A Quantitative Comparison of 31P Magnetic Resonance Spectroscopy RF Coil Sensitivity and SNR between 7T and 10.5T Human MRI Scanners Using a Loop-Dipole 31P-1H Probe
Abstract
:1. Introduction
2. Materials and Methods
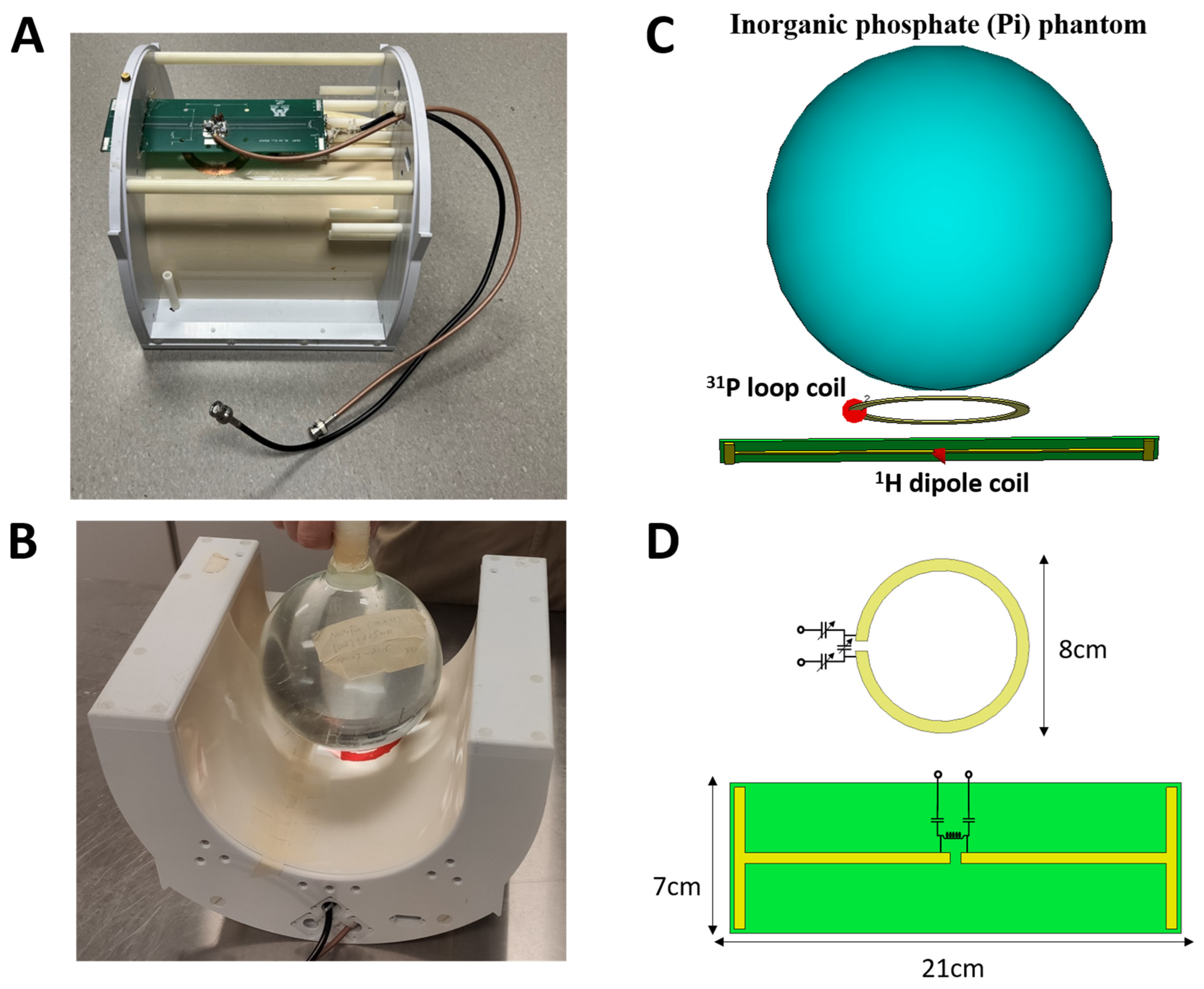
2.1. RF Coil Construction
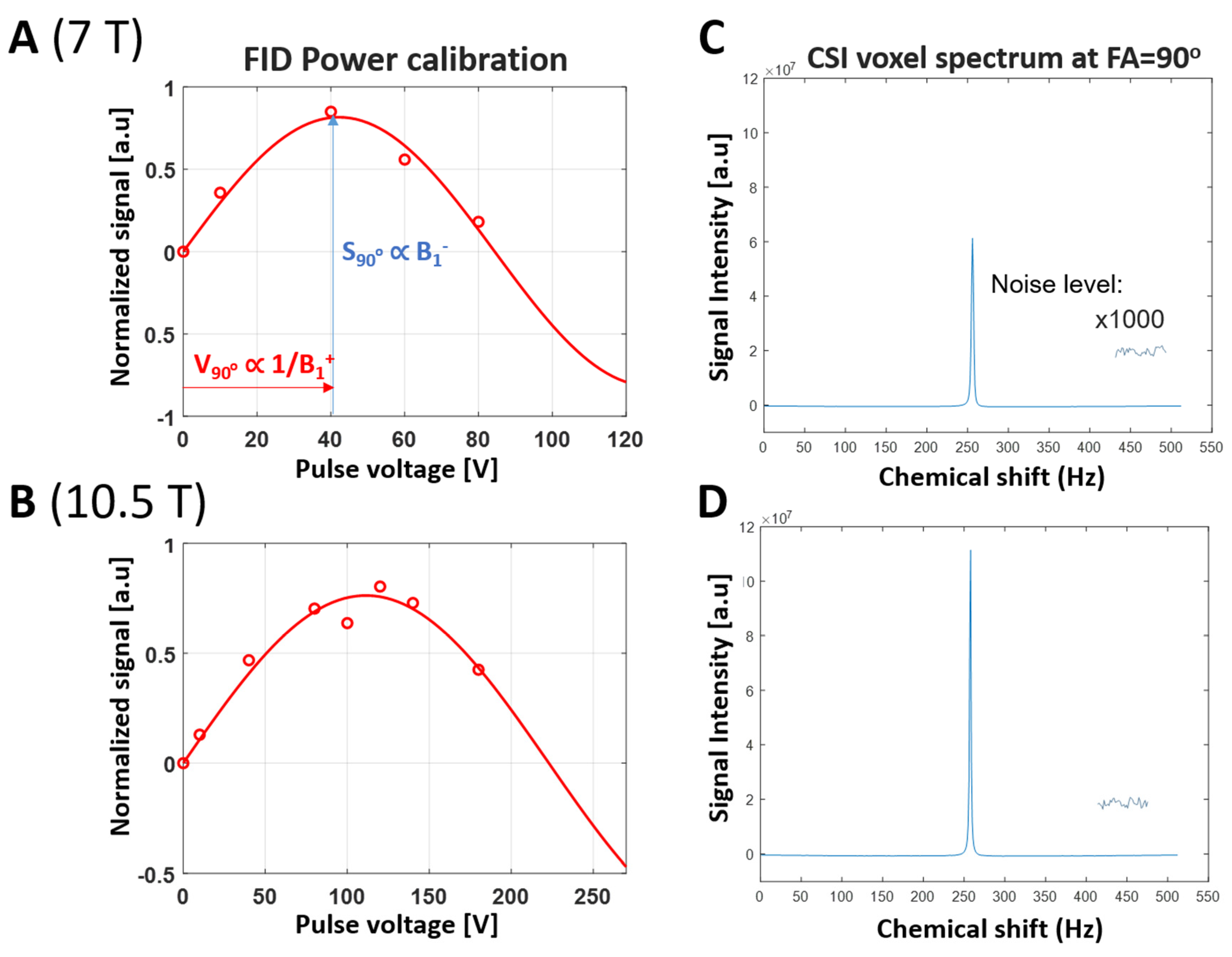
2.2. 31P MRSI Experiment
2.3. SNR Quantification
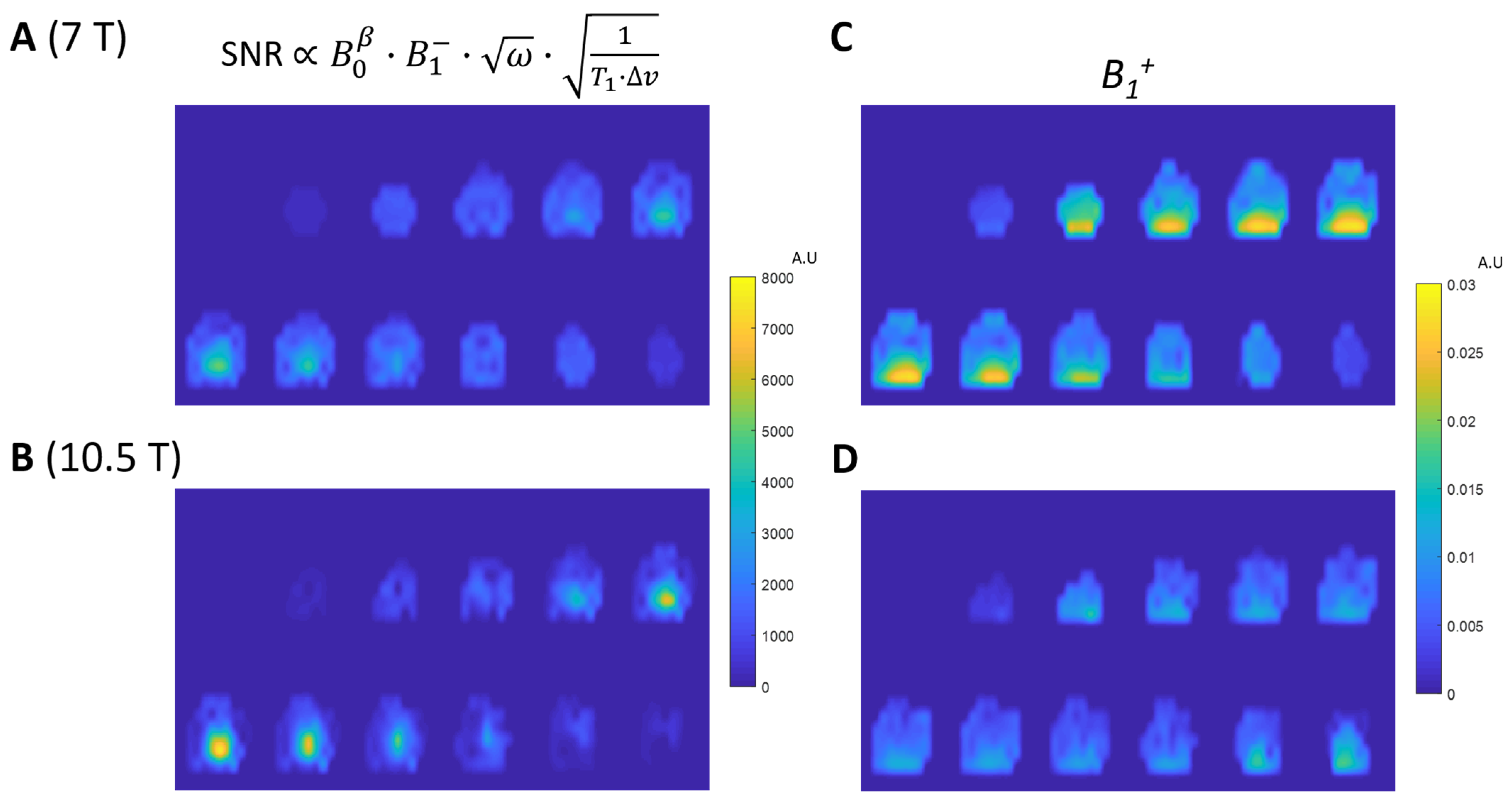
3. Results
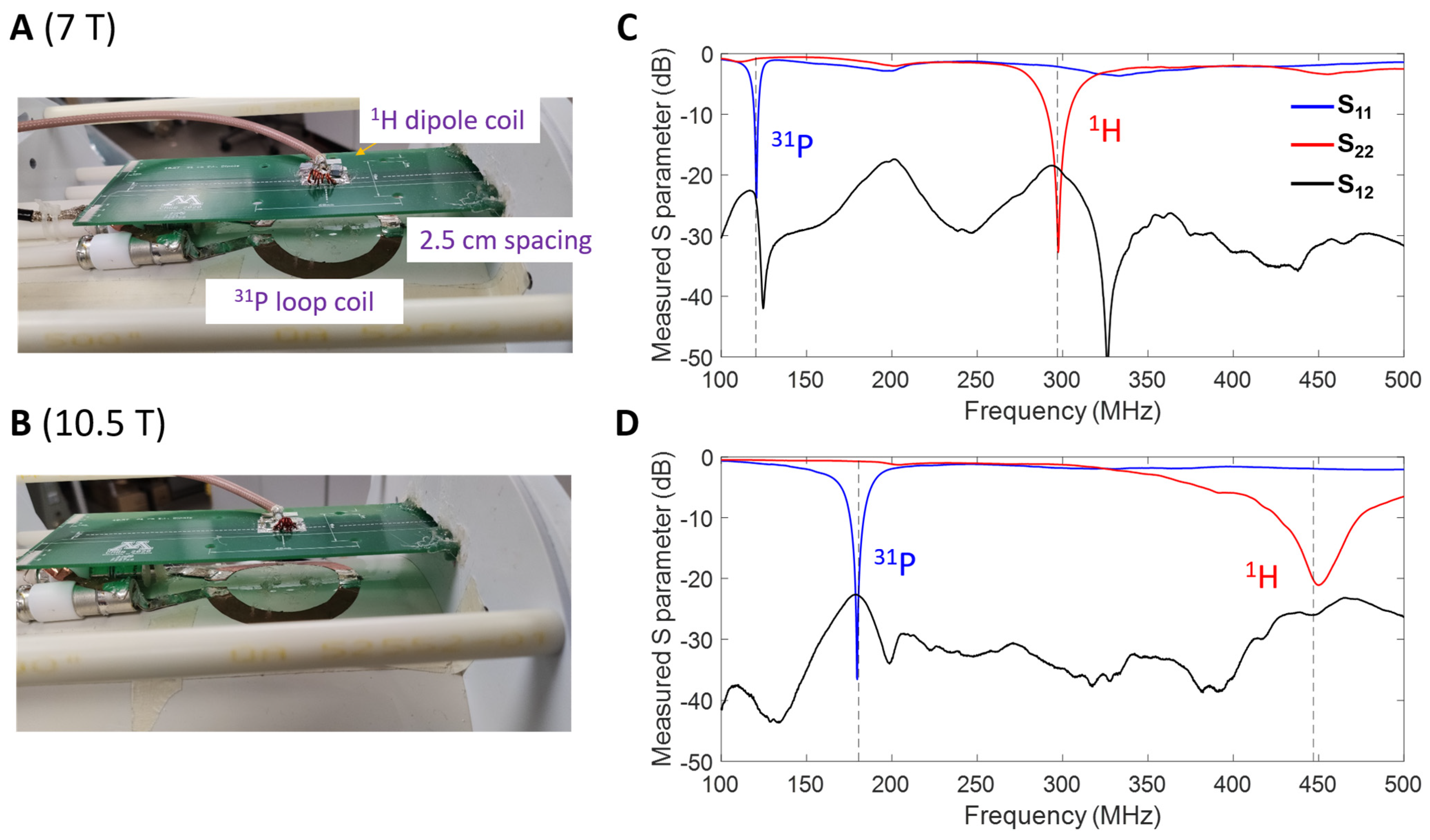
3.1. RF Coil Performance
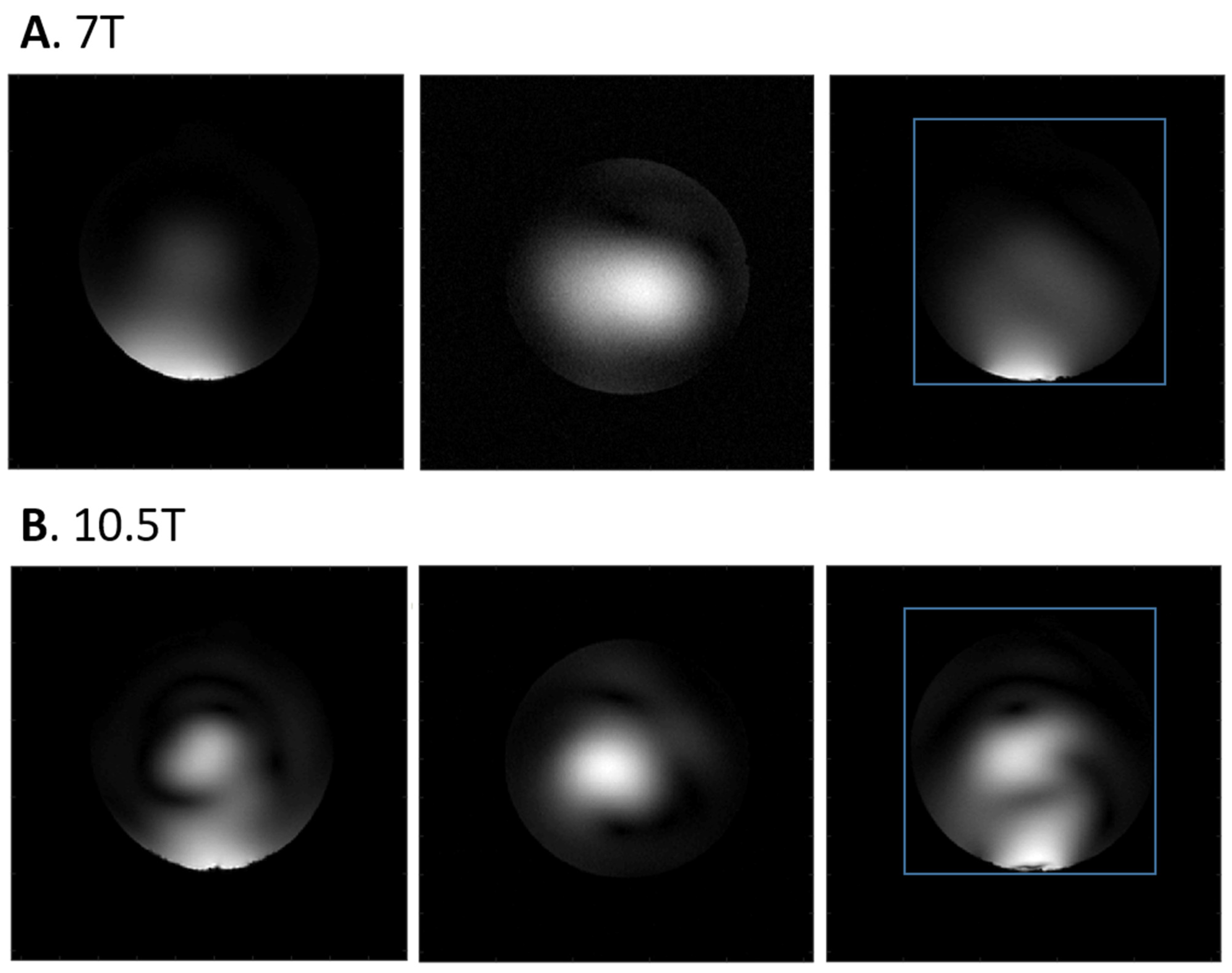
3.2. 1H Imaging and 31P MRSI Results
3.3. SNR Quantification and Comparison between 7T and 10.5T
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rietzler, A.; Steiger, R.; Mangesius, S.; Walchhofer, L.-M.; Gothe, R.M.; Schocke, M.; Gizewski, E.R.; Grams, A.E. Energy metabolism measured by 31P magnetic resonance spectroscopy in the healthy human brain. J. Neuroradiol. 2022, 49, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhu, X.H.; Chen, W. In vivo 31P MRS assessment of intracellular NAD metabolites and NAD+/NADH redox state in human brain at 4 T. NMR Biomed. 2016, 29, 1010–1017. [Google Scholar] [CrossRef]
- Skupienski, R.; Do, K.Q.; Xin, L. In vivo 31P magnetic resonance spectroscopy study of mouse cerebral NAD content and redox state during neurodevelopment. Sci. Rep. 2020, 10, 15623. