Selection of X-ray Tube Settings for Relative Bone Density Quantification in the Knee Joint of Cats Using Computed Digital Absorptiometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
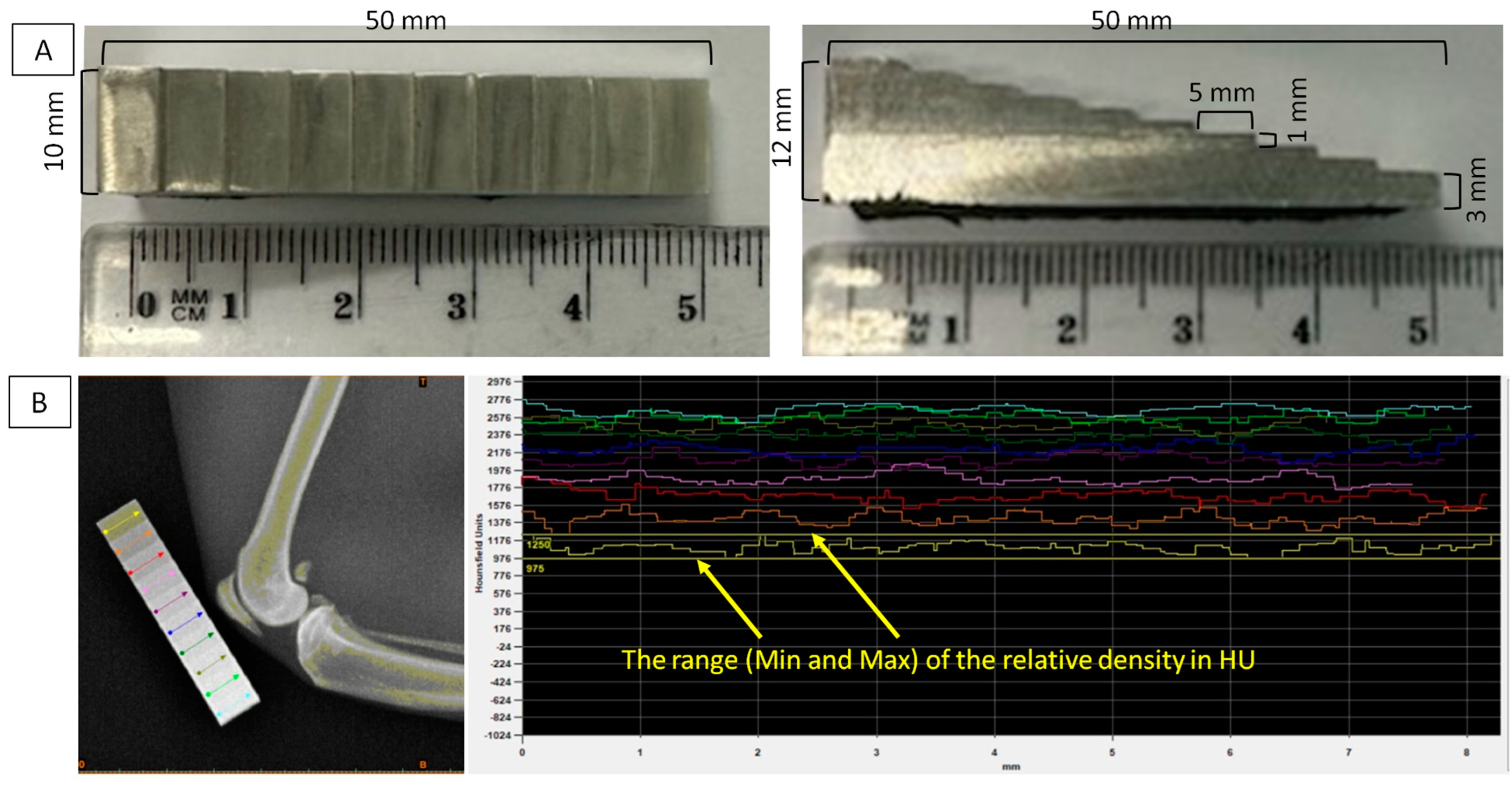
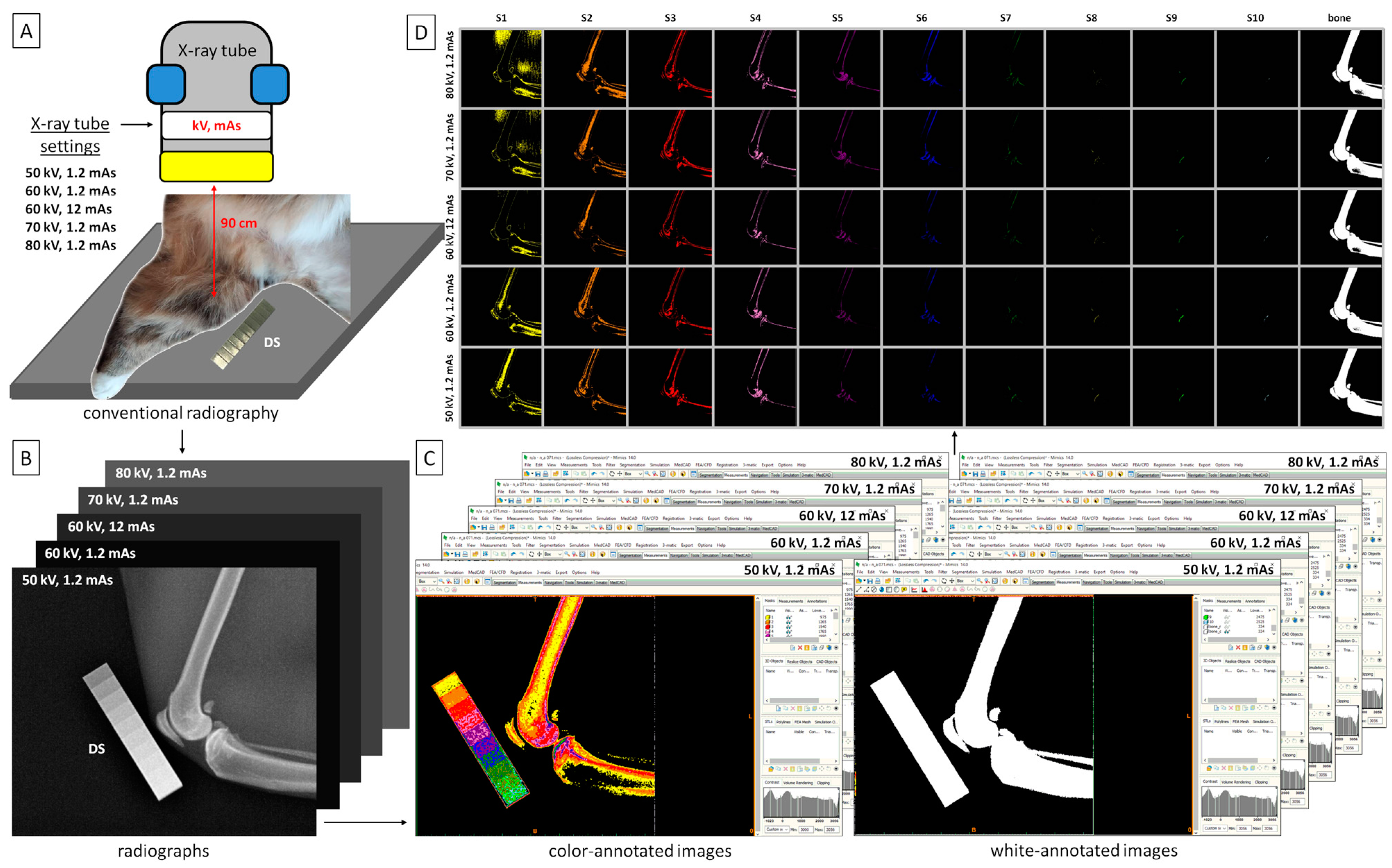
2.2. Image Acquisition
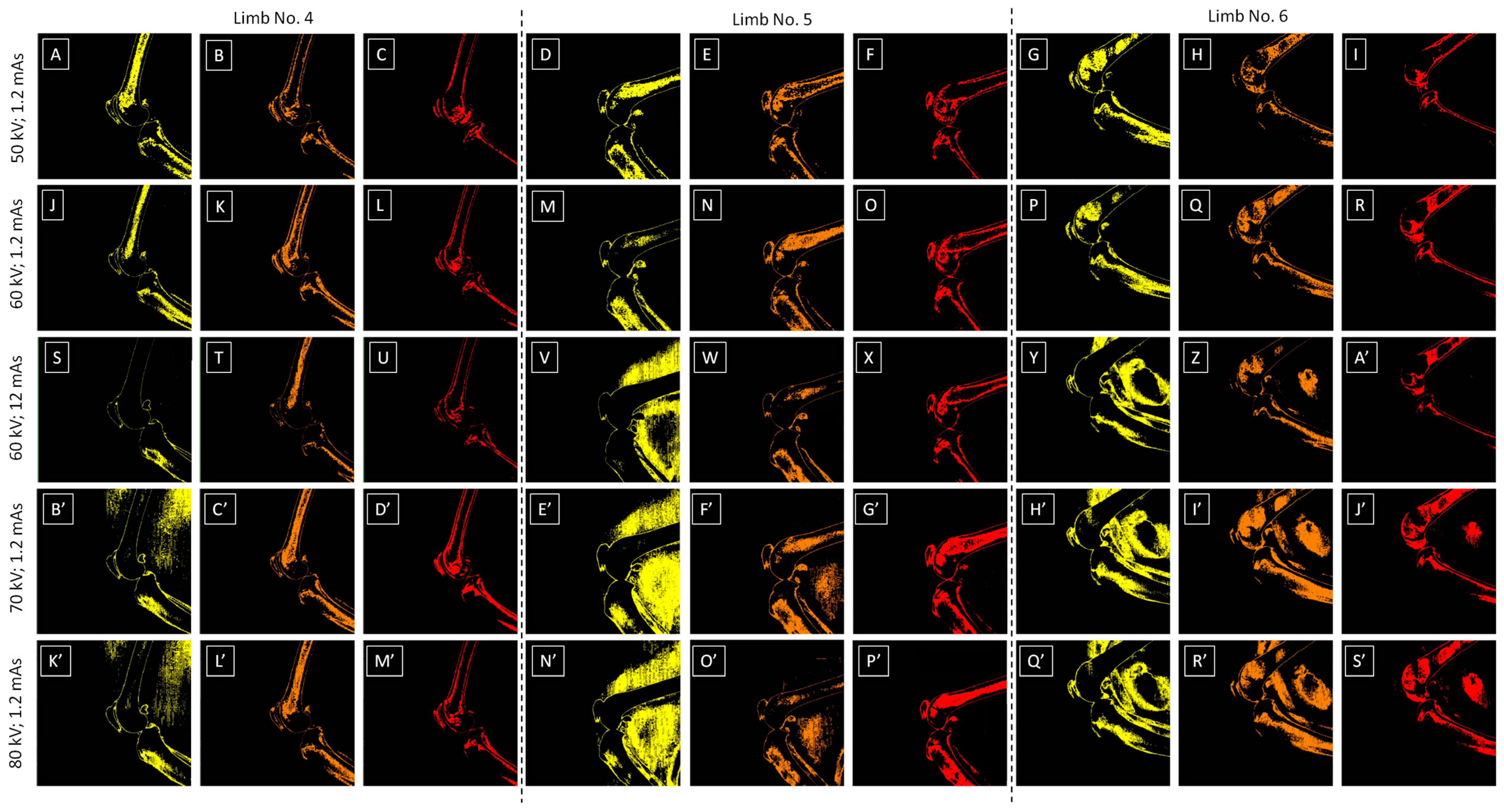
2.3. Image Processing
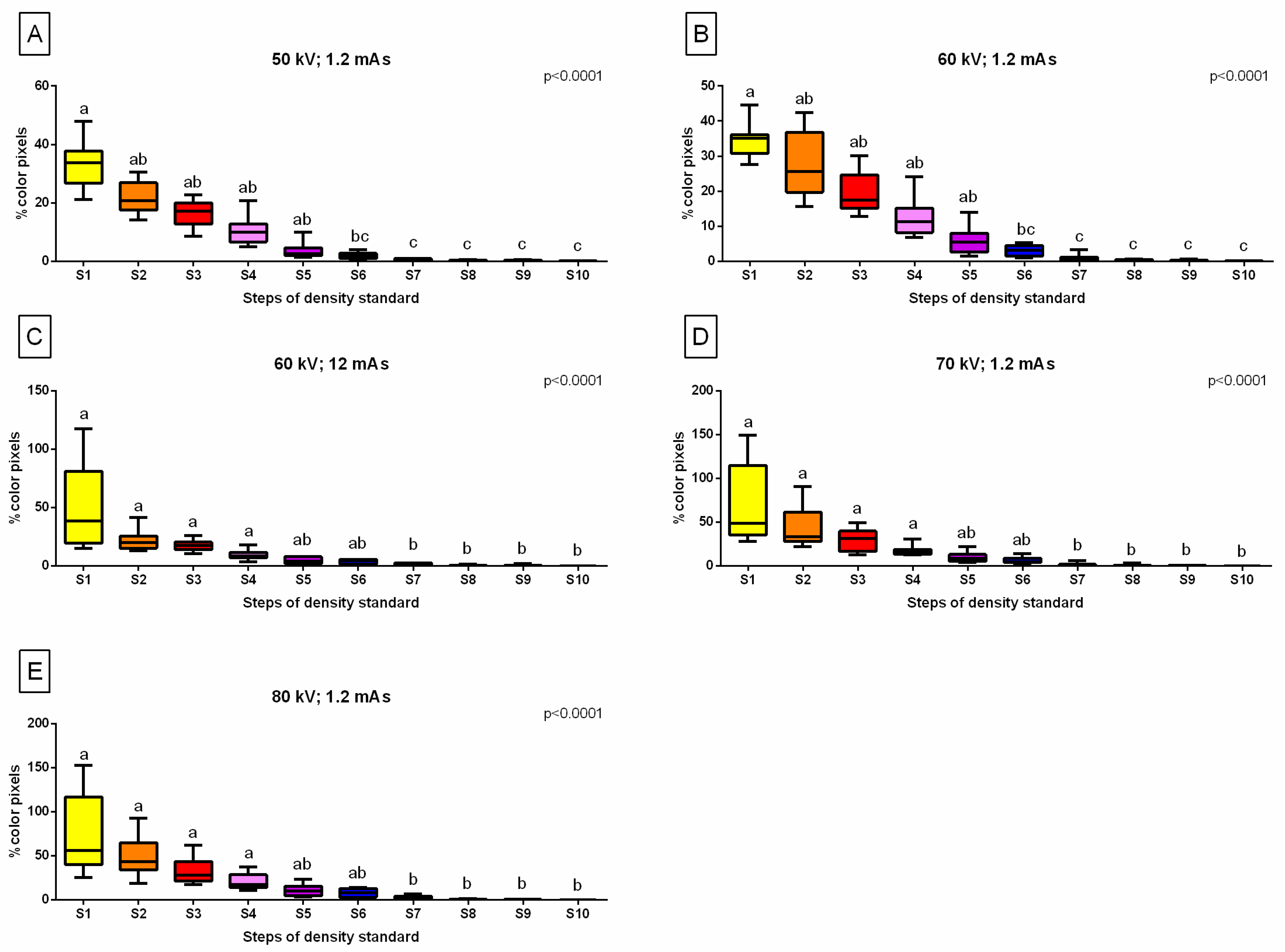
2.4. Color-Pixel-Counting Protocol
2.5. Statistical Analysis
3. Results
3.1. Ranges of the Relative Density
3.2. Relative Bone Density Quantification
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grampp, S.; Jergas, M.; Glüer, C.C.; Lang, P.; Brastow, P.; Genant, H.K. Radiologic diagnosis of osteoporosis: Current methods and perspectives. Radiol. Clin. N. Am. 1993, 31, 1133–1145. [Google Scholar] [CrossRef]
- Ebbesen, E.N.; Thomsen, J.S.; Beck–Nielsen, H.; Nepper–Rasmussen, H.J.; Mosekilde, L. Lumbar vertebral body compressive strength evaluated by dual–energy X–ray absorptiometry, quantitative computed tomography, and ashing. Bone 1999, 25, 713–724. [Google Scholar] [CrossRef]
- Russo, C.R.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Di Iorio, A.; Volpato, S.; Guralnik, J.M.; Harris, T.; Ferrucci, L. Aging bone in men and women: Beyond changes in bone mineral density. Osteoporos. Int. 2003, 14, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Lauten, S.D.; Cox, N.R.; Baker, G.H.; Painter, D.J.; Morrison, N.E.; Baker, H.J. Body composition of growing and adult cats as measured by use of dual energy X-ray absorptiometry. Comp. Med. 2020, 50, 175–183. [Google Scholar]
