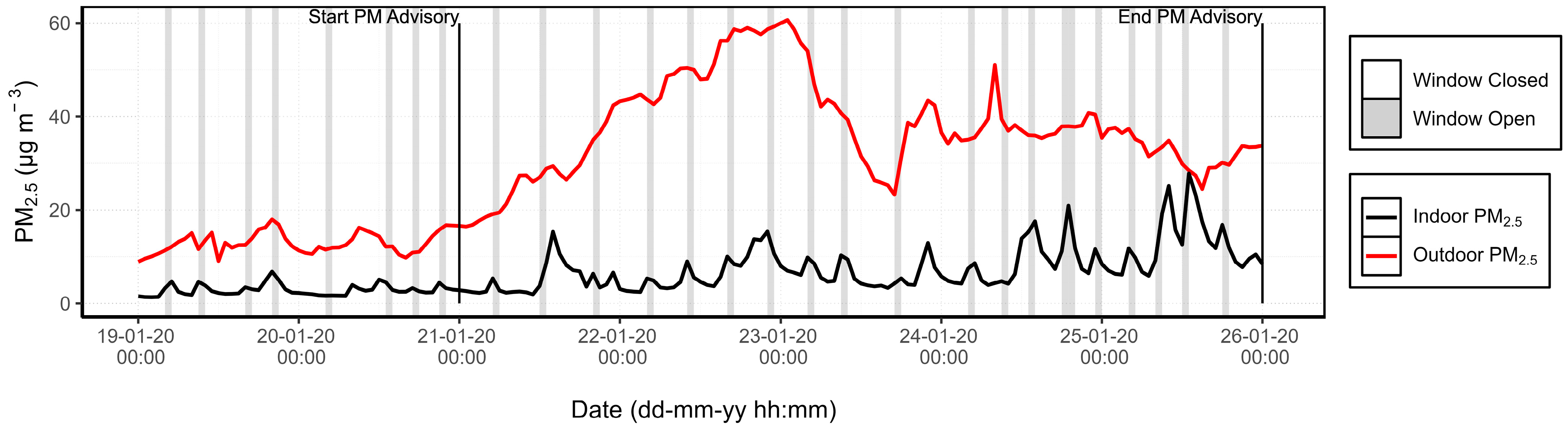
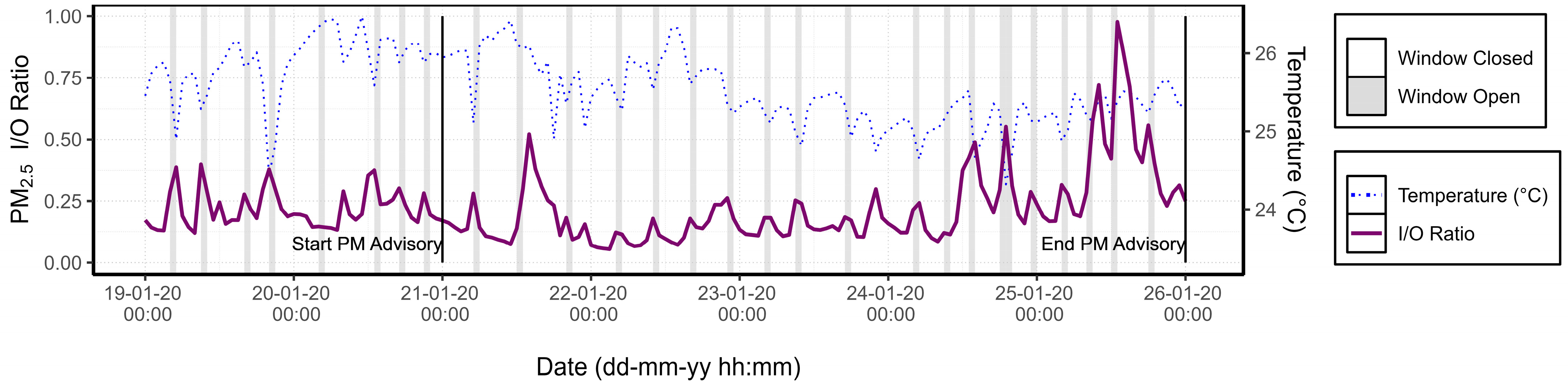
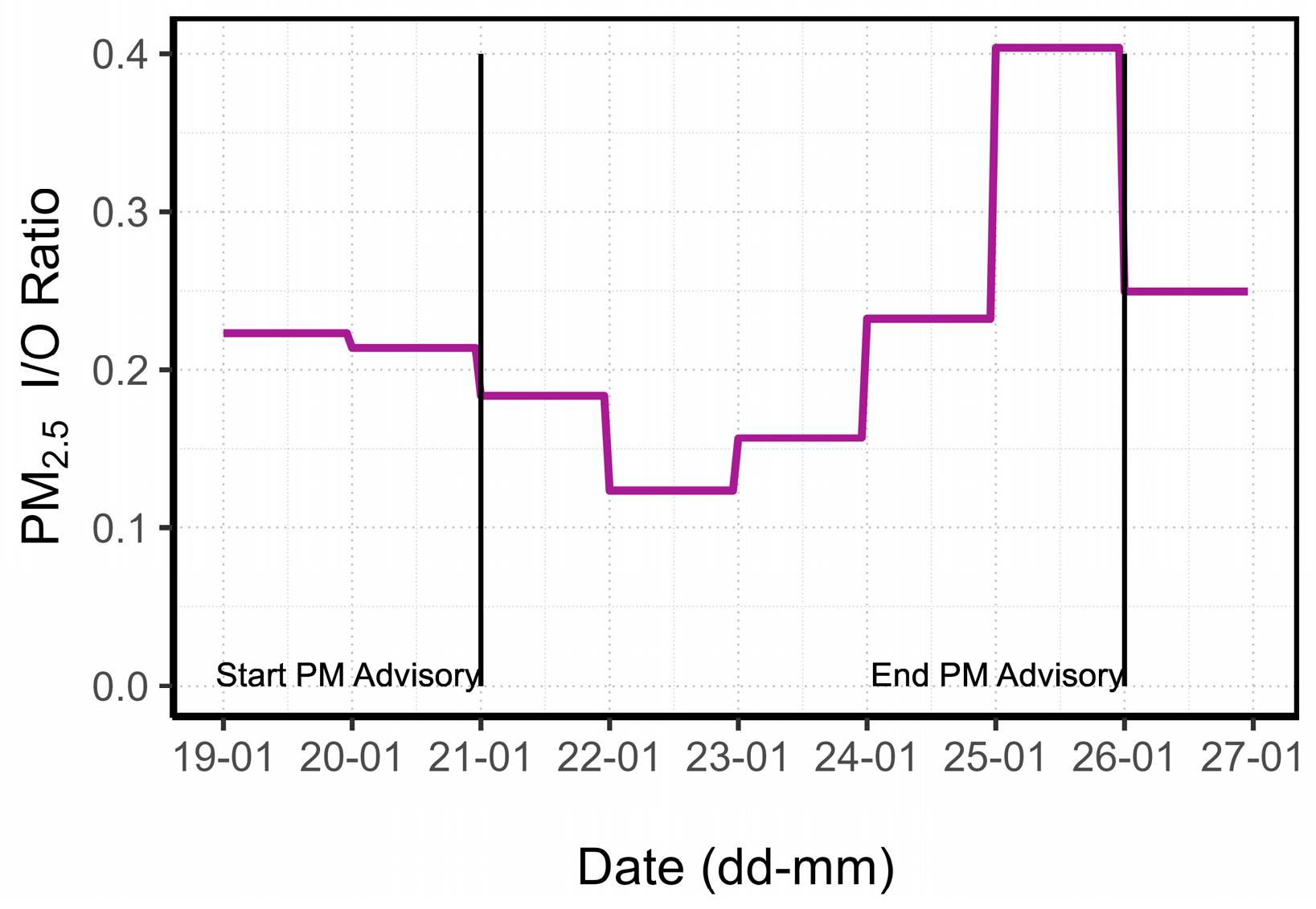
3.1.3. PM Advisory Study
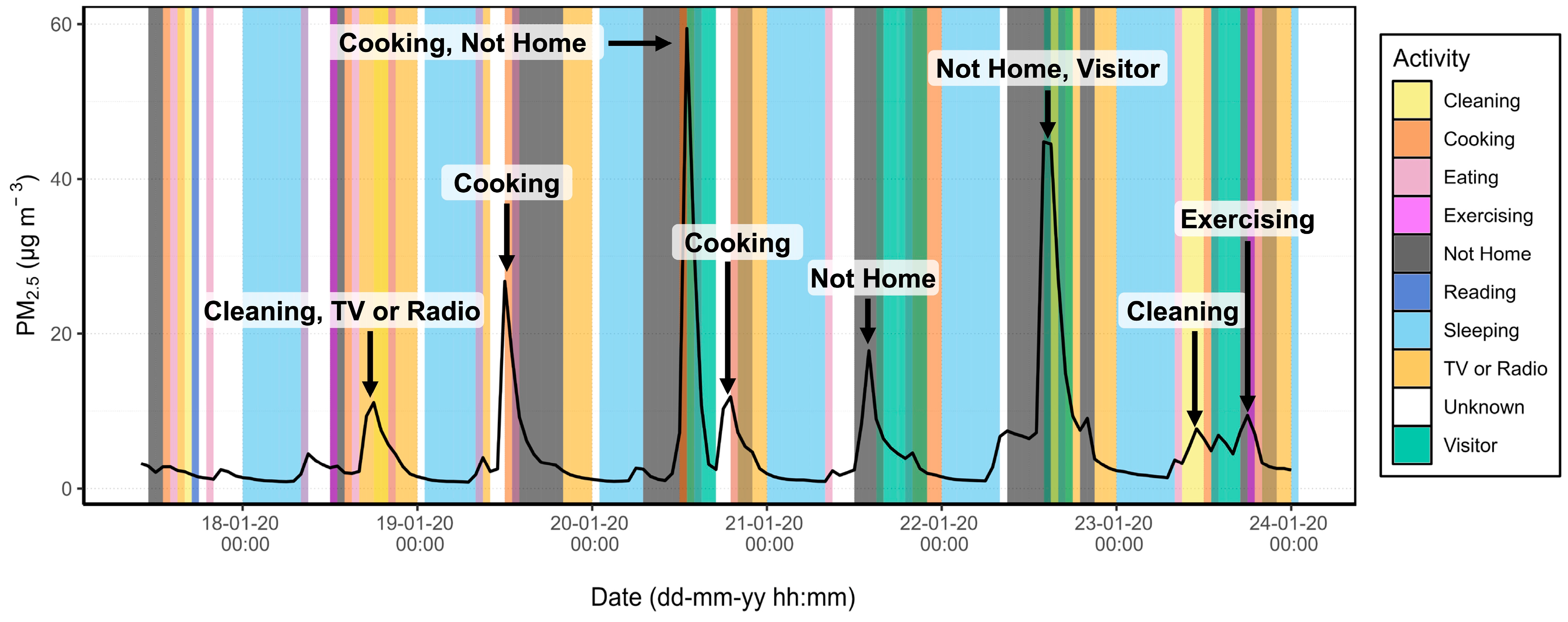
3.2. Source Apportionment
3.3. Symptomatology
3.4. Exposure Assessment