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, R.A.; De Feyter, H.M.; Brown, P.B.; Nixon, T.W.; Rothman, D.L.; Behar, K.L. Detection of cerebral NAD+ in humans at 7T. Magn. Reson. Med. 2017, 78, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Deelchand, D.K.; Nguyen, T.M.; Zhu, X.H.; Mochel, F.; Henry, P.G. Quantification of in vivo 31P NMR brain spectra using LCModel. NMR Biomed. 2015, 28, 633–641. [Google Scholar] [CrossRef]
- Hetherington, H.P.; Pan, J.W.; Spencer, D.D. 1H and 31P spectroscopy and bioenergetics in the lateralization of seizures in temporal lobe epilepsy. J. Magn. Reson. Imaging 2002, 16, 477–483. [Google Scholar] [CrossRef]
- Sutherland, G.R.; Lesiuk, H.; Hazendonk, P.; Peeling, J.; Buist, R.; Kozlowski, P.; Jazinski, A.; Saunders, J.K. Magnetic Resonance Imaging and 31P Magnetic Resonance Spectroscopy Study of the Effect of Temperature on Ischemic Brain Injury. Can. J. Neurol. Sci./J. Can. Des Sci. Neurol. 1992, 19, 317–325. [Google Scholar] [CrossRef]
- Laxer, K.D.; Hubesch, B.; Sappey-Marinier, D.; Weiner, M.W. Increased pH and inorganic phosphate in temporal seizure foci demonstrated by 31P MRS. Epilepsia 1992, 33, 618–623. [Google Scholar] [CrossRef]
- Jett, S.; Boneu, C.; Zarate, C.; Carlton, C.; Kodancha, V.; Nerattini, M.; Battista, M.; Pahlajani, S.; Williams, S.; Dyke, J.P.; et al. Systematic review of 31P-magnetic resonance spectroscopy studies of brain high energy phosphates and membrane phospholipids in aging and Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1183228. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, X.; Zhu, X.