Towards the Instrumentation of Facemasks Used as Personal Protective Equipment for Unobtrusive Breathing Monitoring of Workers
Abstract
:1. Introduction
2. Materials and Methods
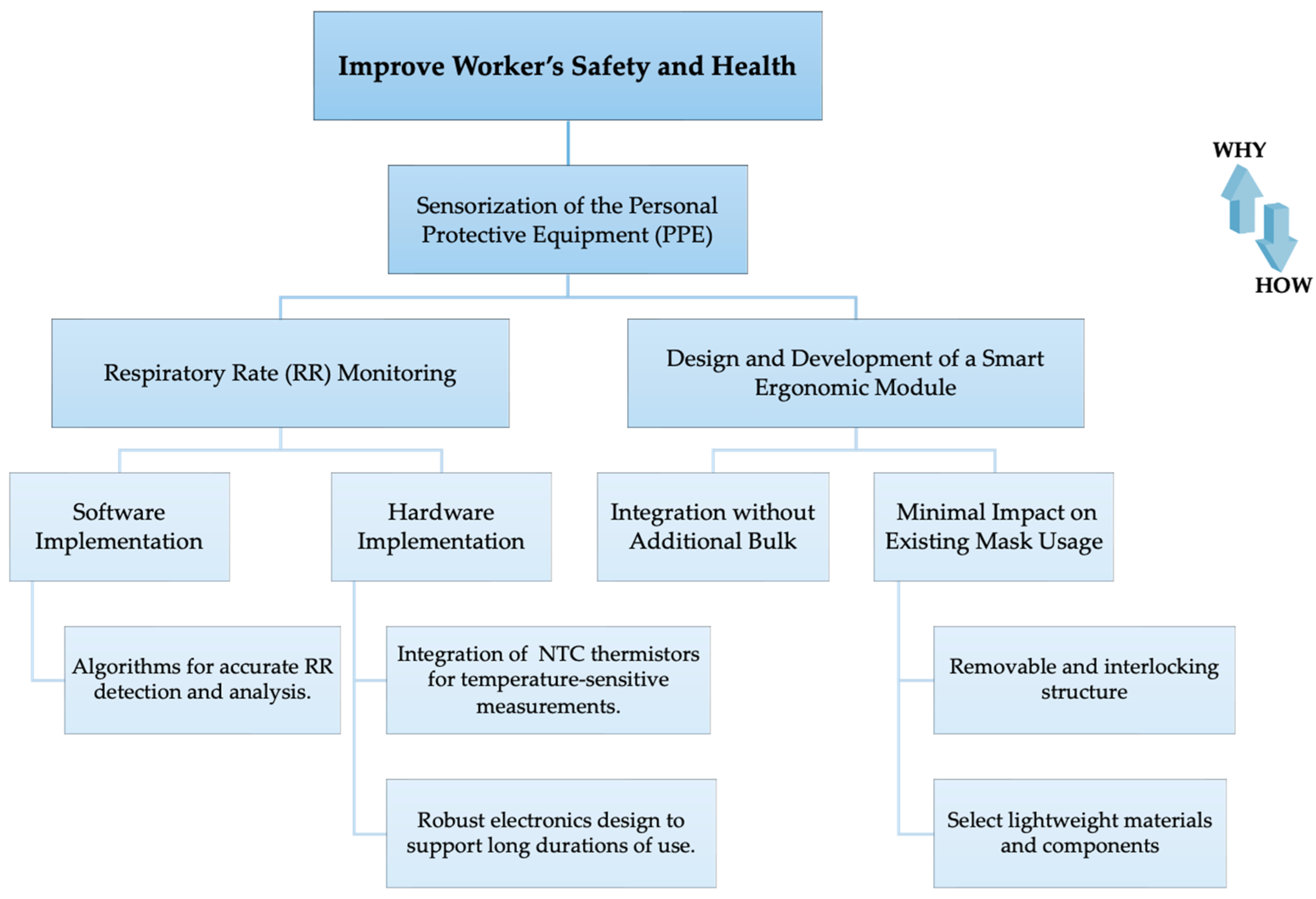
2.1. Smart Module Development Process
- Sensor Selection and Placement: Two NTC thermistors (model number GA10K3MBD1, Mini Betacurve by TE Connectivity, Measurement Specialties, Inc. Berwyn, PA, USA, with diameter of 1.1 mm and mass 0.1 g, resistance at 25 °C equal to 10 kΩ ± 0.2%, response time in liquids: 0.4 s, operating temperature range of −40 °C to 125 °C) were chosen. Within the smart module, sensor t1 is designed to be placed inside the exhalation valve of the mask, positioned in an area expected to have the highest sensitivity [26], and is less subject to external ambient temperature, thus solely responding to temperature variations related to breath. The drawback of this integration is that it requires drilling into the exhalation valve, which is sealed with a particular material that might raise concerns regarding regulatory compliance. Meanwhile, in the second configuration to be tested, sensor t2 is located outside the valve but near the air outlet point, which does not alter the valve structure of the mask, and thus does not require alteration of the mechanical structure of the facemask and its certification.