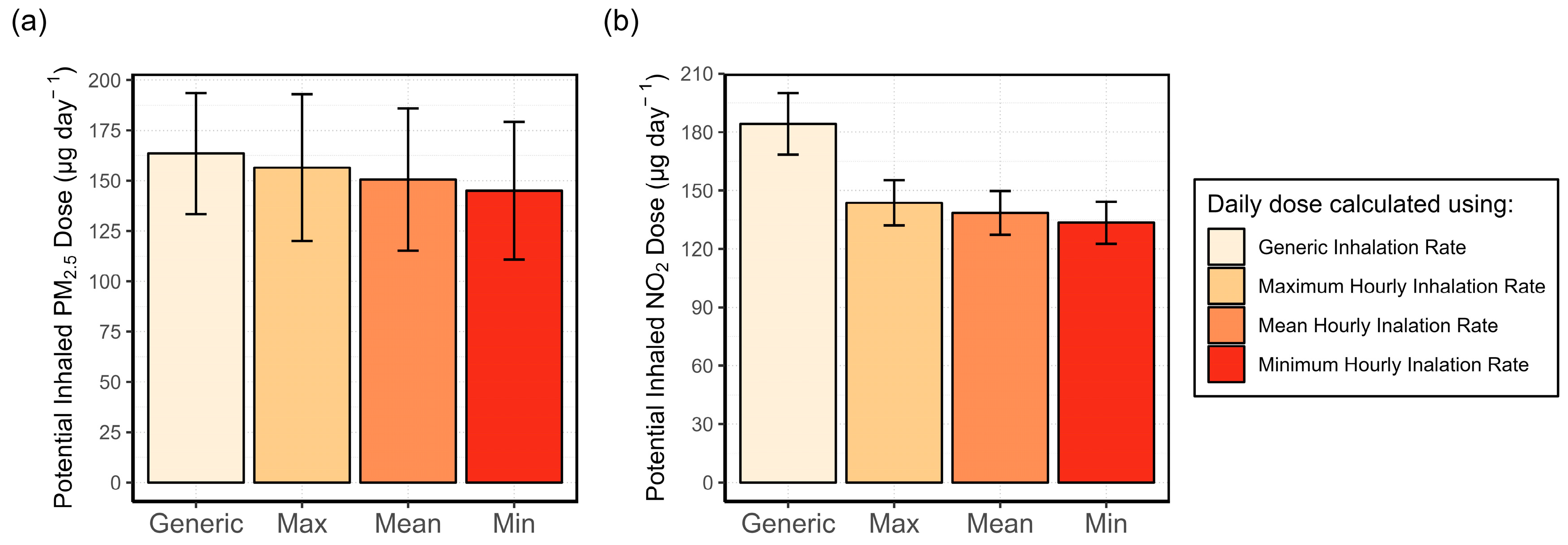
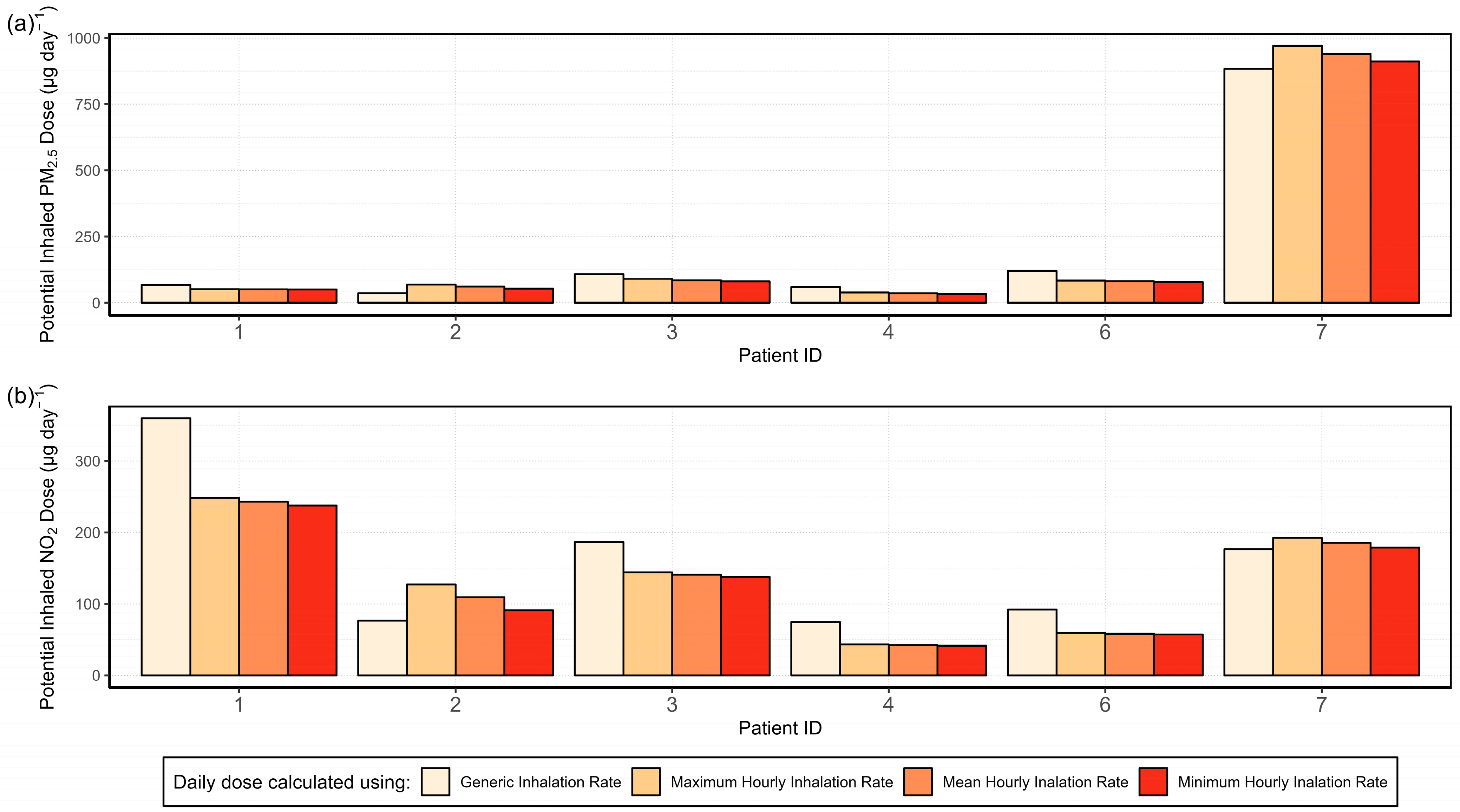
3.4.1. Analysis of the Variability in the Inhalation Rate and Its Effect on the Potential Inhaled Dose
3.4.2. Exposure Misclassification
3.4.3. Activity-Specific Potential Inhaled Dose
4. Discussion
4.1. Air Quality Sensors
4.1.1. Data Loss and Data Quality
4.1.2. Use of Stationary Sensors
4.2. Nature of Participant-Reported Data
4.3. Activity Specific I/O Ratio
4.4. Source Apportionment
4.5. Symptomatology
4.6. Exposure Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K.; de Matteis, S.; Forastiere, F.; Forsberg, B.; Frampton, M.W.; et al. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 1600419. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The 17 Goals. Available online: https://sdgs.un.org/goals (accessed on 18 December 2023).