-H.; Du, F.; Chen, W. In vivo 31P MRS of human brain at high/ultrahigh fields: A quantitative comparison of NMR detection sensitivity and spectral resolution between 4 T and 7 T. Magn. Reson. Imaging 2006, 24, 1281–1286. [Google Scholar] [CrossRef]
- Lu, M.; Chen, W.; Zhu, X.-H. Field dependence study of in vivo brain 31P MRS up to 16.4 T. NMR Biomed. 2014, 27, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Avdievich, N.I.; Hetherington, H.P. 4 T Actively detuneable double-tuned 1H/31P head volume coil and four-channel 31P phased array for human brain spectroscopy. J. Magn. Reson. 2007, 186, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Avdievich, N.I.; Ruhm, L.; Dorst, J.; Scheffler, K.; Korzowski, A.; Henning, A. Double-tuned 31P/1H human head array with high performance at both frequencies for spectroscopic imaging at 9.4T. Magn. Reson. Med. 2020, 84, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Lakshmanan, K.; Madelin, G.; Parasoglou, P. A nested phosphorus and proton coil array for brain magnetic resonance imaging and spectroscopy. NeuroImage 2016, 124, 602–611. [Google Scholar] [CrossRef]
- van de Bank, B.L.; Orzada, S.; Smits, F.; Lagemaat, M.W.; Rodgers, C.T.; Bitz, A.K.; Scheenen, T.W. Optimized 31P MRS in the human brain at 7 T with a dedicated RF coil setup. NMR Biomed. 2015, 28, 1570–1578. [Google Scholar] [CrossRef]
- Rowland, B.C.; Driver, I.D.; Tachrount, M.; Klomp, D.W.J.; Rivera, D.; Forner, R.; Pham, A.; Italiaander, M.; Wise, R.G. Whole brain P MRSI at 7T with a dual-tuned receive array. Magn. Reson. Med. 2020, 83, 765–775. [Google Scholar] [CrossRef]