- Electronics and Data Acquisition: The Nicla Sense ME board (Arduino®, Monza, Italy) was selected for its compact dimensions (22.86 mm × 22.86 mm) and reduced mass (2 g). It includes data acquisition Bluetooth® 4.2 connectivity, an accelerometer, and a gyroscope.To connect the two analog thermistors, t1 and t2, to the board, a voltage divider circuit is commonly employed. A Printed Circuit Board (PCB) was designed to include two voltage dividers each for the 10 kΩ thermistors (Rthermistor). This configuration was chosen because the 10 kΩ NTC thermistors at 25 °C, when paired with a 10 kΩ resistor (Rcircuit) in each voltage divider, create a balanced setup. The output of the voltage divider (Vout) is proportional to the input voltage (Vin), Rcircuit, and Rthermistor, as in Equation (1):Thus, considering a Vin of 5 V:
- At 25 °C, where Rthermistor is 10 kΩ (equal to Rcircuit), the Vout value is 2.5 V.
- For NTC thermistors, the resistance decreases with an increase in temperature. Consequently, at temperatures above 25 °C, Rthermistor will be less than 10 kΩ, resulting in Vout increasing beyond 2.5 V. Conversely, at temperatures below 25 °C, Rthermistor will be greater than 10 kΩ, causing Vout to decrease below 2.5 V.
- Integration of the Smart Module into the Facemask: Successful integration was facilitated by designing two 3D-printed structures using PLA and the Ultimaker S5 3D printer (Ultimaker, Utrecht, The Netherlands). For thermistor t1, an internal quarter-cross-shaped structure was specifically designed to fit into other similar types of valves. This structure has a radius of 13 mm and features a hole with a diameter of 0.65 mm to accommodate the sensor head. For thermistor t2 and the Arduino Nicla Sense ME board, an external interlocking structure was crafted. This structure maintains an open exhalation space and includes a passage for t2. Above this passage, a slot with a diameter of 39 mm and a 4 mm recess aligned with the exhalation part was created for secure mounting of the electronic board. This design allows for the structure to seamlessly interlock with the valve cover, ensuring efficient placement and stability.
- Remote Base Station: Data from the smart module are displayed in real-time within MATLAB to allow for the immediate visualization of waveform shapes, providing crucial feedback on the device’s performance. This real-time data stream is instrumental in verifying the functionality of the sensors and ensuring that they accurately capture the wearer’s breathing pattern.In addition to real-time monitoring, the data are also exported to .csv files for subsequent analysis. The data acquisition for this system was intentionally performed using a non-wireless connection. This approach was chosen to ensure a reliable power supply to the board and uninterrupted data acquisition, which is crucial for long-term monitoring and situations where maintaining consistent data integrity and quality are paramount. The non-wireless setup helps to avoid issues related to battery life and wireless connectivity that might introduce gaps or inconsistencies in the data stream.Despite using a non-wireless setup for the main data acquisition, the board is equipped with Bluetooth capabilities, offering the flexibility to switch to wireless data transmission where appropriate. This wireless functionality is powered by a 3.7 V Li-Po battery, which includes an integrated battery charger, enhancing the device’s portability and ease of use. Including a rechargeable battery system with Bluetooth connectivity makes the device adaptable to various settings, ranging from clinical to field applications, where mobility and minimal encumbrance are essential. This dual-mode capability ensures that the device can be tailored to meet the specific needs of different research or monitoring scenarios, combining robust and reliable data collection with the flexibility of wireless communication when needed.