- Ching, S.V.; Norrdin, R.W.; Fettman, M.J.; LeCouteur, R.A. Trabecular bone remodeling and bone mineral density in the adult cat during chronic dietary acidification with ammonium chloride. J. Bone Miner. Res. 1990, 5, 547–556. [Google Scholar] [CrossRef]
- Cheon, H.; Choi, W.; Lee, Y.; Lee, D.; Kim, J.; Kang, J.H.; Na, K.; Chang, J.; Chang, D. Assessment of trabecular bone mineral density using quantitative computed tomography in normal cats. J. Vet. Med. Sci. 2012, 74, 1461–1467. [Google Scholar] [CrossRef]
- Parker, V.J.; Gilor, C.; Chew, D.J. Feline hyperparathyroidism: Pathophysiology, diagnosis and treatment of primary and secondary disease. J. Feline Med. Surg. 2015, 17, 427–439. [Google Scholar] [CrossRef]
- Hanel, R.M.; Graham, J.P.; Levy, J.K.; Buergelt, C.D.; Creamer, J. Generalized osteosclerosis in a cat. Vet. Radiol. Ultrasound 2004, 45, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Chung, W.J.; Yoon, J. Clinical application of quantitative computed tomography in osteogenesis imperfecta–suspected cat. J. Vet. Sci. 2017, 18, 415–417. [Google Scholar] [CrossRef]
- Turner, A.S.; Norrdin, R.W.; Gaarde, S.; Connally, H.E.; Thrall, M.A. Bone mineral density in feline mucopolysaccharidosis VI measured using dual–energy X-ray absorptiometry. Calcif. Tissue Int. 1995, 57, 191–195. [Google Scholar] [CrossRef]
- Smith, C.; Snead, E.C.; Sukut, S.; Veldhuisen, D. A rare lysosomal storage disorder: Feline mucopolysaccharidosis VII. Vet. Rec. Case Rep. 2022, 10, e445. [Google Scholar] [CrossRef]
- Helm, J.; Morris, J. Musculoskeletal neoplasia: An important differential for lumps or lameness in the cat. J. Feline Med. Surg. 2012, 14, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Shaver, S.L.; Culp, W.T.; Rebhun, R.B. Tumors of bone and joint. In Clinical Small Animal Internal Medicine; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 1327–1332. [Google Scholar]