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003422-8. [Google Scholar]
- Tonne, C. A call for epidemiology where the air pollution is. Lancet Planet. Health 2017, 1, e355–e356. [Google Scholar] [CrossRef] [PubMed]
- Vilcassim, R.; Thurston, G.D. Gaps and future directions in research on health effects of air pollution. EBioMedicine 2023, 93, 104668. [Google Scholar] [CrossRef]
- Martin, R.V.; Brauer, M.; van Donkelaar, A.; Shaddick, G.; Narain, U.; Dey, S. No one knows which city has the highest concentration of fine particulate matter. Atmos. Environ. X 2019, 3, 100040. [Google Scholar] [CrossRef]
- Kornartit, C.; Sokhi, R.S.; Burton, M.A.; Ravindra, K. Activity pattern and personal exposure to nitrogen dioxide in indoor and outdoor microenvironments. Environ. Int. 2010, 36, 36–45. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Bulot, F.M.J.; Russell, H.S.; Rezaei, M.; Johnson, M.S.; Ossont, S.J.J.; Morris, A.K.R.; Basford, P.J.; Easton, N.H.C.; Foster, G.L.; Loxham, M.; et al. Laboratory Comparison of Low-Cost Particulate Matter Sensors to Measure Transient Events of Pollution. Sensors 2020, 20, 2219. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Brook, R.D. The indoor-outdoor air-pollution continuum and the burden of cardiovascular disease: An opportunity for improving global health. Glob. Heart 2012, 7, 207–213. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.-B.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef] [PubMed]
- Okello, G.; Nantanda, R.; Awokola, B.; Thondoo, M.; Okure, D.; Tatah, L.; Bainomugisha, E.; Oni, T. Air quality management strategies in Africa: A scoping review of the content, context, co-benefits and unintended consequences. Environ. Int. 2023, 171, 107709. [Google Scholar] [CrossRef] [PubMed]
- Hasenkopf, C.; Sharma, N.; Kazi, F.; Mukerjee, P.; Greenstone, M. The Case for Closing Global Air Quality Data Gaps with Local Actors: A Golden Opportunity for the Philanthropic Community; EPIC: Chicago, IL, USA, 2023. [Google Scholar]
- Garland, R.M.; Altieri, K.E.; Dawidowski, L.; Gallardo, L.; Mbandi, A.; Rojas, N.Y.; Touré, N.d.E. Opinion: Strengthening research in the Global South—Atmospheric science opportunities in South America and Africa. Atmos. Chem. Phys. 2024, 24, 5757–5764. [Google Scholar] [CrossRef]
- Tonne, C.; Basagaña, X.; Chaix, B.; Huynen, M.; Hystad, P.; Nawrot, T.S.; Slama, R.; Vermeulen, R.; Weuve, J.; Nieuwenhuijsen, M. New frontiers for environmental epidemiology in a changing world. Environ. Int. 2017, 104, 155–162. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M. Use of real-time sensors to characterise human exposures to combustion related pollutants. J. Environ. Monit. 2012, 14, 1824–1837. [Google Scholar] [CrossRef]
- Chatzidiakou, L.; Krause, A.; Popoola, O.A.M.; Di Antonio, A.; Kellaway, M.; Han, Y.; Squires, F.A.; Wang, T.; Zhang, H.; Wang, Q.; et al. Characterising low-cost sensors in highly portable platforms to quantify personal exposure in diverse environments. Atmos. Meas. Tech. 2019, 12, 4643–4657. [Google Scholar] [CrossRef]
- Tékouabou, S.C.K.; Chenal, J.; Azmi, R.; Diop, E.B.; Toulni, H.; Nsegbe, A.d.P. Towards air quality particulate-matter monitoring using low-cost sensor data and visual exploration techniques: Case study of Kisumu, Kenya. Procedia Comput. Sci. 2022, 215, 963–972. [Google Scholar] [CrossRef]
- Bousiotis, D.; Alconcel, L.-N.S.; Beddows, D.C.S.; Harrison, R.M.; Pope, F.D. Monitoring and apportioning sources of indoor air quality using low-cost particulate matter sensors. Environ. Int. 2023, 174, 107907. [Google Scholar] [CrossRef]
- Bertrand, L.; Dawkins, L.; Jayaratne, R.; Morawska, L. How to choose healthier urban biking routes: CO as a proxy of traffic pollution. Heliyon 2020, 6, e04195. [Google Scholar] [CrossRef]
- Chatzidiakou, L.; Krause, A.; Han, Y.; Chen, W.; Yan, L.; Popoola, O.A.M.; Kellaway, M.; Wu, Y.; Liu, J.; Hu, M.; et al. Using low-cost sensor technologies and advanced computational methods to improve dose estimations in health panel studies: Results of the AIRLESS project. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; Donaire-Gonzalez, D.; Popoola, O.; Jones, R.; Cohen, R.C.; Almanza, E.; de Nazelle, A.; Mead, I.; Carrasco-Turigas, G.; Cole-Hunter, T.; et al. Validating novel air pollution sensors to improve exposure estimates for epidemiological analyses and citizen science. Environ. Res. 2017, 158, 286–294. [Google Scholar] [CrossRef]
