Aerogel-Lined Capillaries as Liquid-Core Waveguides for Raman Signal Gain of Aqueous Samples: Advanced Manufacturing and Performance Characterization
Abstract
1. Introduction
2. Methods and Materials for Manufacturing and Characterization of Silica Aerogel-Lined Capillaries
2.1. Preparation of the Alcogel Dispersion
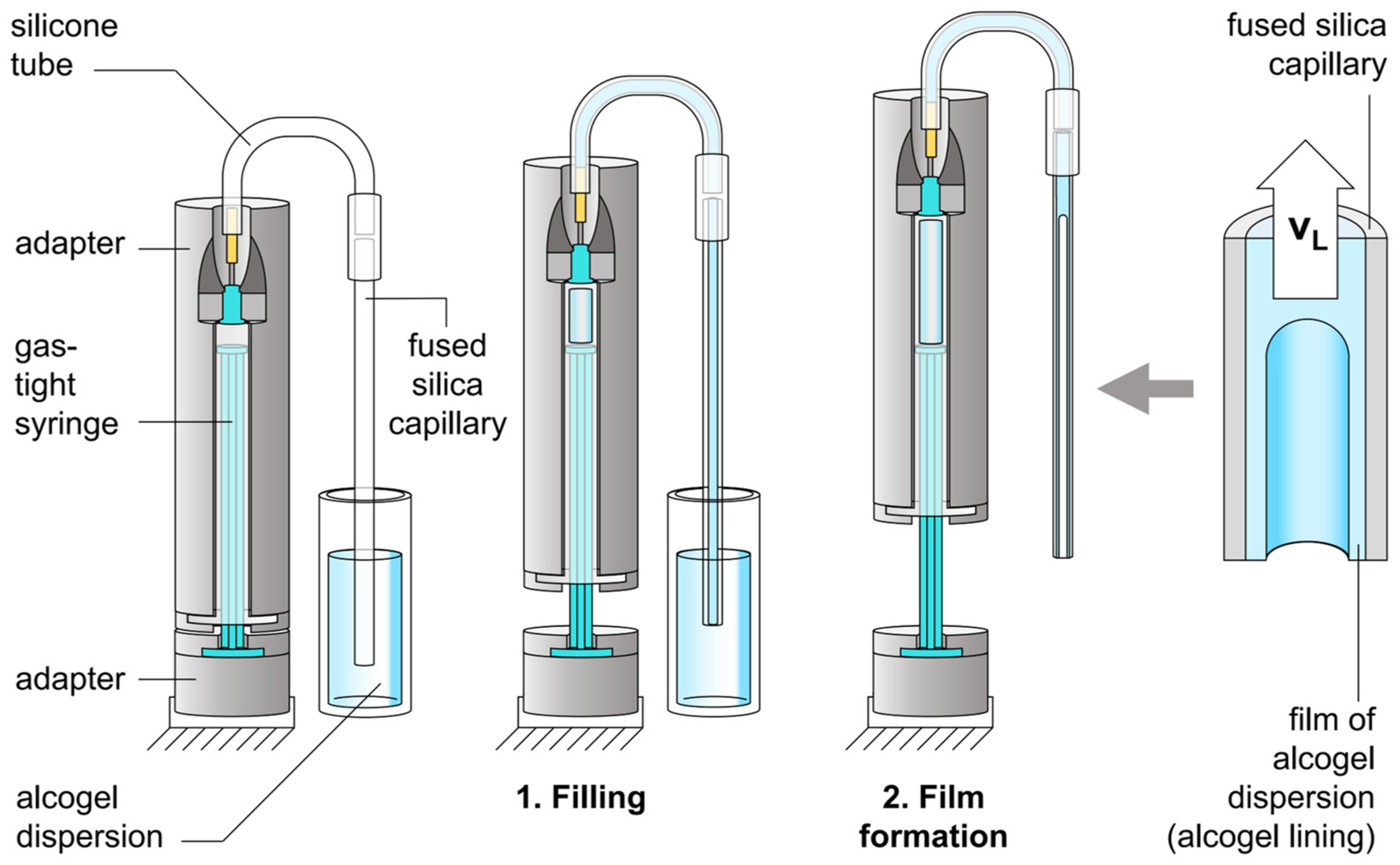
2.2. Lining a Capillary with Alcogel Dispersion
2.3. Convert the Alcogel Lining into an Aerogel Lining
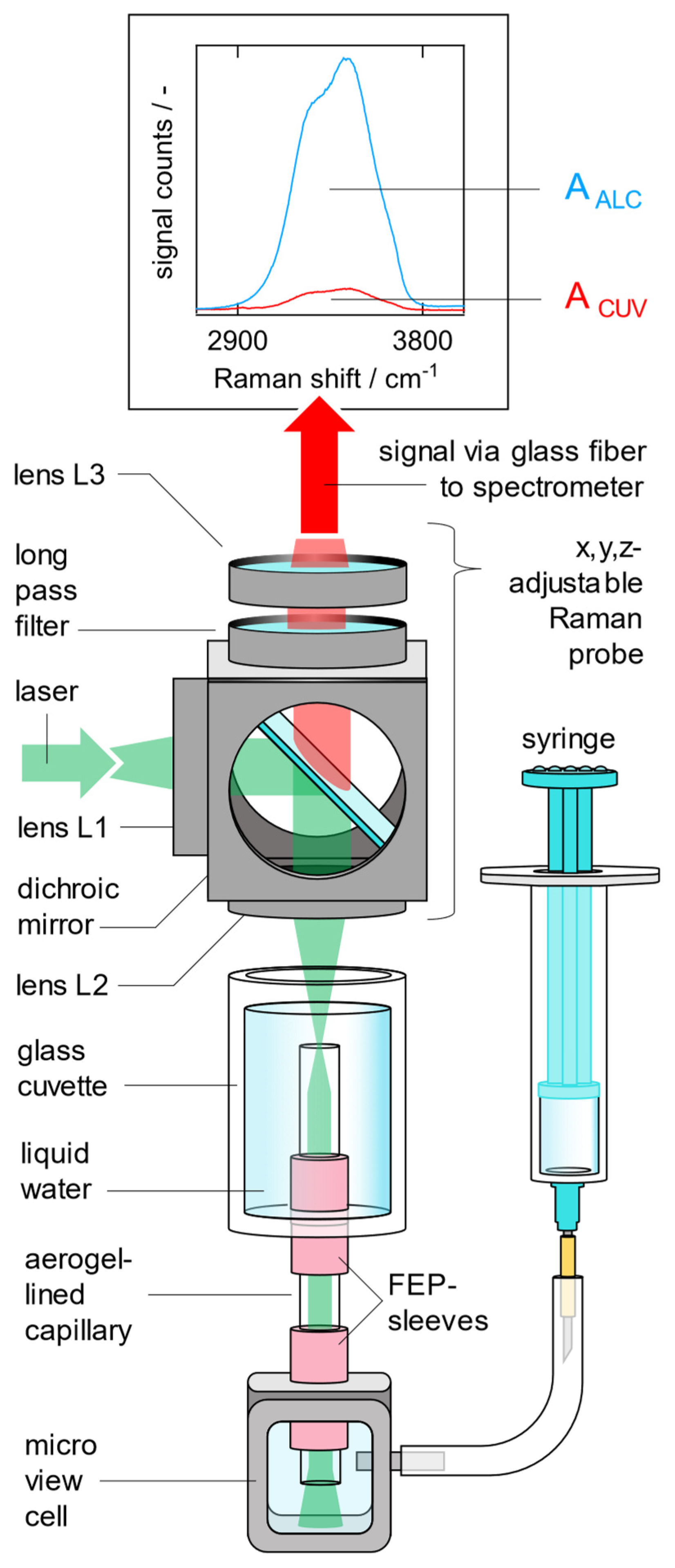
2.4. Quantification of the Raman Signal Gain G
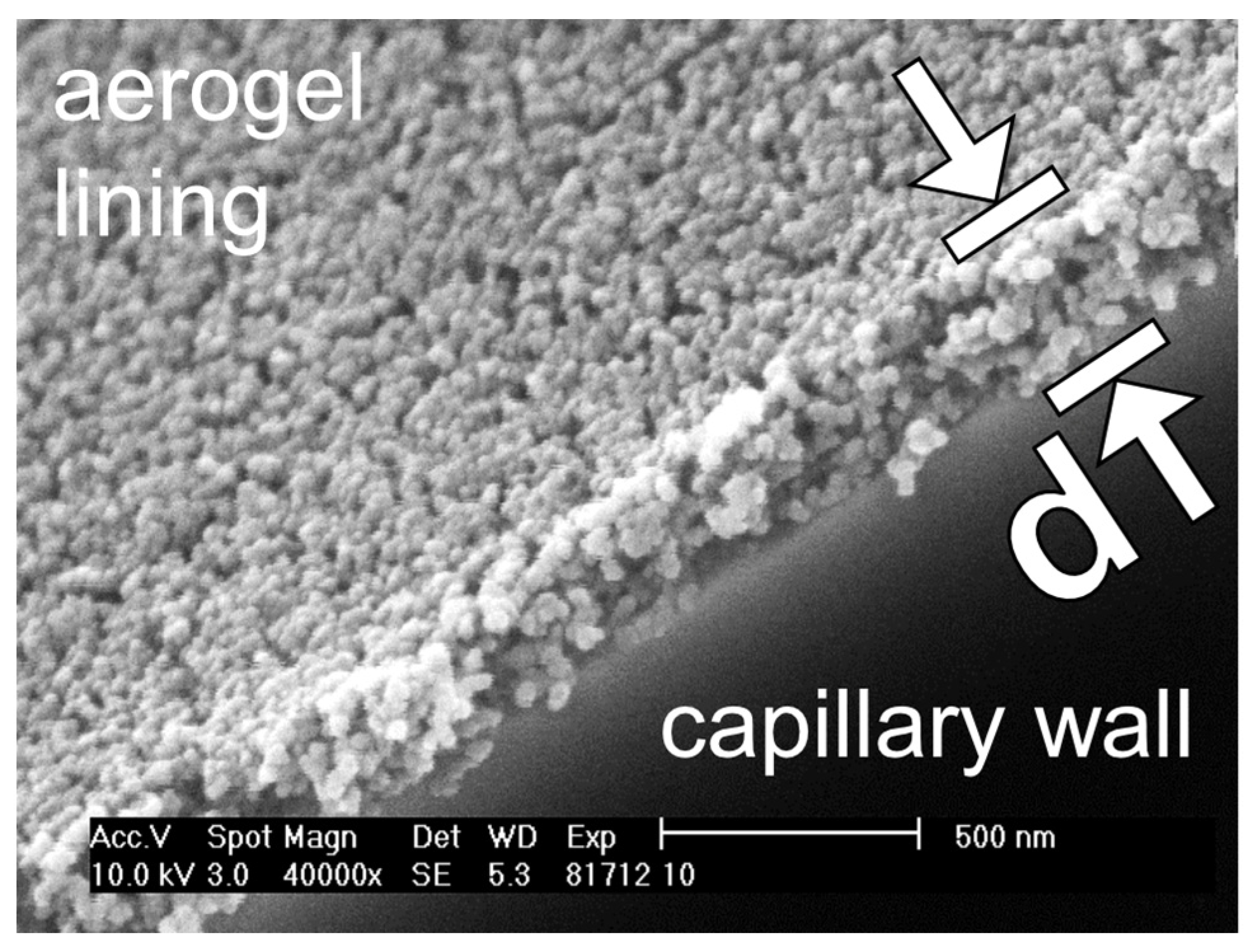
2.5. Characterization of the Thickness and the Topography of an Aerogel Lining
3. Results of the Process Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, G.L.; Lines, A.M.; Bello, J.M.; Bryan, S.A. Online monitoring of solutions within microfluidic chips: Simultaneous raman and uv-vis absorption spectroscopies. ACS Sens. 2019, 4, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.L.; Lackey, H.E.; Bello, J.M.; Felmy, H.M.; Bryan, H.B.; Lamadie, F.; Bryan, S.A.; Lines, A.M. Enabling microscale processing: Combined raman and absorbance spectroscopy for microfluidic on-line monitoring. Anal. Chem. 2021, 93, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Lines, A.M.; Hall, G.B.; Asmussen, S.; Allred, J.; Sinkov, S.; Heller, F.; Gallagher, N.; Lumetta, G.J.; Bryan, S.A. Sensor Fusion: Comprehensive Real-Time, On-Line Monitoring for Process Control via Visible, Near-Infrared, and Raman Spectroscopy. ACS Sens. 2020, 5, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.L.; Asmussen, S.E.; Lines, A.M.; Casella, A.J.; Bottenus, D.R.; Clark, S.B.; Bryan, S.A. Micro-Raman Technology to Interrogate Two-Phase Extraction on a Microfluidic Device. Anal. Chem. 2018, 90, 8345–8353. [Google Scholar] [CrossRef] [PubMed]
- Lines, A.M.; Nelson, G.L.; Casella, A.J.; Bello, J.M.; Clark, S.B.; Bryan, S.A. Multivariate Analysis to Quantify Species in the Presence of Direct Interferents: Micro-Raman Analysis of HNO3 in Microfluidic Devices. Anal. Chem. 2018, 90, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Legner, R.; Rädle, M. Trendbericht Analytische Chemie. Nachrichten Chem. 2024, 72, 59–60. [Google Scholar]