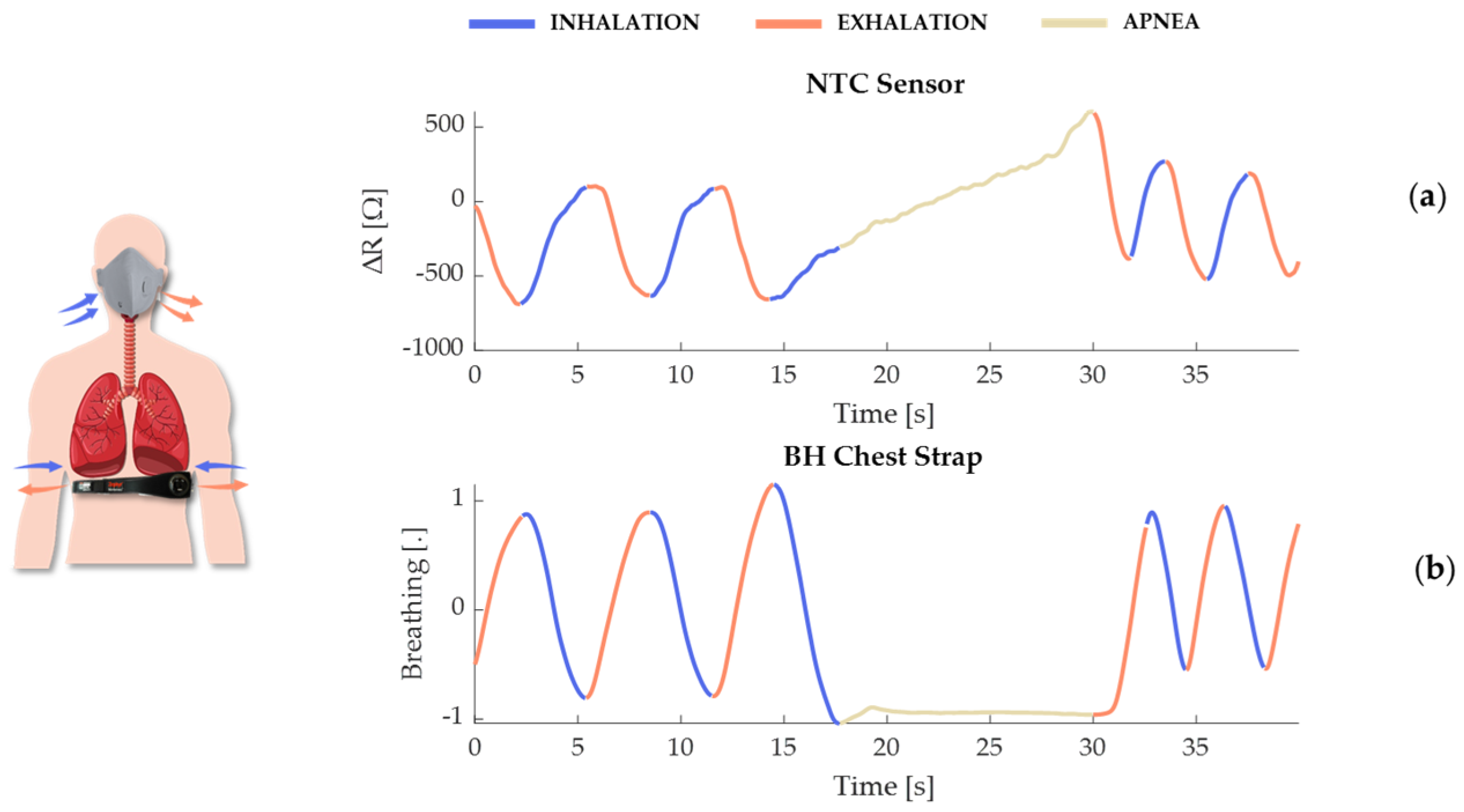
- Selection of the reference system: The Zephyr BioHarness (by Medtronic Inc., Minneapolis, MN, USA, hereinafter BH) was selected for the reference system, as it is widely recognized for physiological monitoring in industrial settings. It embeds a strain sensor for collecting respiratory signals (sampling rate: 25 Hz) and two dry electrodes for capturing single-lead ECG (sampling rate: 250 Hz). The strain sensors used for respiratory monitoring record the chest wall circumference changes due to respiratory effort, converting mechanical deformation into electrical signals. It was placed on the participants’ chest to record respiratory parameters and compare the data with those obtained from the integrated mask module.

2.2. System’s Principle of Working
- If the exhaled TA exceeds the ambient temperature (TE), the measured temperature at the respiratory module increases, consequently lowering the resistance.
- Conversely, if TA is below TE, the measured temperature decreases, thereby increasing the resistance.
- If TA equals TE, both the measured temperature and resistance at the respiratory module remain unchanged.