- Dittmer, K.E.; Pemberton, S. A holistic approach to bone tumors in dogs and cats: Radiographic and histologic correlation. Vet. Pathol. 2021, 58, 841–857. [Google Scholar] [CrossRef]
- Hardie, E.M.; Roe, S.C.; Martin, F.R. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J. Am. Vet. Med. Assoc. 2002, 220, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Mellor, D.; Clements, D.; Gemmill, T.; Farrell, M.; Carmichael, S.; Bennett, D. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet. Rec. 2005, 157, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D. Osteoarthritis in cats: A retrospective radiological study. J. Small Anim. Pract. 2005, 46, 425–429. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Henry, J.B., III; Brown, J.; Robertson, I.; Sumrell, A.T.; Simpson, W.; Wheeler, S.; Hansen, B.D.; Zamprogno, H.; Freire, M.; et al. Cross–sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet. Surg. 2010, 39, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Robertson, I.; Bondell, H.D.; Brown, J.; Hash, J.; Pease, A.P.; Lascelles, B.D.X. Radiographic evaluation of feline appendicular degenerative joint disease vs. macroscopic appearance of articular cartilage. Vet. Radiol. Ultrasound 2011, 52, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Slingerland, L.; Hazewinkel, H.; Meij, B.; Picavet, P.; Voorhout, G. Cross–sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet. J. 2011, 187, 304–309. [Google Scholar] [CrossRef]