- Nachtmann, M.; Paul Keck, S.; Braun, F.; Simon Eckhardt, H.; Mattolat, C.; Gretz, N.; Scholl, S.; Rädle, M. A customized stand-alone photometric Raman sensor applicable in explosive atmospheres: A proof-of-concept study. J. Sens. Sens. Syst. 2018, 7, 543–549. [Google Scholar] [CrossRef]
- Esmonde-White, K.A.; Cuellar, M.; Uerpmann, C.; Lenain, B.; Lewis, I.R. Raman spectroscopy as a process analytical technology for pharmaceutical manufacturing and bioprocessing. Anal. Bioanal. Chem. 2017, 409, 637–649. [Google Scholar] [CrossRef]
- Braeuer, A. In Situ Spectroscopic Techniques at High Pressure; Elsevier: Amsterdam, The Netherlands, 2015; Volume 7, ISBN 9780444634221. [Google Scholar]
- De Beer, T.; Burggraeve, A.; Fonteyne, M.; Saerens, L.; Remon, J.P.; Vervaet, C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int. J. Pharm. 2011, 417, 32–47. [Google Scholar] [CrossRef]
- Puppels, G.J.; Olminkhof, J.H.F.; Segers-Nolten, G.M.J.; Otto, C.; de Mul, F.F.M.; Greve, J. Laser irradiation and Raman spectroscopy of single living cells and chromosomes: Sample degradation occurs with 514.5 nm but not with 660 nm laser light. Exp. Cell Res. 1991, 195, 361–367. [Google Scholar] [CrossRef]
- Batteh, J.H. Damage thresholds in laser-irradiated glass. J. Appl. Phys. 1982, 53, 7537–7544. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, X.; Xu, W.; Xu, S. Waveguide-based Raman enhancement strategies. J. Raman Spectrosc. 2024, 55, 355–376. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Ju, H.; Lu, G. From lab to field: Surface-enhanced Raman scattering-based sensing strategies for on-site analysis. TrAC—Trends Anal. Chem. 2022, 146, 116488. [Google Scholar] [CrossRef]
- Persichetti, G.; Testa, G.; Bernini, R. Optofluidic jet waveguide enhanced Raman spectroscopy. Sens. Actuators B Chem. 2015, 207, 732–739. [Google Scholar] [CrossRef]
