Accelerating the Diagnosis of Pandemic Infection Based on Rapid Sampling Algorithm for Fast-Response Breath Gas Analyzers
Abstract
:1. Introduction
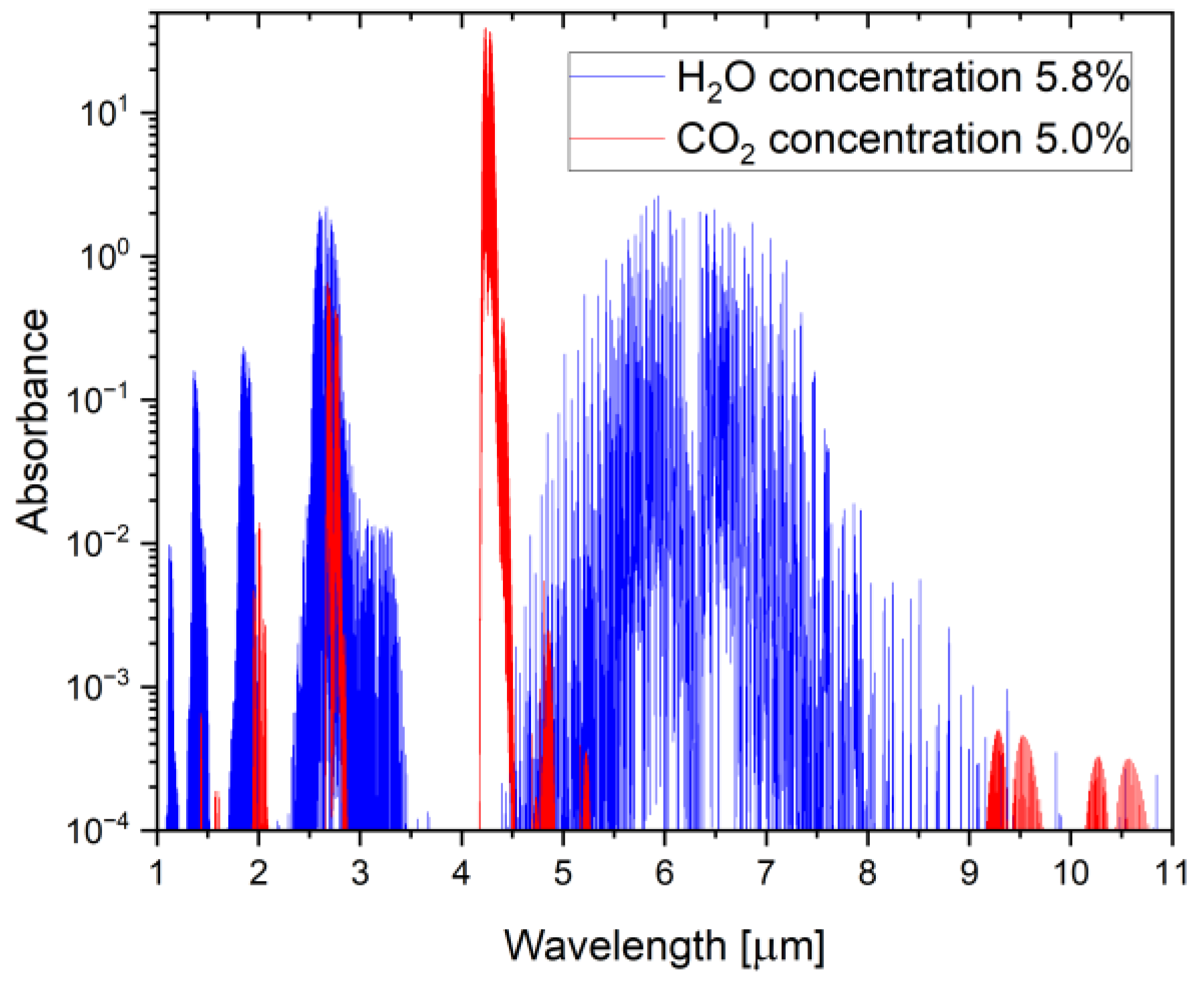
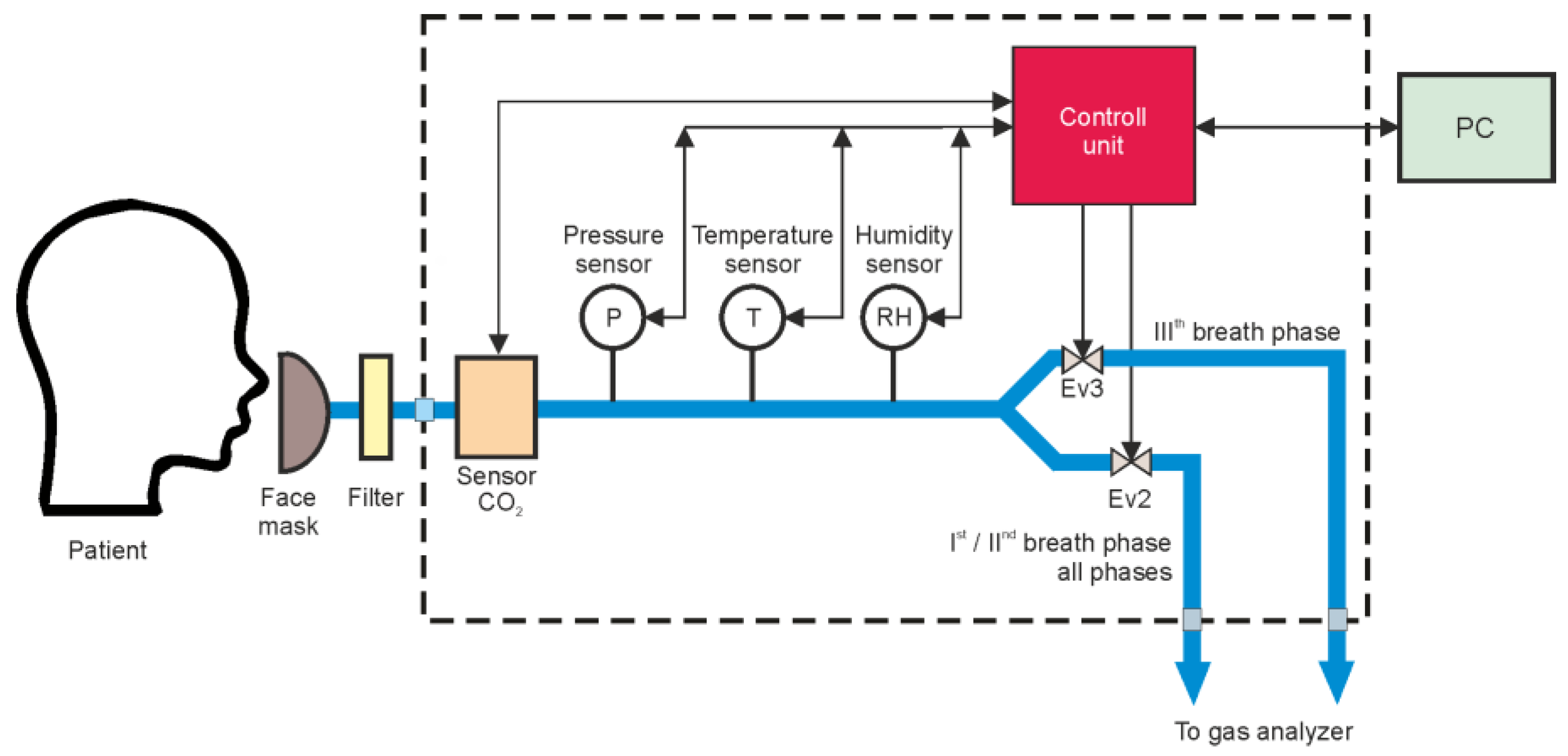