- Sadeghi-Tarakameh, A.; DelaBarre, L.; Lagore, R.L.; Torrado-Carvajal, A.; Wu, X.; Grant, A.; Adriany, G.; Metzger, G.J.; Van de Moortele, P.F.; Ugurbil, K.; et al. In vivo human head MRI at 10.5T: A radiofrequency safety study and preliminary imaging results. Magn. Reson. Med. 2020, 84, 484–496. [Google Scholar] [CrossRef]
- He, X.; Ertürk, M.A.; Grant, A.; Wu, X.; Lagore, R.L.; DelaBarre, L.; Eryaman, Y.; Adriany, G.; Auerbach, E.J.; Van de Moortele, P.F.; et al. First in-vivo human imaging at 10.5T: Imaging the body at 447 MHz. Magn. Reson. Med. 2020, 84, 289–303. [Google Scholar] [CrossRef]
- Schmidt, S.; Ertürk, M.A.; He, X.; Haluptzok, T.; Eryaman, Y.; Metzger, G.J. Improved H body imaging at 10.5 T: Validation and VOP-enabled imaging in vivo with a 16-channel transceiver dipole array. Magn. Reson. Med. 2024, 91, 513–529. [Google Scholar] [CrossRef]
- Tavaf, N.; Lagore, R.L.; Jungst, S.; Gunamony, S.; Radder, J.; Grant, A.; Moeller, S.; Auerbach, E.; Ugurbil, K.; Adriany, G.; et al. A self-decoupled 32-channel receive array for human-brain MRI at 10.5 T. Magn. Reson. Med. 2021, 86, 1759–1772. [Google Scholar] [CrossRef]
