Looping Flexible Fluoropolymer Microcapillary Film Extends Analysis Times for Vertical Microfluidic Blood Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Test Strip Production
2.2. Preparation of Blood Samples
2.3. Flow Dynamics of Looped Microcapillaries
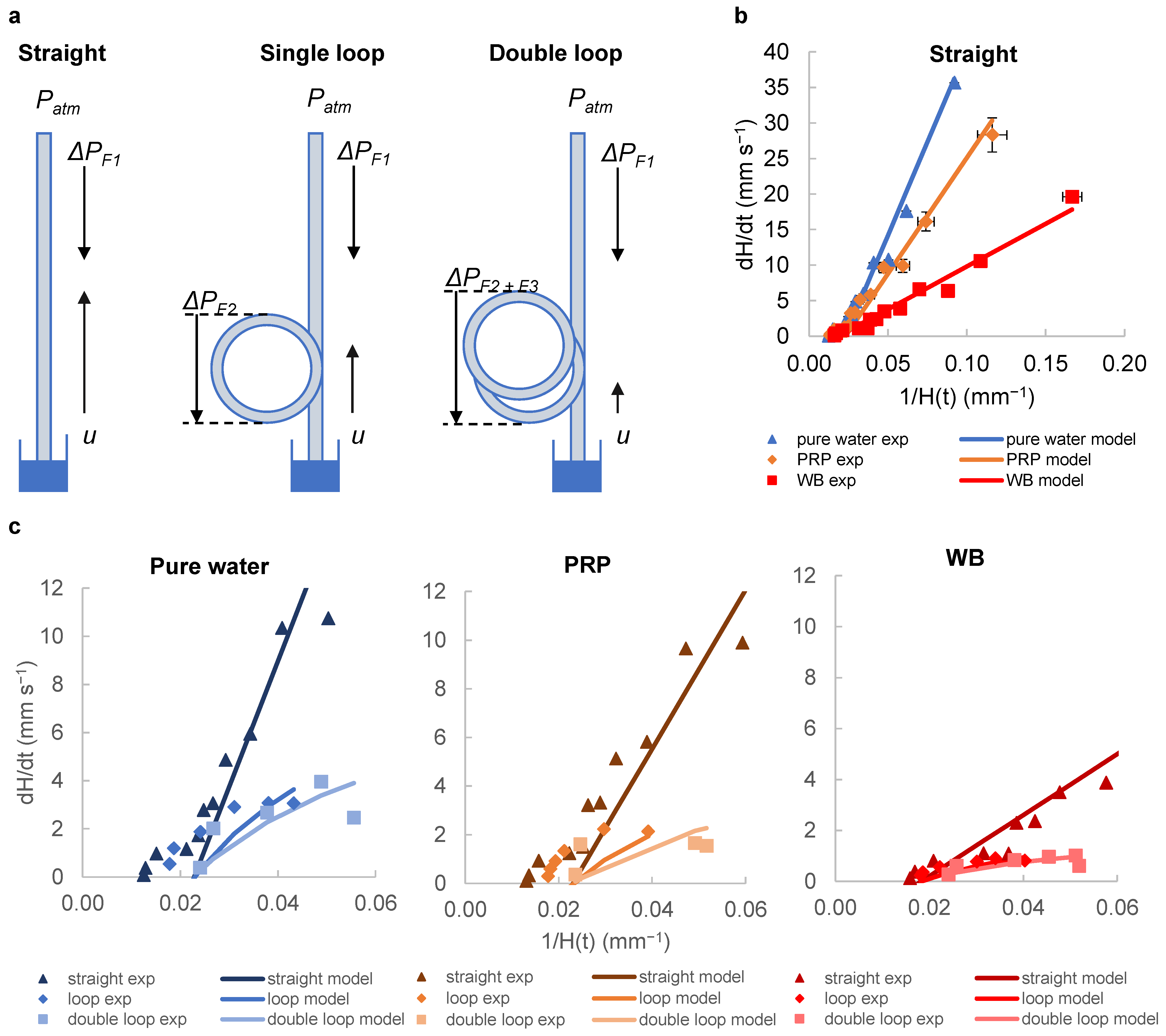
2.4. Analytical Modelling of Fluid Rise in the Straight and Looped Microcapillaries
2.5. Effect of Loop Addition on Capillary Rise of Blood with Stimulation
2.6. Statistical Analyses
3. Results and Discussion
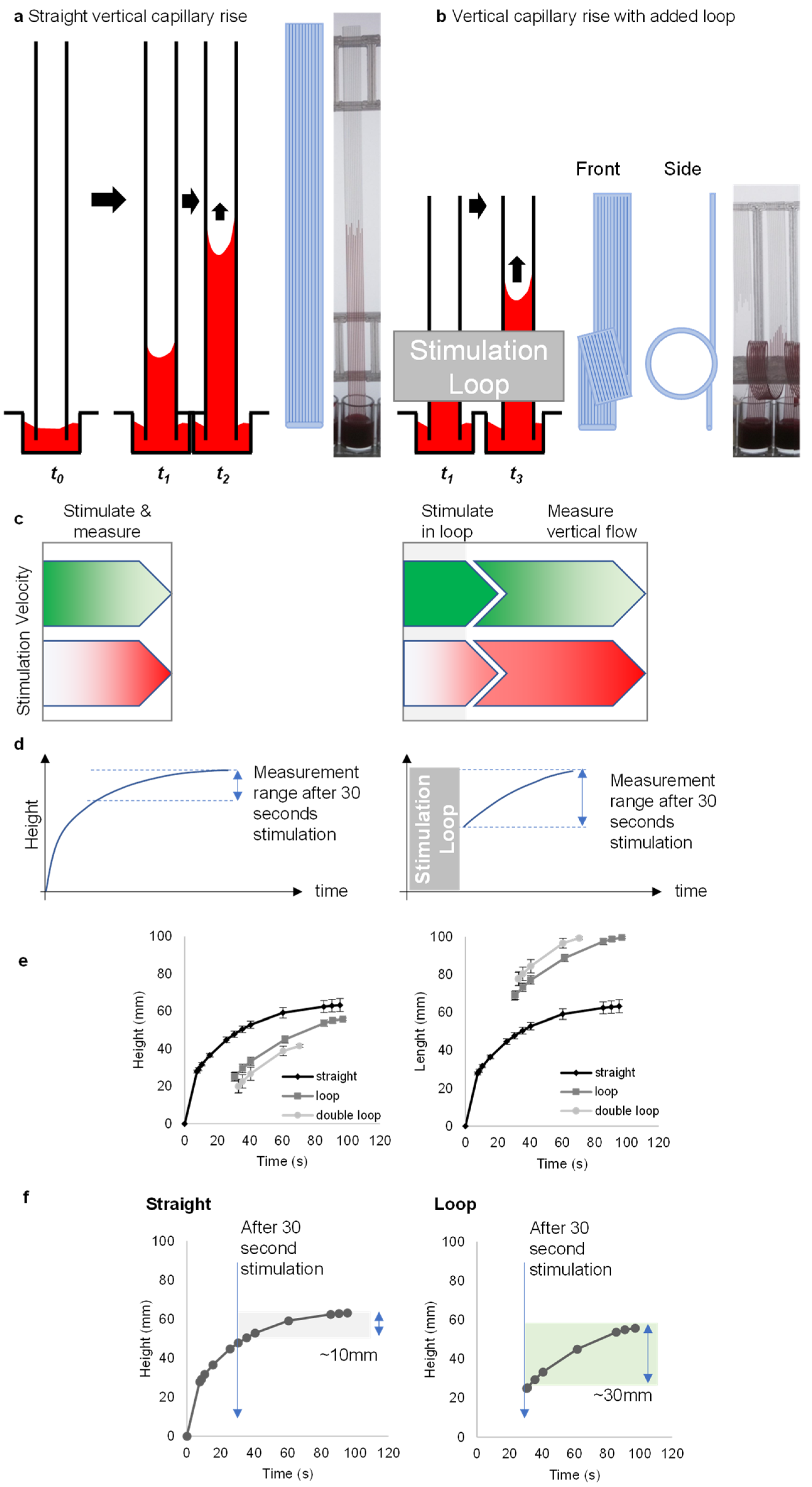
3.1. Loops Introduce Additional Stimulation Zone and Extend Flow Measurement Range
3.2. Capturing Flow Dynamics for Water, Plasma and Blood during Capillary Flow in Vertical and Looped Microcapillaries
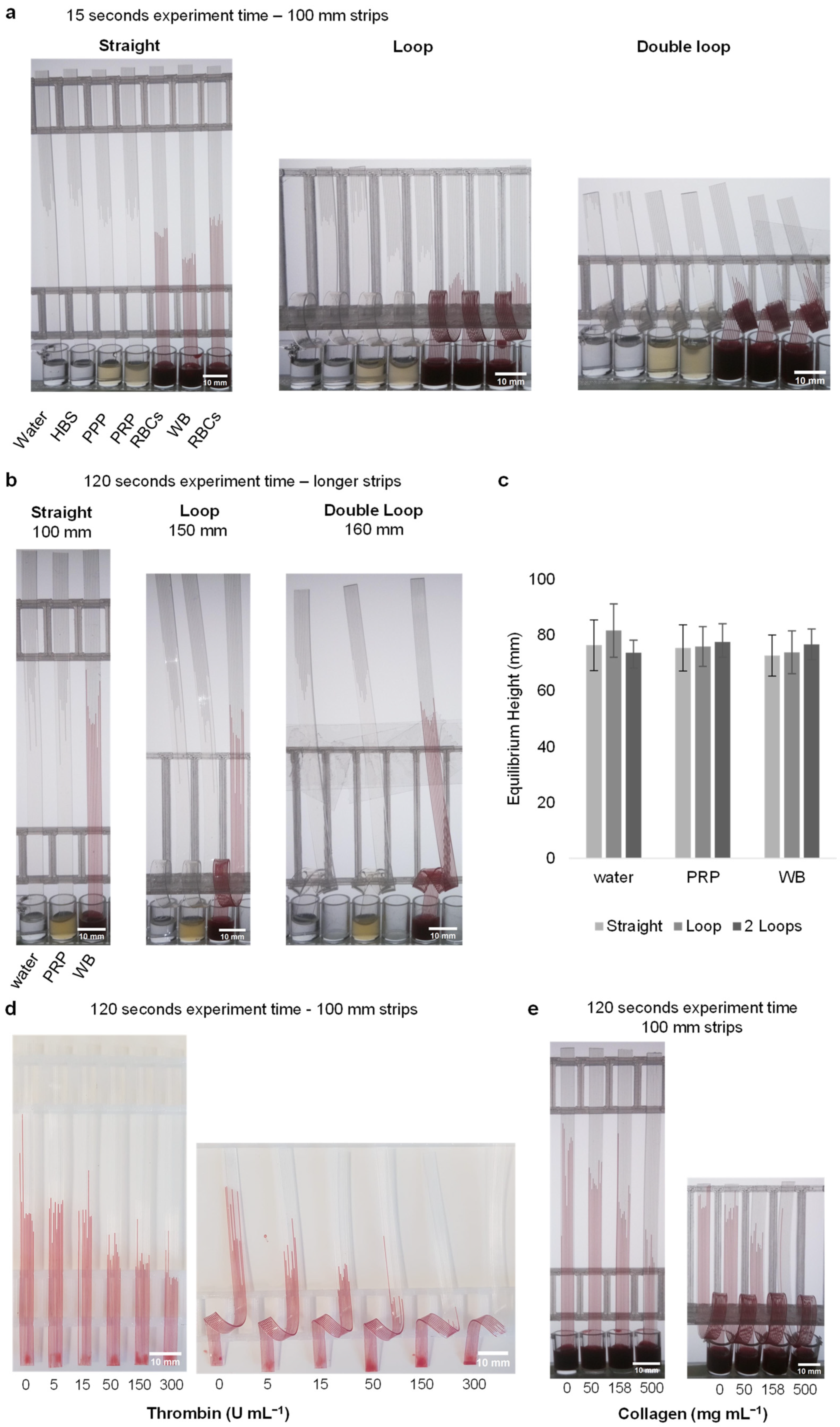
3.3. Loop Design Can Detect Lower Stimulus Concentrations by Increasing Stimulation Time without Affecting Capillary Force
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Capretto, L.; Cheng, W.; Hill, M.; Zhang, X. Micromixing within microfluidic devices. Top. Curr. Chem. 2011, 304, 27–68. [Google Scholar] [PubMed]
- Sivakumar, R.; Lee, N.Y. Microfluidic device fabrication mediated by surface chemical bonding. Analyst 2020, 145, 4096–4110. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef] [PubMed]
- Hitzbleck, M.; Delamarche, E. Reagents in microfluidics: An ‘in’ and ‘out’ challenge. Chem. Soc. Rev. 2013, 42, 8494–8516. [Google Scholar] [CrossRef]
- Gill, K.K.; Liu, Z.; Reis, N.M. Fast prototyping using 3D printed templates and flexible fluoropolymer microcapillary films offers enhanced micromixing in immobilised (bio)catalytic reactions. Chem. Eng. J. 2022, 429, 132266. [Google Scholar] [CrossRef]