- Mazaheri, M.; Clifford, S.; Yeganeh, B.; Viana, M.; Rizza, V.; Flament, R.; Buonanno, G.; Morawska, L. Investigations into factors affecting personal exposure to particles in urban microenvironments using low-cost sensors. Environ. Int. 2018, 120, 496–504. [Google Scholar] [CrossRef]
- Xie, Q.; Dai, Y.; Zhu, X.; Hui, F.; Fu, X.; Zhang, Q. High contribution from outdoor air to personal exposure and potential inhaled dose of PM2.5 for indoor-active university students. Environ. Res. 2022, 215, 114225. [Google Scholar] [CrossRef]
- Amegah, A.K.; Dakuu, G.; Mudu, P.; Jaakkola, J.J.K. Particulate matter pollution at traffic hotspots of Accra, Ghana: Levels, exposure experiences of street traders, and associated respiratory and cardiovascular symptoms. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 333–342. [Google Scholar] [CrossRef]
- Steinle, S.; Reis, S.; Sabel, C.E.; Semple, S.; Twigg, M.M.; Braban, C.F.; Leeson, S.R.; Heal, M.R.; Harrison, D.; Lin, C.; et al. Personal exposure monitoring of PM2.5 in indoor and outdoor microenvironments. Sci. Total Environ. 2015, 508, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Song, J. Towards space-time modelling of PM2.5 inhalation volume with ST-exposure. Sci. Total Environ. 2024, 948, 174888. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Beyond air pollution at home: Assessment of personal exposure to PM2.5 using activity-based travel demand model and low-cost air sensor network data. Environ. Res. 2021, 201, 111549. [Google Scholar] [CrossRef]
- Che, W.; Frey, H.C.; Fung, J.C.H.; Ning, Z.; Qu, H.; Lo, H.K.; Chen, L.; Wong, T.-W.; Wong, M.K.M.; Lee, O.C.W.; et al. PRAISE-HK: A personalized real-time air quality informatics system for citizen participation in exposure and health risk management. Sustain. Cities Soc. 2020, 54, 101986. [Google Scholar] [CrossRef]
- Chatzidiakou, L.; Krause, A.; Kellaway, M.; Han, Y.; Li, Y.; Martin, E.; Kelly, F.J.; Zhu, T.; Barratt, B.; Jones, R.L. Automated classification of time-activity-location patterns for improved estimation of personal exposure to air pollution. Environ. Health 2022, 21, 125. [Google Scholar] [CrossRef]
- Chapizanis, D.; Karakitsios, S.; Gotti, A.; Sarigiannis, D.A. Assessing personal exposure using Agent Based Modelling informed by sensors technology. Environ. Res. 2021, 192, 110141. [Google Scholar] [CrossRef]
- Amegah, A.K. Proliferation of low-cost sensors. What prospects for air pollution epidemiologic research in Sub-Saharan Africa? Environ. Pollut. 2018, 241, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hauryliuk, A.; Malings, C.; Eilenberg, S.R.; Subramanian, R.; Presto, A.A. Characterizing the Aging of Alphasense NO2 Sensors in Long-Term Field Deployments. ACS Sens. 2021, 6, 2952–2959. [Google Scholar] [CrossRef]
- Sesé, L.; Gille, T.; Pau, G.; Dessimond, B.; Uzunhan, Y.; Bouvry, D.; Hervé, A.; Didier, M.; Kort, F.; Freynet, O.; et al. Low-cost air quality portable sensors and their potential use in respiratory health. Int. J. Tuberc. Lung Dis. 2023, 27, 803–809. [Google Scholar] [CrossRef]
- Samad, A.; Kiseleva, O.; Holst, C.C.; Wegener, R.; Kossmann, M.; Meusel, G.; Fiehn, A.; Erbertseder, T.; Becker, R.; Roiger, A.; et al. Meteorological and air quality measurements in a city region with complex terrain: Influence of meteorological phenomena on urban climate. Meteorol. Z. Contrib. Atmos. Sci. 2023, 32, 293–315. [Google Scholar] [CrossRef]
- Schwitalla, T.; Bauer, H.-S.; Warrach-Sagi, K.; Bönisch, T.; Wulfmeyer, V. Turbulence-permitting air pollution simulation for the Stuttgart metropolitan area. Atmos. Chem. Phys. 2021, 21, 4575–4597. [Google Scholar] [CrossRef]
- Chacón-Mateos, M.; Laquai, B.; Vogt, U.; Stubenrauch, C. Evaluation of a low-cost dryer for a low-cost optical particle counter. Atmos. Meas. Tech. 2022, 15, 7395–7410. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Exposure Factors Handbook: 2011 Edition; EPA/600/R-09/052F; U.S. Environmental Protection Agency: Washington, DC, USA, 2011. Available online: https://www.epa.gov/expobox/about-exposure-factors-handbook (accessed on 23 December 2023).