- Speakman, J.R.; Booles, D.; Butterwick, R. Validation of dual energy X-ray absorptiometry (DXA) by comparison with chemical analysis of dogs and cats. Int. J. Obes. 2001, 25, 439–447. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kirpensteijn, J.; Nielsen, D.H.; Buelund, L.; Hansen, M.S. Nutritional secondary hyperparathyroidism in two cats. Vet. Comp. Orthop. Traumatol. 2010, 23, 56–61. [Google Scholar]
- Buelund, L.E.; Nielsen, D.H.; McEvoy, F.J.; Svalastoga, E.L.; Bjornvad, C.R. Measurement of body composition in cats using computed tomography and dual energy X-ray absorptiometry. Vet. Radiol. Ultrasound 2011, 52, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G.; Witzel, A.L.; Moyers, T.D.; Kirk, C.A. Body composition of lean outdoor intact cats vs. lean indoor neutered cats using dual–energy X-ray absorptiometry. J. Feline Med. Surg. 2019, 21, 459–464. [Google Scholar] [CrossRef]
- Bewes, J.M.; Morphett, A.; Pate, F.D.; Henneberg, M.; Low, A.J.; Kruse, L.; Craig, B.; Hindson, A.; Adams, E. Imaging ancient and mummified specimens: Dual–energy CT with effective atomic number imaging of two ancient Egyptian cat mummies. J. Archaeol. Sci. Rep. 2016, 8, 173–177. [Google Scholar] [CrossRef]
- Krokowski, E.; Schlungbaum, W. Die Objektivierung der röntgenologischen Diagnose “Osteoporose”. RöFo-Fortschritte Geb. Röntgenstrahlen Bildgeb. Verfahr. 1959, 91, 740–746. [Google Scholar] [CrossRef]
- Górski, K.; Borowska, M.; Turek, B.; Pawlikowski, M.; Jankowski, K.; Bereznowski, A.; Polkowska, I.; Domino, M. An application of the density standard and scaled–pixel–counting protocol to assess the radiodensity of equine incisor teeth affected by resorption and hypercementosis: Preliminary advancement in dental radiography. BMC Vet. Res. 2023, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Turek, B.; Borowska, M.; Jankowski, K.; Skierbiszewska, K.; Pawlikowski, M.; Jasiński, T.; Domino, M. A Preliminary Protocol of Radiographic Image Processing for Quantifying the Severity of Equine Osteoarthritis in the Field: A Model of Bone Spavin. Appl. Sci. 2024, 14, 5498. [Google Scholar] [CrossRef]