- Yang, A.-S.; Chuang, F.-C.; Chen, C.-K.; Lee, M.-H.; Chen, S.-W.; Su, T.-L.; Yang, Y.-C. A high-performance micromixer using three-dimensional Tesla structures for bio-applications. Chem. Eng. J. 2015, 263, 444–451. [Google Scholar] [CrossRef]
- Femmer, T.; Eggersdorfer, M.L.; Kuehne, A.J.C.; Wessling, M. Efficient gas–liquid contact using microfluidic membrane devices with staggered herringbone mixers. Lab Chip 2015, 15, 3132–3137. [Google Scholar] [CrossRef]
- Lee, H.; Kim, G.; Lim, C.; Lee, B.; Shin, S. A simple method for activating the platelets used in microfluidic platelet aggregation tests: Stirring-induced platelet activation. Biomicrofluidics 2016, 10, 064118. [Google Scholar] [CrossRef]
- Seo, H.S.; Choi, S.H.; Han, M.; Kim, K.A.; Cho, C.H.; An, S.S.A.; Lim, C.S.; Shin, S. Measurement of platelet aggregation functions using whole blood migration ratio in a microfluidic chip. Clin. Hemorheol. Microcirc. 2016, 62, 151–163. [Google Scholar] [CrossRef]
- Xu, S.; Piao, J.; Lee, B.; Lim, C.; Shin, S. Platelet thrombus formation by upstream activation and downstream adhesion of platelets in a microfluidic system. Biosens. Bioelectron. 2020, 165, 112395. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, D.; Pauletti, G.M.; Steckl, A.J. Blood coagulation screening using a paper-based microfluidic lateral flow device. Lab Chip 2014, 14, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.M.; Pivetal, J.; Loo-Zazueta, A.L.; Barros, J.M.S.; Edwards, A.D. Lab on a stick: Multi-analyte cellular assays in a microfluidic dipstick. Lab Chip 2016, 16, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Murallidharan, J.S.; Sharma, A.; Agarwal, A. Microfluidics geometries involved in effective blood plasma separation. Microfluid. Nanofluidics 2022, 26, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; Li, W.; Alici, G.; Nguyen, N.-T. High throughput extraction of plasma using a secondary flow-aided inertial microfluidic device. RSC Adv. 2014, 4, 33149–33159. [Google Scholar] [CrossRef]
- Robinson, M.; Marks, H.; Hinsdale, T.; Maitland, K.; Coté, G. Rapid isolation of blood plasma using a cascaded inertial microfluidic device. Biomicrofluidics 2017, 11, 24109. [Google Scholar] [CrossRef]
- Nivedita, N.; Papautsky, I. Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 2013, 7, 054101. [Google Scholar] [CrossRef]
- Sollier, E.; Rostaing, H.; Pouteau, P.; Fouillet, Y.; Achard, J.-L. Passive microfluidic devices for plasma extraction from whole human blood. Sens. Actuators B Chem. 2009, 141, 617–624. [Google Scholar] [CrossRef]
- Di Carlo, D.; Edd, J.F.; Irimia, D.; Tompkins, R.G.; Toner, M. Equilibrium Separation and Filtration of Particles Using Differential Inertial Focusing. Anal. Chem. 2008, 80, 2204–2211. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Liu, C.; Li, H.; Chen, J. Continuous flow microfluidic device for cell separation, cell lysis and DNA purification. Anal. Chim. Acta 2007, 584, 237–243. [Google Scholar] [CrossRef]
- Blattert, C.; Jurischka, R.; Tahhan, I.; Schoth, A.; Kerth, P.; Menz, W. Separation of blood in microchannel bends. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar]