- Zhu, X.H.; Merkle, H.; Kwag, J.H.; Ugurbil, K.; Chen, W. 17O relaxation time and NMR sensitivity of cerebral water and their field dependence. Magn. Reson. Med. 2001, 45, 543–549. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, R.A.; Hendriks, A.D.; Klomp, D.W.J.; Kumaragamage, C.; Welting, D.; Arteaga de Castro, C.S.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Prompers, J.J.; et al. On the magnetic field dependence of deuterium metabolic imaging. NMR Biomed. 2020, 33, e4235. [Google Scholar] [CrossRef] [PubMed]
- Pohmann, R.; Speck, O.; Scheffler, K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn. Reson. Med. 2016, 75, 801–809. [Google Scholar] [CrossRef]
- Pohmann, R.; Avdievich, N.I.; Scheffler, K. Signal-to-noise ratio versus field strength for small surface coils. NMR Biomed. 2024, e5168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wiesner, H.M.; Waks, M.; Zhu, X.-H.; Chen, W. Quantitative Comparison of 31P MRS Imaging Performances between 7T and 10.5T Human Scanners Using a Loop-dipole 31P-1H Probe. In Proceedings of the 2021 ISMRM & SMRT Annual Meeting & Exhibition, Online, 15–20 May 2021; Volume 2503. [Google Scholar]
- Shajan, G.; Mirkes, C.; Buckenmaier, K.; Hoffmann, J.; Pohmann, R.; Scheffler, K. Three-layered radio frequency coil arrangement for sodium MRI of the human brain at 9.4 Tesla. Magn. Reson. Med. 2016, 75, 906–916. [Google Scholar] [CrossRef]
- Yan, X.; Xue, R.; Zhang, X. A monopole/loop dual-tuned RF coil for ultrahigh field MRI. Quant. Imaging Med. Surg. 2014, 4, 225–231. [Google Scholar] [CrossRef]
- Avdievich, N.I.; Solomakha, G.; Ruhm, L.; Henning, A.; Scheffler, K. 9.4 T double-tuned 13C/1H human head array using a combination of surface loops and dipole antennas. NMR Biomed. 2021, 34, e4577. [Google Scholar] [CrossRef]