- Persichetti, G.; Bernini, R. Water monitoring by optofluidic Raman spectroscopy for in situ applications. Talanta 2016, 155, 145–152. [Google Scholar] [CrossRef]
- Walrafen, G.E.; Stone, J. Intensification of Spontaneous Raman Spectra by Use of Liquid Core Optical Fibers. Appl. Spectrosc. 1972, 26, 585–589. [Google Scholar] [CrossRef]
- Altkorn, R.; Malinsky, M.D.; Van Duyne, R.P.; Koev, I. Intensity considerations in liquid core optical fiber Raman spectroscopy. Appl. Spectrosc. 2001, 55, 373–381. [Google Scholar] [CrossRef]
- Pelletier, M.J.; Altkorn, R. Efficient elimination of fluorescence background from Raman spectra collected in a liquid core optical fiber. Appl. Spectrosc. 2000, 54, 1837–1841. [Google Scholar] [CrossRef]
- Altkorn, R.; Koev, I.; Van Duyne, R.P.; Litorja, M. Low-loss liquid-core optical fiber for low-refractive-index liquids: Fabrication, characterization, and application in Raman spectroscopy. Appl. Opt. 1997, 36, 8992–8998. [Google Scholar] [CrossRef]
- Holtz, M.; Dasgupta, P.K.; Zhang, G. Small-volume Raman spectroscopy with a liquid core waveguide. Anal. Chem. 1999, 71, 2934–2938. [Google Scholar] [CrossRef]
- Pelletier, M.J.; Altkorn, R. Raman sensitivity enhancement for aqueous protein samples using a liquid-core optical-fiber cell. Anal. Chem. 2001, 73, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Altkorn, R.; Koev, I.; Pelletier, M.J. Raman performance characteristics of Teflon-AF 2400 liquid-core optical-fiber sample cells. Appl. Spectrosc. 1999, 53, 1169–1176. [Google Scholar] [CrossRef]
- Mu, B.; Li, X.; Liu, J.; Mao, X. Review on liquid-core waveguide technology and its application for spectroscopic analysis. Microchem. J. 2024, 199, 109935. [Google Scholar] [CrossRef]
- Dijkstra, R.J.; Slooten, C.J.; Stortelder, A.; Buijs, J.B.; Ariese, F.; Brinkman, U.A.T.; Gooijer, C. Liquid-core waveguide technology for coupling column liquid chromatography and Raman spectroscopy. J. Chromatogr. A 2001, 918, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, L.; Zuo, J.; Li, Z.; Gao, S.; Lu, G. Raman sensitivity enhancement for aqueous absorbing sample using Teflon-AF 2400 liquid core optical fibre cell. Anal. Chim. Acta 2007, 581, 154–158. [Google Scholar] [CrossRef]