3. Feasibility Assessment of the Smart Module
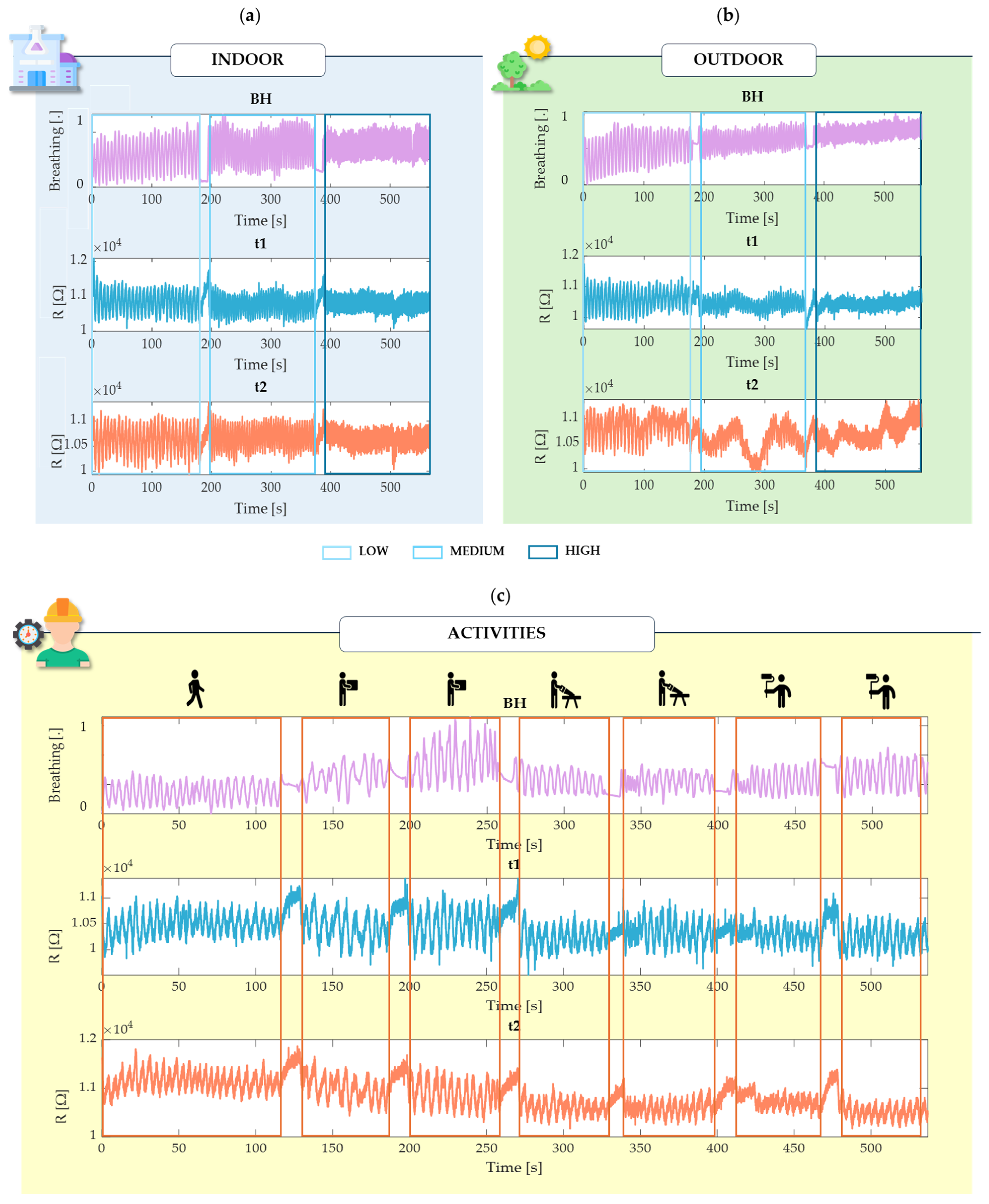
3.1. Experimental Setup and Protocol
- ○
- Low rate: 10 breaths per minute (bpm).
- ○
- Medium rate: 15 bpm.
- ○
- High rate: 30 bpm.
- Walking (2 min).
- Lifting Boxes (1 min slow and 1 min fast).
- Cutting (1 min slow and 1 min fast).
- Painting (1 min slow and 1 min fast).
3.2. Data Processing
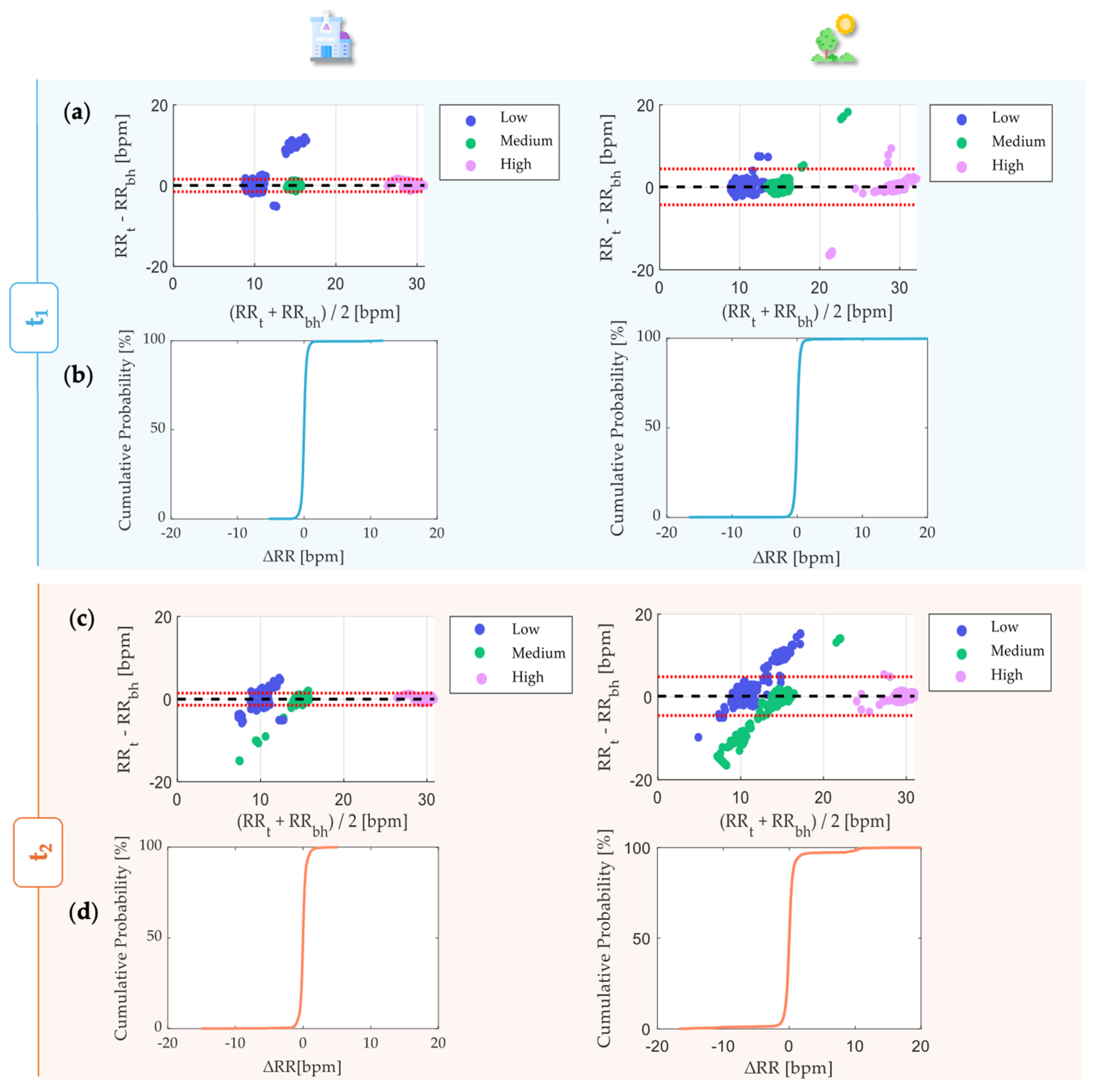
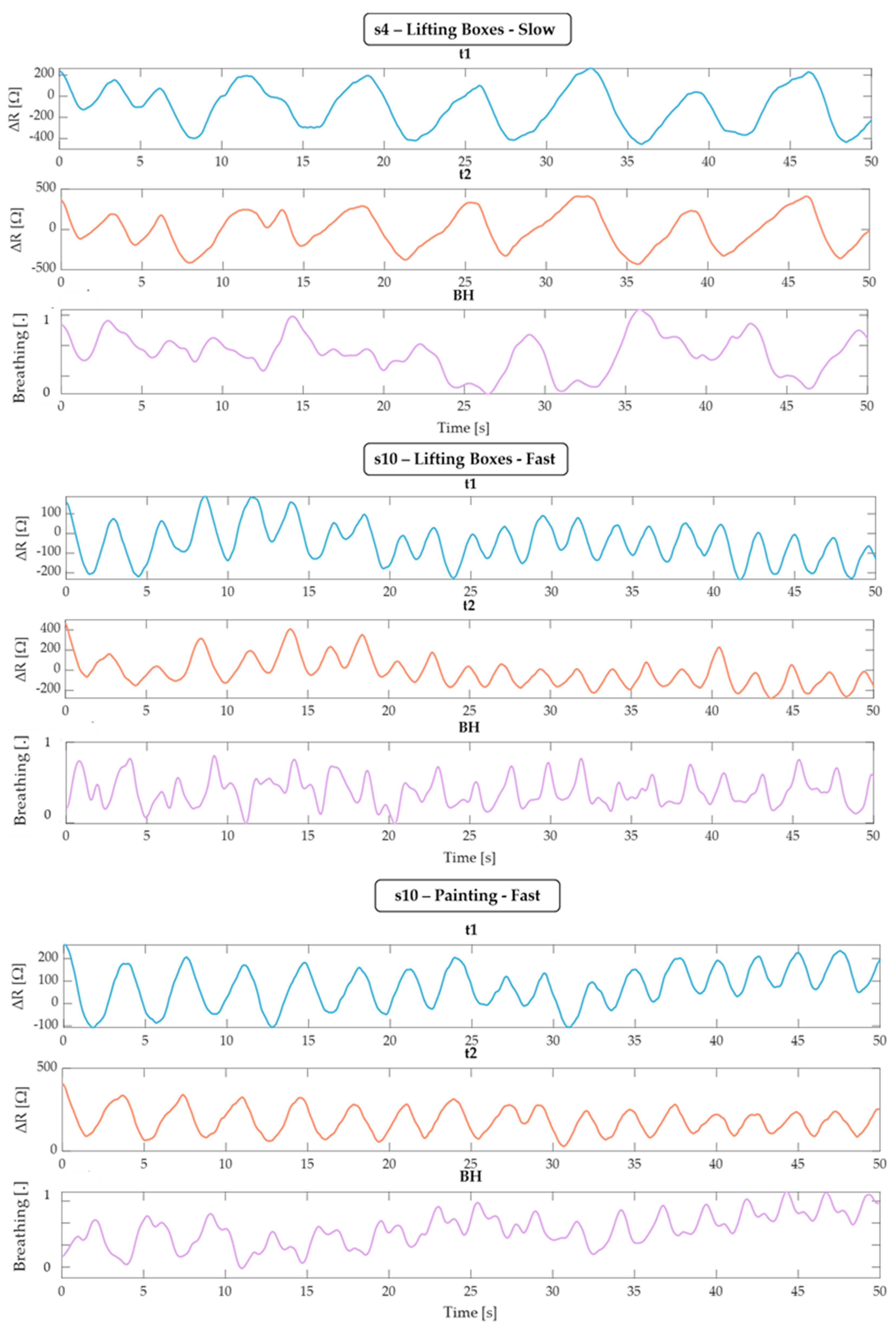
4. Results
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senthilselvan, A.; Coonghe, W.V.L.; Beach, J. Respiratory health, occupation and the healthy worker effect. Occup. Med. 2020, 70, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Balkhyour, M.A. Occupational exposure and respiratory health of workers at small scale industries. Saudi J. Biol. Sci. 2020, 27, 985–990. [Google Scholar] [CrossRef]