- Bowen, A.J.; Burd, M.A.; Craig, J.J.; Craig, M. Radiographic calibration for analysis of bone mineral density of the equine third metacarpal bone. J. Equine Vet. Sci. 2013, 33, 1131–1135. [Google Scholar] [CrossRef]
- Yamada, K.; Sato, F.; Higuchi, T.; Nishihara, K.; Kayano, M.; Sasaki, N.; Nambo, Y. Experimental investigation of bone mineral density in Thoroughbreds using quantitative computed tomography. J. Equine Sci. 2015, 26, 81–87. [Google Scholar] [CrossRef]
- Leijon, A.; Ley, C.J.; Corin, A.; Ley, C. Cartilage lesions in feline stifle joints–Associations with articular mineralizations and implications for osteoarthritis. Res. Vet. Sci. 2017, 114, 186–193. [Google Scholar] [CrossRef]
- Bonecka, J.; Skibniewski, M.; Zep, P.; Domino, M. Knee Joint Osteoarthritis in Overweight Cats: The Clinical and Radiographic Findings. Animals 2023, 13, 2427. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Dong, Y.H.; Marcellin–Little, D.J.; Thomson, A.; Wheeler, S.; Correa, M. Relationship of orthopedic examination, goniometric measurements, and radiographic signs of degenerative joint disease in cats. BMC Vet. Res. 2012, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Prosé, P. Anatomy of the knee joint of the cat. Cells Tissues Organs 1984, 119, 40–48. [Google Scholar] [CrossRef]
- Freeman, M.A.; Wyke, B. The innervation of the knee joint. An anatomical and histological study in the cat. J. Anat. 1967, 101, 505. [Google Scholar] [PubMed]