- Schwab, S.D.; McCreery, R.L. Remote, Long-Pathlength Cell for High-Sensitivity Raman Spectroscopy. Appl. Spectrosc. 1987, 41, 126–130. [Google Scholar] [CrossRef]
- Groeneveld, I.; Bagdonaite, I.; Beekwilder, E.; Ariese, F.; Somsen, G.W.; Van Bommel, M.R. Liquid Core Waveguide Cell with in Situ Absorbance Spectroscopy and Coupled to Liquid Chromatography for Studying Light-Induced Degradation. Anal. Chem. 2022, 94, 7647–7654. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Kelble, C.; Millero, F.J. Gas-segmented continuous flow analysis of iron in water with a long liquid waveguide capillary flow cell. Anal. Chim. Acta 2001, 438, 49–57. [Google Scholar] [CrossRef]
- Yao, W.; Byrne, R.H.; Waterbury, R.D. Determination of nanomolar concentrations of nitrite and nitrate in natural waters using long path length absorbance spectroscopy. Environ. Sci. Technol. 1998, 32, 2646–2649. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Chi, J. Automated analysis of nanomolar concentrations of phosphate in natural waters with liquid waveguide. Environ. Sci. Technol. 2002, 36, 1048–1053. [Google Scholar] [CrossRef]
- Yao, W.; Byrne, R.H. Determination of trace chromium(VI) and molybdenum(VI) in natural and bottled mineral waters using long pathlength absorbance spectroscopy (LPAS). Talanta 1999, 48, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, K.-I.; Nomura, A.; Yamada, J.; Nishi, S. Possibility of signal enhancement in liquid absorption spectrometry with a long capillary cell utilizing successive total reflection at the outer cell surface. Appl. Spectrosc. 1989, 43, 49–55. [Google Scholar] [CrossRef]
- Stone, J. Measurements of the Absorption of Light in Low-Loss Liquids. J. Opt. Soc. Am. 1972, 62, 327. [Google Scholar] [CrossRef]