- Raaijmakers, A.J.E.; Ipek, O.; Klomp, D.W.J.; Possanzini, C.; Harvey, P.R.; Lagendijk, J.J.W.; van den Berg, C.A.T. Design of a radiative surface coil array element at 7 T: The single-side adapted dipole antenna. Magn. Reson. Med. 2011, 66, 1488–1497. [Google Scholar] [CrossRef]
- Vincent, J.M.; Rispoli, J.V. Conductive Thread-Based Stretchable and Flexible Radiofrequency Coils for Magnetic Resonance Imaging. IEEE Trans. Biomed. Eng. 2020, 67, 2187–2193. [Google Scholar] [CrossRef]
- Brown, T.R.; Kincaid, B.M.; Ugurbil, K. NMR chemical shift imaging in three dimensions. Proc. Natl. Acad. Sci. USA 1982, 79, 3523–3526. [Google Scholar] [CrossRef]
- Hendrich, K.; Hu, X.P.; Menon, R.S.; Merkle, H.; Camarata, P.; Heros, R.; Ugurbil, K. Spectroscopic Imaging of Circular Voxels with a Two-Dimensional Fourier-Series Window Technique. J. Magn. Reson. Ser. B 1994, 105, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.R.; Bodenhausen, G.; Wokaun, A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions; Oxford University Press: Oxford, UK, 1990. [Google Scholar] [CrossRef]
- Ernst, R.R.; Anderson, W.A. Application of Fourier Transform Spectroscopy to Magnetic Resonance. Rev. Sci. Instrum. 1966, 37, 93–102. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Zhu, X.-H.; Rupprecht, S.; Lanagan, M.T.; Yang, Q.X.; Chen, W. Large improvement of RF transmission efficiency and reception sensitivity for human in vivo 31P MRS imaging using ultrahigh dielectric constant materials at 7 T. Magn. Reson. Imaging 2017, 42, 158–163. [Google Scholar] [CrossRef]