- Hounsfield, G.N. Computed medical imaging. J. Comput. Assist. Tomo. 1980, 4, 665–674. [Google Scholar] [CrossRef]
- Preston, T.; Glyde, M.; Hosgood, G.; Snow, L. Morphometric description of the feline radius and ulna generated from computed tomography. J. Feline Med. Surg. 2015, 17, 991–999. [Google Scholar] [CrossRef]
- Thrall, D.E. Textbook of Veterinary Diagnostic Radiology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Huda, W.; Abrahams, R.B. Radiographic techniques, contrast, and noise in X-ray imaging. Am. J. Roentgenol. 2015, 204, W126–W131. [Google Scholar] [CrossRef]
- Seibert, J.A.; Boone, J.M.; Lindfors, K.K. Flat-field correction technique for digital detectors. In Medical Imaging 1998: Physics of Medical Imaging; SPIE: Bellingham, WA, USA, 1998; pp. 348–354. [Google Scholar]
- Kim, H.K.; Lee, S.C.; Cho, M.H.; Lee, S.Y.; Cho, G. Use of a flat-panel detector for microtomography: A feasibility study for small-animal imaging. IEEE Trans. Nucl. Sci. 2005, 52, 193–198. [Google Scholar]
- Boyd, S.K.; Müller, R.; Leonard, T.; Herzog, W. Long–term periarticular bone adaptation in a feline knee injury model for post–traumatic experimental osteoarthritis. Osteoarthr. Cartil. 2005, 13, 235–242. [Google Scholar] [CrossRef]
- Bartling, S.H.; Stiller, W.; Semmler, W.; Kiessling, F. Small animal computed tomography imaging. Curr. Med. Imaging Rev. 2007, 3, 45–59. [Google Scholar] [CrossRef]
- Dendere, R.; Whiley, S.P.; Douglas, T.S. Computed digital absorptiometry for measurement of phalangeal bone mineral mass on a slot-scanning digital radiography system. Osteoporos. Int. 2014, 25, 2625–2630. [Google Scholar] [CrossRef] [PubMed]


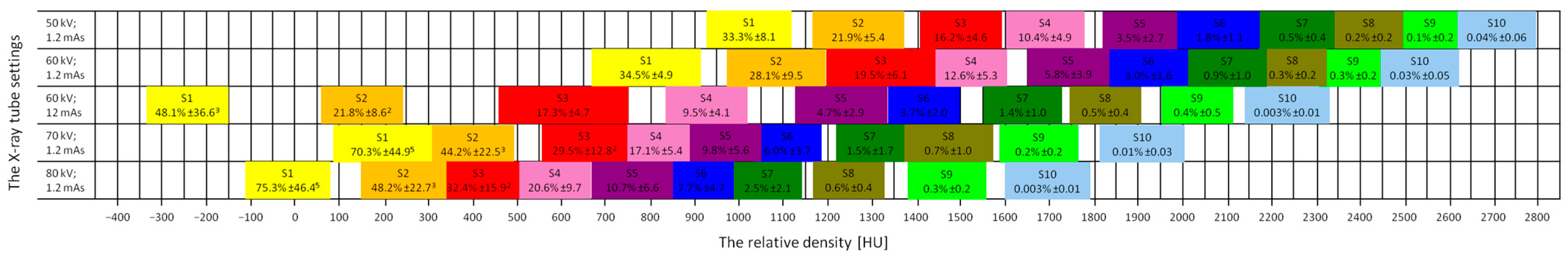