- Chamberlain, R.; Windolf, H.; Burckhardt, B.B.; Breitkreutz, J.; Fischer, B. Embedding a Sensitive Liquid-Core Waveguide UV Detector into an HPLC-UV System for Simultaneous Quantification of Differently Dosed Active Ingredients during Drug Release. Pharmaceutics 2022, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- Rubles, T.; Paige, D.; Anastasio, C. Lens-coupled liquid core waveguide for ultraviolet-visible absorption spectroscopy. Rev. Sci. Instrum. 2006, 77, 073103. [Google Scholar] [CrossRef]
- Kottke, D.; Burckhardt, B.B.; Breitkreutz, J.; Fischer, B. Application and validation of a coaxial liquid core waveguide fluorescence detector for the permeation analysis of desmopressin acetate. Talanta 2021, 226, 122145. [Google Scholar] [CrossRef]
- Byrne, R.H.; Yao, W.; Kaltenbacher, E.; Waterbury, R.D. Construction of a compact spectrofluorometer/spectrophotometer system using a flexible liquid core waveguide. Talanta 2000, 50, 1307–1312. [Google Scholar] [CrossRef]
- Chu, Q.; Jin, Z.; Yu, X.; Li, C.; Zhang, W.; Ji, W.; Lin, B.; Shum, P.P.; Zhang, X.; Wang, G. Volumetric enhancement of Raman scattering for fast detection based on a silver-lined hollow-core fiber. Opt. Express 2019, 27, 10370. [Google Scholar] [CrossRef]
- Voß, D.; Ponce, S.; Wesinger, S.; Etzold, B.J.M.; Albert, J. Combining autoclave and LCWM reactor studies to shed light on the kinetics of glucose oxidation catalyzed by doped molybdenum-based heteropoly acids. RSC Adv. 2019, 9, 29347–29356. [Google Scholar] [CrossRef]
- Ponce, S.; Trabold, M.; Drochner, A.; Albert, J.; Etzold, B.J.M. Insights into the redox kinetics of vanadium substituted heteropoly acids through liquid core waveguide membrane microreactor studies. Chem. Eng. J. 2019, 369, 443–450. [Google Scholar] [CrossRef]
- Ponce, S.; Christians, H.; Drochner, A.; Etzold, B.J.M. An Optical Microreactor Enabling In Situ Spectroscopy Combined with Fast Gas-Liquid Mass Transfer. Chem. Ing. Tech. 2018, 90, 1855–1863. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed]
- özbakir, Y.; Jonas, A.; Kiraz, A.; Erkey, C. Aerogels for optofluidic waveguides. Micromachines 2017, 8, 98. [Google Scholar] [CrossRef]