- Respiratory Findings in Workers Employed in the Brick-Manufacturing Industry: Journal of Occupational and Environmental Medicine. Available online: https://journals.lww.com/joem/abstract/1998/09000/respiratory_findings_in_workers_employed_in_the.11.aspx (accessed on 21 May 2024).
- Seliga, R.; Bhattacharya, A.; Succop, P.; Wickstrom, R.; Smith, D.; Willeke, K. Effect of work loan and respirator wear on postural stability, heart rate, and perceived exertion. Am. Ind. Hyg. Assoc. J. 1991, 52, 417–422. [Google Scholar] [CrossRef] [PubMed]
- 1910.134—Respiratory Protection|Occupational Safety and Health Administration. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 (accessed on 3 June 2024).
- Maestrelli, P.; Boschetto, P.; Carta, P.; Corradi, M.; De Zotti, R.; Di Lorenzo, L.; Ferrari, M.; Guarnieri, G.; Imbriani, M.; Innocenti, A.; et al. Linee Guida per la Formazione Continua e L’accreditamento del Medico del Lavoro Linee Guida per la Sorveglianza Sanitaria di Lavoratori Esposti ad Irritanti e Tossici per L’apparato Respiratorio; Società Italiana di Medicina del Lavoro e Igiene Industriale: Bologna, Italy, 2009. [Google Scholar]
- Inam, S.H.A. Physiological Impacts of Personal Protective Equipment on Health Care Workers. Indones. J. Occup. Saf. Health 2021, 10, 1–5. [Google Scholar]
- Ergonomic Guidelines for Manual Material Handling. Available online: https://www.cdc.gov/niosh/media/pdfs/Ergonomic-Guidelines-for-Manual-Material-Handling_2007-131.pdf (accessed on 21 May 2024).