- U.S. Environmental Protection Agency. Guidelines for Exposure Assessment; U.S. Environmental Protection Agency: Washington, DC, USA, 1992; Volume 57, pp. 22888–22938. [Google Scholar]
- Krause, A. Using Novel Portable Air Quality Monitors to Improve Personal Exposure and Dose Estimations for Health Studies; Apollo-University of Cambridge Repository: Cambridge, UK, 2021. [Google Scholar]
- Chacón-Mateos, M.; Vogt, U.; Laquai, B.; García-Salamero, H.; Witt, C.; Liebers, U.; Heimann, F. Evaluation of Air Quality Sensors for Environmental Epidemiology; EGU General Assembly: Vienna, Austria, 2023. [Google Scholar]
- García Salamero, H. Evaluation of Long-Term Measurements of NO2 Sensors for Epidemiological Studies. Master’s Thesis, University of Stuttgart, Stuttgart, Germany, 2021. [Google Scholar]
- Fortmann, R.; Kariher, P.; Clayton, R. Indoor Air Quality: Residential Cooking Exposures: Final Report; ARCADIS Geraghty & Miller, Inc.: Sacramento, CA, USA, 2001. [Google Scholar]
- Stamp, S.; Burman, E.; Chatzidiakou, L.; Cooper, E.; Wang, Y.; Mumovic, D. A critical evaluation of the dynamic nature of indoor-outdoor air quality ratios. Atmos. Environ. 2022, 273, 118955. [Google Scholar] [CrossRef]
- Stasiulaitiene, I.; Krugly, E.; Prasauskas, T.; Ciuzas, D.; Kliucininkas, L.; Kauneliene, V.; Martuzevicius, D. Infiltration of outdoor combustion-generated pollutants to indoors due to various ventilation regimes: A case of a single-family energy efficient building. Build. Environ. 2019, 157, 235–241. [Google Scholar] [CrossRef]
- Faria, T.; Martins, V.; Correia, C.; Canha, N.; Diapouli, E.; Manousakas, M.; Eleftheriadis, K.; Almeida, S.M. Children’s exposure and dose assessment to particulate matter in Lisbon. Build. Environ. 2020, 171, 106666. [Google Scholar] [CrossRef]
- deSouza, P.; Kahn, R.; Stockman, T.; Obermann, W.; Crawford, B.; Wang, A.; Crooks, J.; Li, J.; Kinney, P. Calibrating networks of low-cost air quality sensors. Atmos. Meas. Tech. 2022, 15, 6309–6328. [Google Scholar] [CrossRef]
- Diapouli, E.; Eleftheriadis, K.; Karanasiou, A.A.; Vratolis, S.; Hermansen, O.; Colbeck, I.; Lazaridis, M. Indoor and Outdoor Particle Number and Mass Concentrations in Athens. Sources, Sinks and Variability of Aerosol Parameters. Aerosol Air Qual. Res. 2011, 11, 632–642. [Google Scholar] [CrossRef]
- Vernon, M.K.; Bell, J.A.; Wiklund, I.; Dale, P.; Chapman, K.R. Asthma Control and Asthma Triggers. J. Asthma Allergy Educ. 2013, 4, 155–164. [Google Scholar] [CrossRef]
- Jiang, H.; Han, J.; Zhu, Z.; Xu, W.; Zheng, J.; Zhu, Y. Patient compliance with assessing and monitoring of asthma. J. Asthma 2009, 46, 1027–1031. [Google Scholar] [CrossRef]