- Yalizay, B.; Morova, Y.; Dincer, K.; Özbakir, Y.; Jonas, A.; Erkey, C.; Kiraz, A.; Akturk, S. Versatile liquid-core optofluidic waveguides fabricated in hydrophobic silica aerogels by femtosecond-laser ablation. Opt. Mater. 2015, 47, 478–483. [Google Scholar] [CrossRef]
- Yalizay, B.; Morova, Y.; Ozbakir, Y.; Jonas, A.; Erkey, C.; Kiraz, A.; Akturk, S. Optofluidic waveguides written in hydrophobic silica aerogels with a femtosecond laser. In Proceedings of the Integrated Optics: Devices, Materials, and Technologies XIX, San Francisco, CA, USA, 27 February 2015; p. 936518. [Google Scholar] [CrossRef]
- Özbakır, Y.; Jonáš, A.; Kiraz, A.; Erkey, C. Total internal reflection-based optofluidic waveguides fabricated in aerogels. J. Sol-Gel Sci. Technol. 2017, 84, 522–534. [Google Scholar] [CrossRef]
- Özbakir, Y.; Jonas, A.; Kiraz, A.; Erkey, C. Application of Aerogels in Optical Devices. In Handbook of Aerogels; Springer: Cham, Switzerland, 2023; pp. 1431–1454. [Google Scholar]
- Fraval, H.R.; Brinker, C.J. Fluid Light Guide Having Hydrophobic Aerogel Cladding Layer. U.S. Patent 6,983,093 B2, 1–4 January 2006. [Google Scholar]
- Feng, X.; Shi, Y.; Yin, X.; Wang, X. Transparent superhydrophobic film with anti-fouling and anti-scaling ability by facile method of dip-coating SiO2Sol. Mater. Res. Express 2022, 9, 016404. [Google Scholar] [CrossRef]
- Spiske, F.; Dirauf, M.P.; Braeuer, A.S. Aerogel-Lined Capillaries for Raman Signal Gain of Aqueous Mixtures. Sensors 2022, 22, 4388. [Google Scholar] [CrossRef]
- He, X.; Cheng, X.; Zhang, Y.; Shao, Z. Multiscale structural characterization of methyltriethoxysilane-based silica aerogels. J. Mater. Sci. 2018, 53, 994–1004. [Google Scholar] [CrossRef]
- Veres, P.; Kéri, M.; Bányai, I.; Lázár, I.; Fábián, I.; Domingo, C.; Kalmár, J. Mechanism of drug release from silica-gelatin aerogel—Relationship between matrix structure and release kinetics. Colloids Surf. B Biointerfaces 2017, 152, 229–237. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Feng, J.; Zhang, S.; Peng, F.; Xiao, Y.; Li, L.; Feng, J. Preparation of silica aerogels with high temperature resistance and low thermal conductivity by monodispersed silica sol. Mater. Des. 2020, 191, 108640. [Google Scholar] [CrossRef]