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef]
- Anwer, S.; Li, H.; Antwi-Afari, M.F.; Umer, W.; Wong, A.Y.L. Evaluation of Physiological Metrics as Real-Time Measurement of Physical Fatigue in Construction Workers: State-of-the-Art Review. J. Constr. Eng. Manag. 2021, 147, 03121001. [Google Scholar] [CrossRef]
- Silva, L.; Dias, M.; Folgado, D.; Nunes, M.; Namburi, P.; Anthony, B.; Carvalho, D.; Carvalho, M.; Edelman, E.; Gamboa, H. Respiratory Inductance Plethysmography to Assess Fatigability during Repetitive Work. Sensors 2022, 22, 4247. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, C.; Nicolo, A.; Sacchetti, M.; Schena, E. Contactless methods for measuring respiratory rate: A review. IEEE Sens. J. 2020, 21, 12821–12839. [Google Scholar] [CrossRef]
- Erik, V.; Igual, R.; Plaza, I. Sensing systems for respiration monitoring: A technical systematic review. Sensors 2020, 20, 5446. [Google Scholar] [CrossRef]
- Di Tocco, J.; Raiano, L.; Sabbadini, R.; Massaroni, C.; Formica, D.; Schena, E. A wearable system with embedded conductive textiles and an imu for unobtrusive cardio-respiratory monitoring. Sensors 2021, 21, 3018. [Google Scholar] [CrossRef]
- Elfaramawy, T.; Fall, C.L.; Arab, S.; Morissette, M.; Lellouche, F.; Gosselin, B. A wireless respiratory monitoring system using a wearable patch sensor network. IEEE Sens. J. 2018, 19, 650–657. [Google Scholar] [CrossRef]
- Al-Khalidi, F.Q.; Saatchi, R.; Burke, D.; Elphick, H.; Tan, S. Respiration rate monitoring methods: A review. Pediatr. Pulmonol. 2011, 46, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Schena, E.; Silvestri, S.; Massaroni, C. Non-contact respiratory monitoring using an RGB camera for real-world applications. Sensors 2021, 21, 5126. [Google Scholar] [CrossRef]
- Molinaro, N.; Schena, E.; Silvestri, S.; Bonotti, F.; Aguzzi, D.; Viola, E.; Buccolini, F.; Massaroni, C. Contactless vital signs monitoring from videos recorded with digital cameras: An overview. Front. Physiol. 2022, 13, 801709. [Google Scholar] [CrossRef]
- Chan, P.Y.; Ryan, N.P.; Chen, D.; McNeil, J.; Hopper, I. Novel wearable and contactless heart rate, respiratory rate, and oxygen saturation monitoring devices: A systematic review and meta-analysis. Anaesthesia 2022, 77, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, R.; Di Tocco, J.; Massaroni, C.; Schena, E.; Carassiti, M. A smart face mask based on photoplethysmography for cardiorespiratory monitoring in occupational settings. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2021, Lausanne, Switzerland, 23–25 June 2021. [Google Scholar] [CrossRef]
- Zhong, J.; Li, Z.; Takakuwa, M.; Inoue, D.; Hashizume, D.; Jiang, Z.; Shi, Y.; Ou, L.; Nayeem, M.O.G.; Umezu, S.; et al. Smart Face Mask Based on an Ultrathin Pressure Sensor for Wireless Monitoring of Breath Conditions. Adv. Mater. 2022, 34, 2107758. [Google Scholar] [CrossRef]
- Suo, J.; Liu, Y.; Wu, C.; Chen, M.; Huang, Q.; Liu, Y.; Yao, K.; Chen, Y.; Pan, Q.; Chang, X.; et al. Wide-Bandwidth Nanocomposite-Sensor Integrated Smart Mask for Tracking Multiphase Respiratory Activities. Adv. Sci. 2022, 9, e2203565. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; Faramarzi, N.; Ali, B.; Ballam, L.R.; D’Aloia, A.G.; Tamburrano, A.; Sarto, M.S. Wearable Graphene-based smart face mask for Real-Time human respiration monitoring. Mater. Des. 2023, 230, 111970. [Google Scholar] [CrossRef]
- Romano, C.; Nicolò, A.; Innocenti, L.; Sacchetti, M.; Schena, E.; Massaroni, C. Design and Testing of a Smart Facemask for Respiratory Monitoring during Cycling Exercise. Biosensors 2023, 13, 369. [Google Scholar] [CrossRef]
- Giorgi, L.; Di Marco, F.; Presti, D.L.; Massaroni, C.; Romano, C.; Moffa, A.; Casale, M.; Schena, E. An innovative smart face mask for the estimation of respiratory rate: Design, development and feasibility assessment. In Proceedings of the 2023 IEEE International Workshop on Metrology for Industry 4.0 and IoT, MetroInd4.0 and IoT 2023, Brescia, Italy, 6–8 June 2023; pp. 189–193. [Google Scholar] [CrossRef]
- Lazaro, M.; Lazaro, A.; Villarino, R.; Girbau, D. Smart Face Mask with an Integrated Heat Flux Sensor for Fast and Remote People’s Healthcare Monitoring. Sensors 2021, 21, 7472. [Google Scholar] [CrossRef]