- Côté, J.; Cartier, A.; Malo, J.L.; Rouleau, M.; Boulet, L.P. Compliance with peak expiratory flow monitoring in home management of asthma. Chest 1998, 113, 968–972. [Google Scholar] [CrossRef]
- Dąbrowiecki, P.; Chciałowski, A.; Dąbrowiecka, A.; Badyda, A. Ambient Air Pollution and Risk of Admission Due to Asthma in the Three Largest Urban Agglomerations in Poland: A Time-Stratified, Case-Crossover Study. Int. J. Environ. Res. Public Health 2022, 19, 5988. [Google Scholar] [CrossRef]
- Corlin, L.; Woodin, M.; Amaravadi, H.; Henderson, N.; Brugge, D.; Durant, J.L.; Gute, D.M. A field study to estimate inhalation rates for use in a particle inhalation rate exposure metric. Sci. Total Environ. 2019, 696, 133919. [Google Scholar] [CrossRef]
- Véliz, K.D.; Alcantara-Zapata, D.E.; Chomalí, L.; Vargas, J. Gender-differentiated impact of PM2.5 exposure on respiratory and cardiovascular mortality: A review. Air Qual. Atmos. Health 2024, 17, 1565–1586. [Google Scholar] [CrossRef]


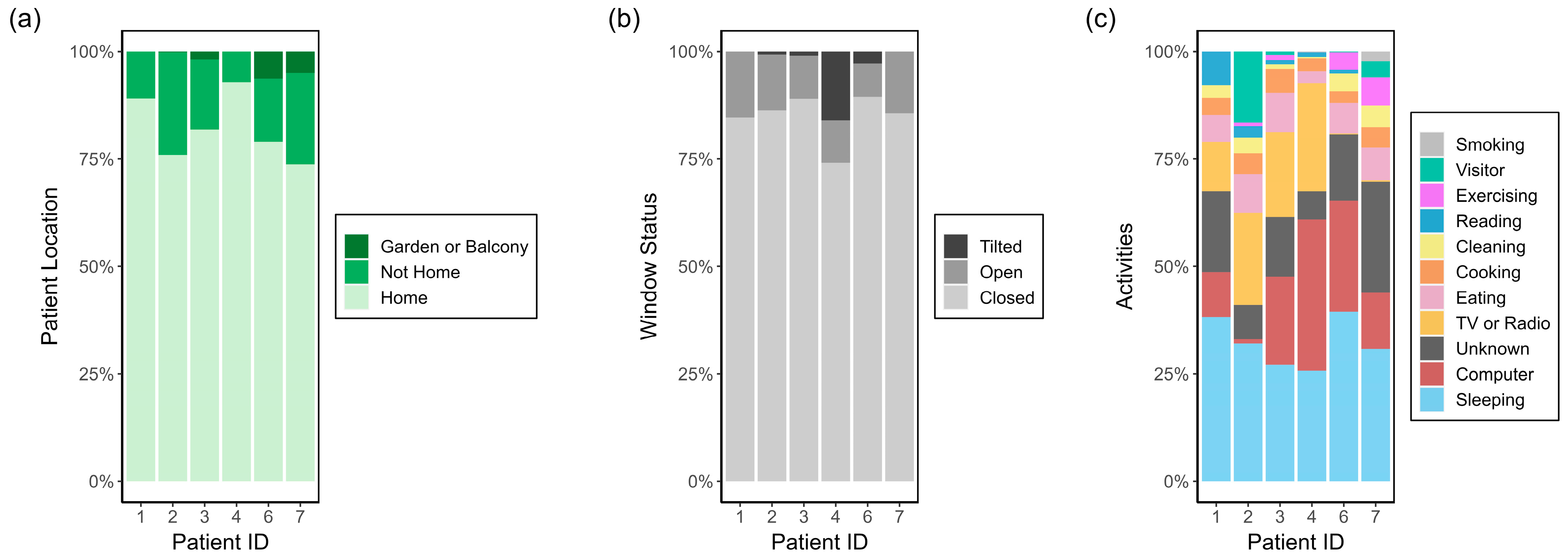


| Category | Possible Values | Default Value |
|---|---|---|
| Patient location | Home Not home Garden or balcony | Home |
| Window status | Window closed Window tilted Window open | Window closed |
| Activity | Sleeping Exercising Reading Computer TV or radio Cooking Eating Visitor Cleaning | Unknown |
| Patient ID | Health Score (0–28) | PEF (L min−1) | ||||
|---|---|---|---|---|---|---|
| Minimum | Mean | Maximum | Minimum | Mean | Maximum | |
| 1 | 10 | 14.5 | 20 | - | - | - |
| 2 | - | - | - | - | - | - |
| 3 | 6 | 11.1 | 18 | 500 | 540 | 580 |
| 4 | 0 | 1.6 | 4 | 370 | 400 | 430 |
| 5 | - | - | - | - | - | - |
| 6 | 0 | 1.0 | 5 | 800 | 800 | 800 |
| 7 | 0 | 1.2 | 4 | 290 | 324 | 370 |
| Patient ID | Hourly Mean IR (L min−1) | |
|---|---|---|
| Activity Adjusted | Generic | |
| 1 | 7.0 | 9 |
| 2 | 9.2 | 6.8 |
| 3 | 8.3 | 9.9 |
| 4 | 5.3 | 8.5 |
| 6 | 9.5 | 12.1 |
| 7 | 9.5 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Mateos, M.; Remy, E.; Liebers, U.; Heimann, F.; Witt, C.; Vogt, U. Feasibility Study on the Use of NO2 and PM2.5 Sensors for Exposure Assessment and Indoor Source Apportionment at Fixed Locations. Sensors 2024, 24, 5767. https://doi.org/10.3390/s24175767
Chacón-Mateos M, Remy E, Liebers U, Heimann F, Witt C, Vogt U. Feasibility Study on the Use of NO2 and PM2.5 Sensors for Exposure Assessment and Indoor Source Apportionment at Fixed Locations. Sensors. 2024; 24(17):5767. https://doi.org/10.3390/s24175767
Chicago/Turabian StyleChacón-Mateos, Miriam, Erika Remy, Uta Liebers, Frank Heimann, Christian Witt, and Ulrich Vogt. 2024. "Feasibility Study on the Use of NO2 and PM2.5 Sensors for Exposure Assessment and Indoor Source Apportionment at Fixed Locations" Sensors 24, no. 17: 5767. https://doi.org/10.3390/s24175767