| Decomposition | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Color | Yellow | Orange | Red | Light purple | Dark purple | Dark blue | Dark green | Navy green | Light green | Light blue |
| HEX code | #FFFF00 | #E08000 | #FF0000 | #E080C0 | #800080 | #0000FF | #008000 | #808000 | #00FF00 | #A6CAF0 |
| X-ray Tube Settings | Range | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 kV; 1.2 mAs | Min | 880.6 ± 129.0 | 1173.9 ± 143.5 | 1417.8 ± 147.2 | 1614.4 ± 155.8 | 1826.1 ± 142.2 | 1985.0 ± 109.0 | 2157.2 ± 104.0 | 2318.9 ± 60.1 | 2447.2 ± 56.8 | 2478.3 ± 90.8 |
| Max | 1130.6 ± 69.6 | 1367.8 ± 122.3 | 1590.8 ± 70.8 | 1841.7 ± 112.4 | 1982.8 ± 93.9 | 2182.8 ± 99.7 | 2379.4 ± 111.4 | 2470.6 ± 126.5 | 2588.9 ± 116.2 | 2798.9 ± 47.4 | |
| 60 kV; 1.2 mAs | Min | 672.8 ± 142.5 | 928.9 ± 155.9 | 1194.4 ± 154.6 | 1423.9 ± 163.3 | 1658.9 ± 157.7 | 1848.3 ± 158.0 | 2012.8 ± 157.7 | 2149.4 ± 139.6 | 2280.6 ± 129.0 | 2363.3 ± 96.8 |
| Max | 919.4 ± 88.3 | 1191.7 ± 122.1 | 1446.7 ± 103.9 | 1659.4 ± 112.8 | 1821.1 ± 115.0 | 2018.9 ± 104.8 | 2181.1 ± 142.7 | 2268.3 ± 107.1 | 2381.1 ± 143.7 | 2539.4 ± 94.0 | |
| 60 kV; 12 mAs | Min | −330.0 ± 104.1 | 63.3 ± 170.8 | 465.0 ± 148.5 | 837.8 ± 137.7 | 1125.6 ± 116.9 | 1291.9 ± 195.1 | 1554.4 ± 133.8 | 1741.1 ± 149.6 | 1965.6 ± 159.1 | 2156.7 ± 166.1 |
| Max | −148.6 ± 68.3 | 231.1 ± 120.4 | 747.8 ± 119.7 | 1078.9 ± 150.4 | 1365.0 ± 115.1 | 1507.2 ± 156.3 | 1727.2 ± 158.9 | 1910.0 ± 142.2 | 2115.0 ± 156.3 | 2306.7 ± 143.2 | |
| 70 kV; 1.2 mAs | Min | 86.1 ± 52.2 | 294.7 ± 78.7 | 546.7 ± 78.8 | 732.9 ± 81.9 | 871.1 ± 80.5 | 976.7 ± 148.1 | 1255.6 ± 122.5 | 1379.4 ± 187.2 | 1590.6 ± 240.1 | 1815.0 ± 212.1 |
| Max | 302.8 ± 120.2 | 490.0 ± 128.7 | 762.8 ± 82.4 | 885.0 ± 80.2 | 1045.6 ± 183.3 | 1161.1 ± 189.9 | 1342.8 ± 196.3 | 1570.0 ± 235.9 | 1760.6 ± 207.5 | 2007.2 ± 196.1 | |