- Hurtado, D.E.; Abusleme, A.; Chávez, J.A.P. Non-invasive continuous respiratory monitoring using temperature-based sensors. J. Clin. Monit. Comput. 2020, 34, 223–231. [Google Scholar] [CrossRef]
- Akre, O.S.H. Diagnosing respiratory events and tracing air flow by internal thermistors. Acta Oto-Laryngol. 2000, 120, 414–419. [Google Scholar] [CrossRef]
- Raiano, L.; Di Tocco, J.; Massaroni, C.; Di Pino, G.; Schena, E.; Formica, D. Respiratory Rate Estimation During Walking/Running Activities Using Principal Components Estimated from Signals Recorded by a Smart Garment Embedding Piezoresistive Sensors. In Proceedings of the 2021 IEEE International Workshop on Metrology for Industry 4.0 and IoT, Rome, Italy, 7–9 June 2021; pp. 544–549. [Google Scholar] [CrossRef]
- Norman, R.G.; Ahmed, M.M.; Walsleben, J.A.; Rapoport, D.M. Detection of respiratory events during NPSG: Nasal cannula/pressure sensor versus thermistor. Sleep 1997, 20, 1175–1184. [Google Scholar] [CrossRef]
- Ermer, S.; Brewer, L.; Orr, J.; Egan, T.D.; Johnson, K. Comparison of 7 Different Sensors for Detecting Low Respiratory Rates Using a Single Breath Detection Algorithm in Nonintubated, Sedated Volunteers. Anesth. Analg. 2019, 129, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Q.; Tang, Z.; Liu, J.; Xiang, B. Towards Accurate, Cost-Effective, Ultra-Low-Power and Non-Invasive Respiration Monitoring: A Reusable Wireless Wearable Sensor for an Off-the-Shelf KN95 Mask. Sensors 2021, 21, 6698. [Google Scholar] [CrossRef] [PubMed]
- Hameed, K.; Shahrukh, S.I.; Ateeq, I.S. Design of Monitoring System for Respiratory Diagnosis. In Proceedings of the 2022 IEEE International IOT, Electronics and Mechatronics Conference, IEMTRONICS, Toronto, ON, Canada, 1–4 June 2022. [Google Scholar] [CrossRef]
- Rao, K.M.; Sudarshan, B.G. Design and development of real time respiratory rate monitor using non-invasive biosensor. Int. J. Res. Eng. Technol. 2015, 4, 437–442. [Google Scholar]
- De Tommasi, F.; Presti, D.L.; Caponero, M.A.; Carassiti, M.; Schena, E.; Massaroni, C. Smart mattress based on multipoint fiber Bragg gratings for respiratory rate monitoring. IEEE Trans. Instrum. Meas. 2022, 72, 4000710. [Google Scholar] [CrossRef]
- Chon, K.H.; Dash, S.; Ju, K. Estimation of respiratory rate from photoplethysmogram data using time–frequency spectral estimation. IEEE Trans. Biomed. Eng. 2009, 56, 2054–2063. [Google Scholar] [CrossRef]
- Martin, B.J.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]




| Sensor t1 | Sensor t2 | |||
|---|---|---|---|---|
| INDOOR | OUTDOOR | INDOOR | OUTDOOR | |
| MOD [bpm] | 0.03 | 0.11 | −0.01 | 0.17 |
| LOA1 [bpm] | +1.57 | +4.45 | +1.45 | +4.86 |
| LOA2 [bpm] | −1.51 | −4.24 | −1.47 | −4.51 |
| % of RR within ±2 bpm [%] | 99.6 | 99.4 | 99.2 | 96.7 |
| MAPE [%] | ||
|---|---|---|
| Activities | Sensor t1 | Sensor t2 |
| WALKING | 2.49 | 2.76 |
| LIFTING BOXES—SLOW | 6.46 | 7.24 |
| LIFTING BOXES—FAST | 2.93 | 2.22 |
| CUTTING—SLOW | 4.25 | 4.68 |
| CUTTING—FAST | 2.86 | 2.61 |
| PAINTING—SLOW | 2.53 | 2.41 |
| PAINTING—FAST | 3.84 | 3.26 |
| Sensor t1 | Sensor t2 | |
|---|---|---|
| ACTIVITY | ACTIVITY | |
| MOD [bpm] | −0.21 | −0.05 |
| LOA1 [bpm] | +3.34 | +2.25 |
| LOA2 [bpm] | −3.76 | −2.36 |
| % of RR within ±2 bpm [%] | 97.54 | 97.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinnelli, M.; Lo Presti, D.; Silvestri, S.; Setola, R.; Schena, E.; Massaroni, C. Towards the Instrumentation of Facemasks Used as Personal Protective Equipment for Unobtrusive Breathing Monitoring of Workers. Sensors 2024, 24, 5815. https://doi.org/10.3390/s24175815
Pinnelli M, Lo Presti D, Silvestri S, Setola R, Schena E, Massaroni C. Towards the Instrumentation of Facemasks Used as Personal Protective Equipment for Unobtrusive Breathing Monitoring of Workers. Sensors. 2024; 24(17):5815. https://doi.org/10.3390/s24175815
Chicago/Turabian StylePinnelli, Mariangela, Daniela Lo Presti, Sergio Silvestri, Roberto Setola, Emiliano Schena, and Carlo Massaroni. 2024. "Towards the Instrumentation of Facemasks Used as Personal Protective Equipment for Unobtrusive Breathing Monitoring of Workers" Sensors 24, no. 17: 5815. https://doi.org/10.3390/s24175815