- Vaughan, J.T.; Garwood, M.; Collins, C.M.; Liu, W.; DelaBarre, L.; Adriany, G.; Andersen, P.; Merkle, H.; Goebel, R.; Smith, M.B.; et al. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn. Reson. Med. 2001, 46, 24–30. [Google Scholar] [CrossRef]
- Li, X.; Wiesner, H.M.; Zhu, X.-H.; Chen, W. Large benefit of RF coil B1 efficiency for X-nuclear MRS at ultrahigh field beyond the gyromagnetic ratio advantage of proton. In Proceedings of the 31st Annual Meeting of ISMRM, London, UK, 7–12 May 2022; Volume 4508. [Google Scholar]
- Hoult, D.I.; Lauterbur, P.C. The sensitivity of the zeugmatographic experiment involving human samples. J. Magn. Reson. (1969) 1979, 34, 425–433. [Google Scholar] [CrossRef]
- Hoult, D.I.; Richards, R.E. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson. (1969) 1976, 24, 71–85. [Google Scholar] [CrossRef]
- Edelstein, W.A.; Glover, G.H.; Hardy, C.J.; Redington, R.W. The intrinsic signal-to-noise ratio in NMR imaging. Magn. Reson. Med. 1986, 3, 604–618. [Google Scholar] [CrossRef]
- Ocali, O.; Atalar, E. Ultimate intrinsic signal-to-noise ratio in MRI. Magn. Reson. Med. 1998, 39, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Kopanoglu, E.; Erturk, V.B.; Atalar, E. Analytic expressions for the ultimate intrinsic signal-to-noise ratio and ultimate intrinsic specific absorption rate in MRI. Magn. Reson. Med. 2011, 66, 846–858. [Google Scholar] [CrossRef]