- Haeberle, S.; Brenner, T.; Zengerle, R.; Ducrée, J. Centrifugal extraction of plasma from whole blood on a rotating disk. Lab Chip 2006, 6, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Burklund, A.; Tadimety, A.; Nie, Y.; Hao, N.; Zhang, J.X. Chapter One—Advances in diagnostic microfluidics. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–72. [Google Scholar]
- Davì, G.; Patrono, C. Platelet Activation and Atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Sarıyer, R.M.; Gill, K.; Needs, S.H.; Hodge, D.; Reis, N.M.; Jones, C.I.; Edwards, A.D. Time- and distance-resolved robotic imaging of fluid flow in vertical microfluidic strips: A new technique for quantitative, multiparameter measurement of global haemostasis. Sens. Diagn. 2023, 2, 1623–1637. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.L.; Dunster, J.L.; Kriek, N.; Unsworth, A.J.; Sage, T.; Mohammed, Y.M.; De Simone, I.; Taylor, K.A.; Bye, A.P.; Ólafsson, G.; et al. The rate of platelet activation determines thrombus size and structure at arterial shear. J. Thromb. Haemost. 2023, 21, 2248–2259. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Reis, N.M.; Slater, N.K.H.; Mackley, M.R. A simple device for multiplex ELISA made from melt-extruded plastic microcapillary film. Lab Chip 2011, 11, 4267–4273. [Google Scholar] [CrossRef]
- Hallmark, B.; Gadala-Maria, F.; Mackley, M.R. The melt processing of polymer microcapillary film (MCF). J. Non-Newton. Fluid Mech. 2005, 128, 83–98. [Google Scholar] [CrossRef]
- Sarıyer, R.M.; Edwards, A.D.; Needs, S.H. Open Hardware for Microfluidics: Exploiting Raspberry Pi Singleboard Computer and Camera Systems for Customisable Laboratory Instrumentation. Biosensors 2023, 13, 948. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98–99, 50–60. [Google Scholar] [CrossRef]