| 80 kV; 1.2 mAs | Min | −103.3 ± 133.6 | 140.6 ± 83.1 | 328.3 ± 95.9 | 513.3 ± 102.6 | 682.2 ± 83.8 | 797.2 ± 115.3 | 960.0 ± 156.9 | 1177.8 ± 119.3 | 1380.0 ± 177.3 | 1608.9 ± 165.9 |
| Max | 92.2 ± 135.9 | 359.4 ± 121.1 | 487.8 ± 178.1 | 663.9 ± 117.6 | 850.6 ± 130.9 | 975.6 ± 179.3 | 1142.2 ± 135.2 | 1339.4 ± 201.2 | 1563.9 ± 185.7 | 1786.7 ± 162.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonecka, J.; Turek, B.; Jankowski, K.; Borowska, M.; Jasiński, T.; Skierbiszewska, K.; Domino, M. Selection of X-ray Tube Settings for Relative Bone Density Quantification in the Knee Joint of Cats Using Computed Digital Absorptiometry. Sensors 2024, 24, 5774. https://doi.org/10.3390/s24175774
Bonecka J, Turek B, Jankowski K, Borowska M, Jasiński T, Skierbiszewska K, Domino M. Selection of X-ray Tube Settings for Relative Bone Density Quantification in the Knee Joint of Cats Using Computed Digital Absorptiometry. Sensors. 2024; 24(17):5774. https://doi.org/10.3390/s24175774
Chicago/Turabian StyleBonecka, Joanna, Bernard Turek, Krzysztof Jankowski, Marta Borowska, Tomasz Jasiński, Katarzyna Skierbiszewska, and Małgorzata Domino. 2024. "Selection of X-ray Tube Settings for Relative Bone Density Quantification in the Knee Joint of Cats Using Computed Digital Absorptiometry" Sensors 24, no. 17: 5774. https://doi.org/10.3390/s24175774
APA StyleBonecka, J., Turek, B., Jankowski, K., Borowska, M., Jasiński, T., Skierbiszewska, K., & Domino, M. (2024). Selection of X-ray Tube Settings for Relative Bone Density Quantification in the Knee Joint of Cats Using Computed Digital Absorptiometry. Sensors, 24(17), 5774. https://doi.org/10.3390/s24175774