- Lee, H.H.; Sodickson, D.K.; Lattanzi, R. An analytic expression for the ultimate intrinsic SNR in a uniform sphere. Magn. Reson. Med. 2018, 80, 2256–2266. [Google Scholar] [CrossRef]

| B0 | T1 (ms) (with Gd) | ∆v (Hz) | Qloaded | SNR (10 Voxels Average) | (10 Voxels Average) | |
|---|---|---|---|---|---|---|
| 10.5T | 220 | 27.6 | 40.9 | 5.57 ± 0.89 × 103 | 10.9 × 10−3 | 1.9 × 104 |
| 7T | 300 | 29.0 | 75.3 | 3.82 ± 0.69 × 103 | 24.4 × 10−3 | 1.4 × 104 |
| ratio | 0.73 | 0.95 | 0.543 | 1.48 ± 0.24 | 0.45 | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, X.-H.; Chen, W. A Quantitative Comparison of 31P Magnetic Resonance Spectroscopy RF Coil Sensitivity and SNR between 7T and 10.5T Human MRI Scanners Using a Loop-Dipole 31P-1H Probe. Sensors 2024, 24, 5793. https://doi.org/10.3390/s24175793
Li X, Zhu X-H, Chen W. A Quantitative Comparison of 31P Magnetic Resonance Spectroscopy RF Coil Sensitivity and SNR between 7T and 10.5T Human MRI Scanners Using a Loop-Dipole 31P-1H Probe. Sensors. 2024; 24(17):5793. https://doi.org/10.3390/s24175793
Chicago/Turabian StyleLi, Xin, Xiao-Hong Zhu, and Wei Chen. 2024. "A Quantitative Comparison of 31P Magnetic Resonance Spectroscopy RF Coil Sensitivity and SNR between 7T and 10.5T Human MRI Scanners Using a Loop-Dipole 31P-1H Probe" Sensors 24, no. 17: 5793. https://doi.org/10.3390/s24175793