- Prakash, S.S.; Brinker, C.J.; Hurd, A.J.; Rao, S.M. Ambient-pressure silica aerogel films. J. Non-Cryst. Solids 1995, 190, 264–275. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Taylor, G.I. Deposition of a viscous fluid on the wall of a tube. J. Fluid Mech. 1961, 10, 161–165. [Google Scholar] [CrossRef]
- Aussillous, P.; Quere, D. Quick deposition of a fluid on the wall of a tube. Phys. Fluids 2000, 12, 2367–2371. [Google Scholar] [CrossRef]
- Boulogne, F.; Giorgiutti-Dauphiné, F.; Pauchard, L. Surface patterns in drying films of silica colloidal dispersions. Soft Matter 2015, 3, 102–108. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiske, F.; Jakob, L.S.; Lippold, M.; Rahimi, P.; Joseph, Y.; Braeuer, A.S. Aerogel-Lined Capillaries as Liquid-Core Waveguides for Raman Signal Gain of Aqueous Samples: Advanced Manufacturing and Performance Characterization. Sensors 2024, 24, 5979. https://doi.org/10.3390/s24185979
Spiske F, Jakob LS, Lippold M, Rahimi P, Joseph Y, Braeuer AS. Aerogel-Lined Capillaries as Liquid-Core Waveguides for Raman Signal Gain of Aqueous Samples: Advanced Manufacturing and Performance Characterization. Sensors. 2024; 24(18):5979. https://doi.org/10.3390/s24185979
Chicago/Turabian StyleSpiske, Felix, Lara Sophie Jakob, Maximilian Lippold, Parvaneh Rahimi, Yvonne Joseph, and Andreas Siegfried Braeuer. 2024. "Aerogel-Lined Capillaries as Liquid-Core Waveguides for Raman Signal Gain of Aqueous Samples: Advanced Manufacturing and Performance Characterization" Sensors 24, no. 18: 5979. https://doi.org/10.3390/s24185979
APA StyleSpiske, F., Jakob, L. S., Lippold, M., Rahimi, P., Joseph, Y., & Braeuer, A. S. (2024). Aerogel-Lined Capillaries as Liquid-Core Waveguides for Raman Signal Gain of Aqueous Samples: Advanced Manufacturing and Performance Characterization. Sensors, 24(18), 5979. https://doi.org/10.3390/s24185979