- Trudnowski, R.J.; Rico, R.C. Specific Gravity of Blood and Plasma at 4 and 37 °C. Clin. Chem. 1974, 20, 615–616. [Google Scholar] [CrossRef]
- Stuart, J.; Kenny, M.W. Blood rheology. J. Clin. Pathol. 1980, 33, 417. [Google Scholar] [CrossRef]
- Khnouf, R.; Karasneh, D.; Abdulhay, E.; Abdelhay, A.; Sheng, W.; Fan, Z.H. Microfluidics-based device for the measurement of blood viscosity and its modeling based on shear rate, temperature, and heparin concentration. Biomed. Microdevices 2019, 21, 80. [Google Scholar] [CrossRef]
- Guo, W.; Hansson, J.; van der Wijngaart, W. Capillary pumping independent of the liquid surface energy and viscosity. Microsyst. Nanoeng. 2018, 4, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıyer, R.M.; Gill, K.K.; Needs, S.H.; Reis, N.M.; Jones, C.I.; Edwards, A.D. Looping Flexible Fluoropolymer Microcapillary Film Extends Analysis Times for Vertical Microfluidic Blood Testing. Sensors 2024, 24, 5870. https://doi.org/10.3390/s24185870
Sarıyer RM, Gill KK, Needs SH, Reis NM, Jones CI, Edwards AD. Looping Flexible Fluoropolymer Microcapillary Film Extends Analysis Times for Vertical Microfluidic Blood Testing. Sensors. 2024; 24(18):5870. https://doi.org/10.3390/s24185870
Chicago/Turabian StyleSarıyer, Rüya Meltem, Kirandeep K. Gill, Sarah H. Needs, Nuno M. Reis, Chris I. Jones, and Alexander Daniel Edwards. 2024. "Looping Flexible Fluoropolymer Microcapillary Film Extends Analysis Times for Vertical Microfluidic Blood Testing" Sensors 24, no. 18: 5870. https://doi.org/10.3390/s24185870