2. Materials and Methods
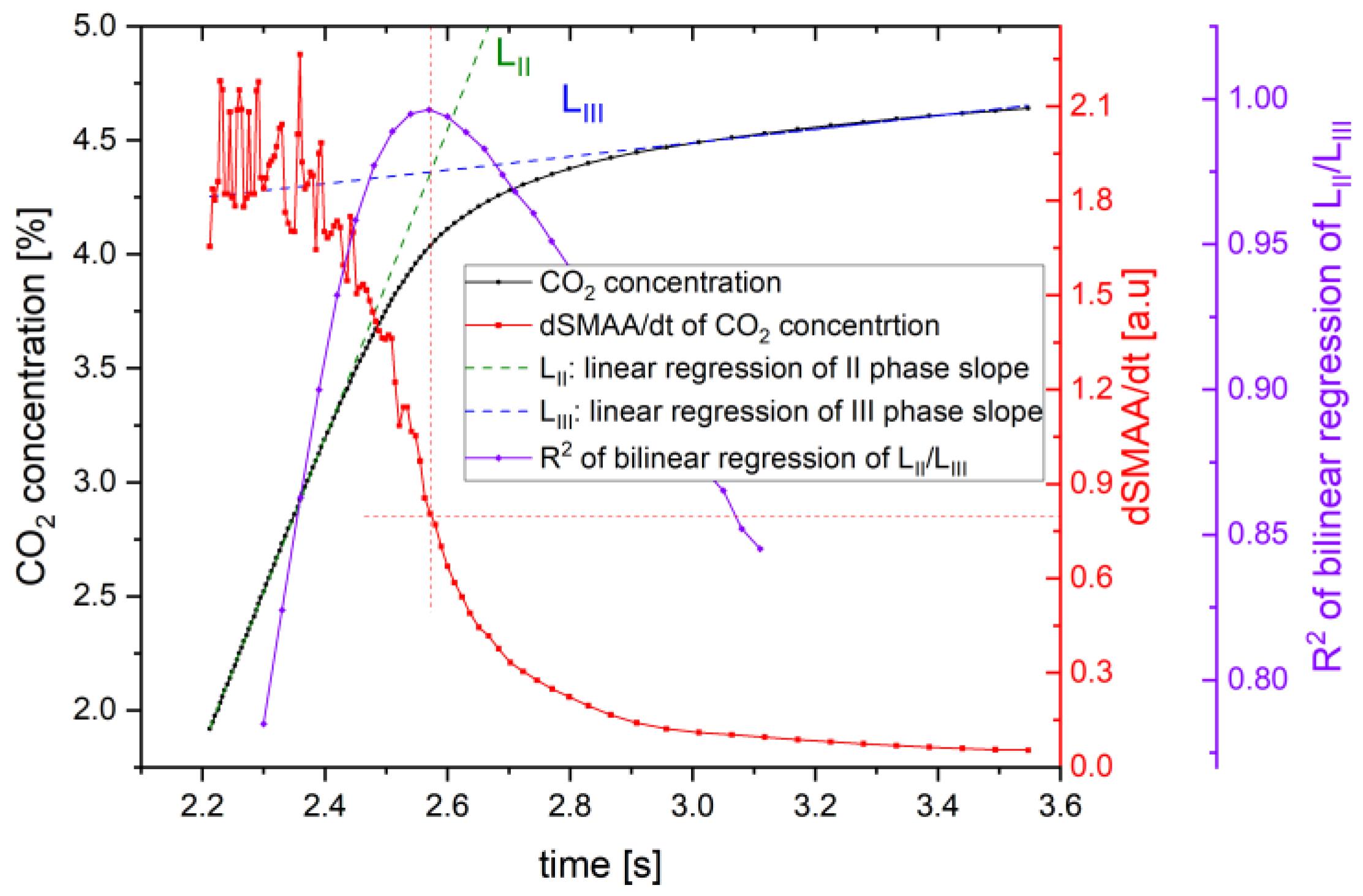
3. Developed Breath’s Third Phase Detection Algorithm
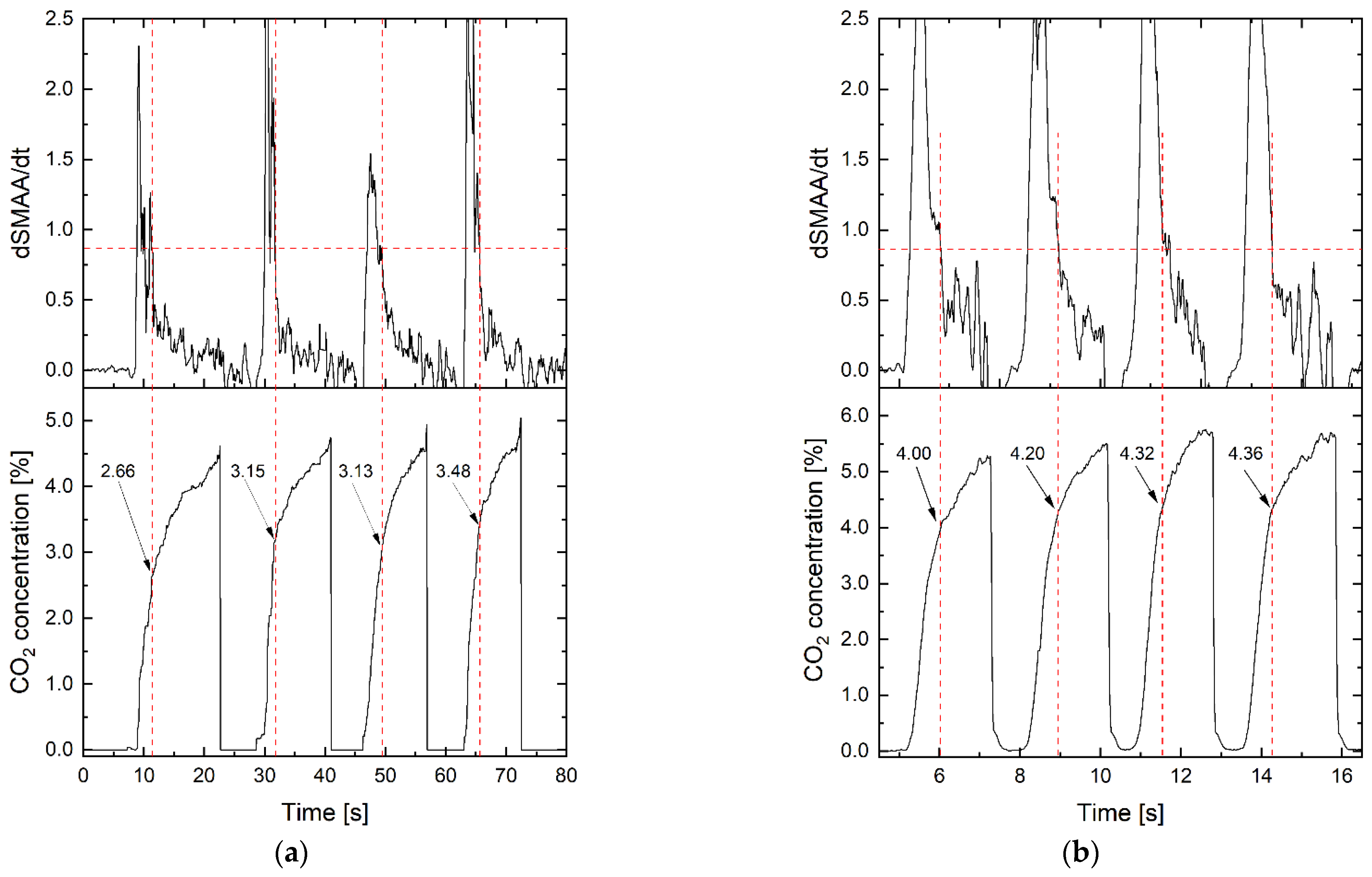
4. Experiments
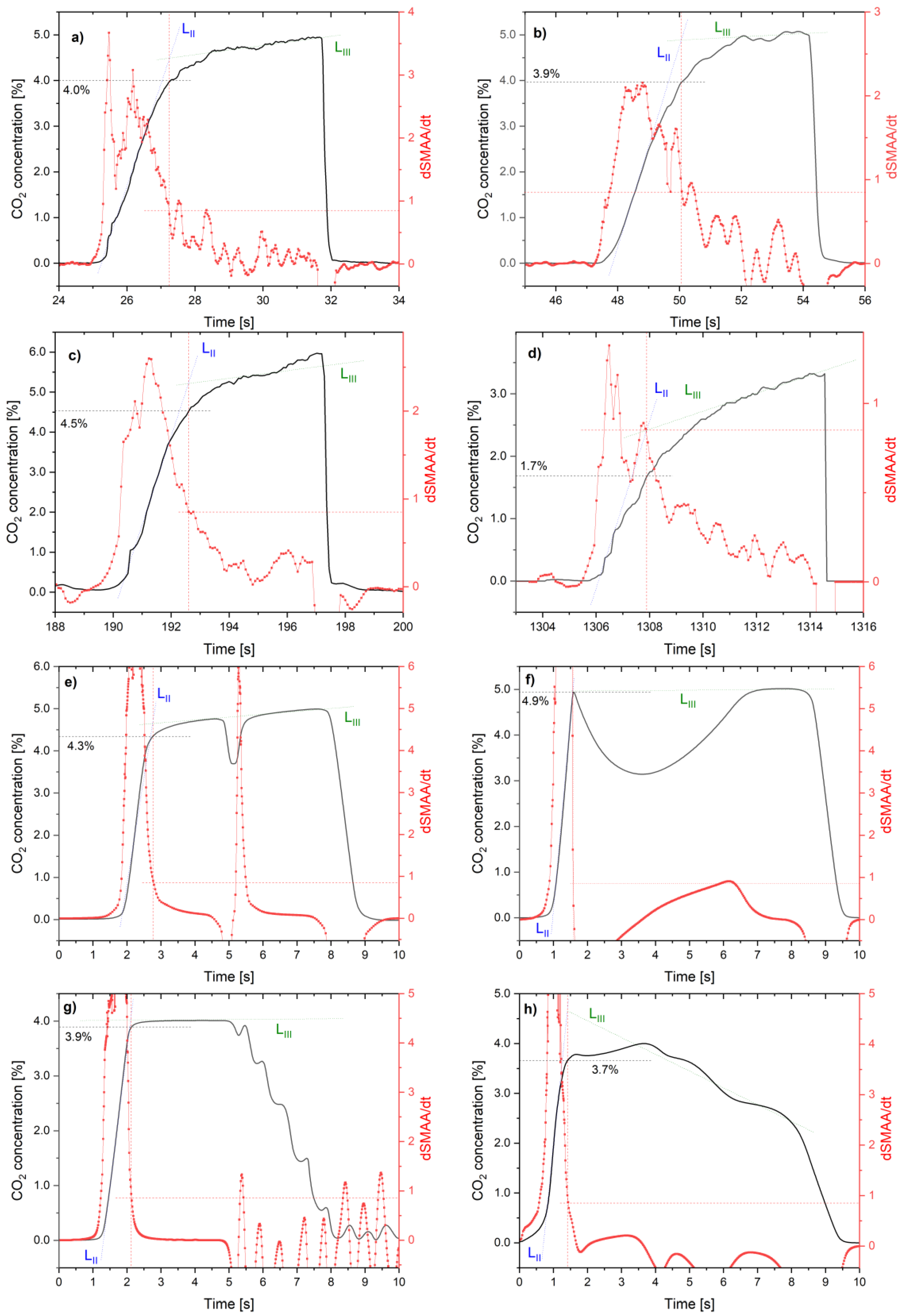
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buszewski, B.; Kesy, M.; Ligor, T.A.A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Szabra, D.; Prokopiuk, A.; Mikołajczyk, J.; Ligor, T.; Buszewski, B.; Bielecki, Z. Air sampling unit for breath analyzers. Rev. Sci. Instrum. 2017, 88, 115006. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, L.; Edwards, J.; Kennington, E.; Aurora, P.; Baraldi, E.; Carraro, S.; Gappa, M.; Louis, R.; Moreno-Galdo, A.; Peroni, D.G.; et al. Priorities for future research into asthma diagnostic tools: A PAN-EU consensus exercise from the European asthma research innovation partnership (EARIP). Clin. Exp. Allergy 2018, 48, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiao, Y.; Luo, J.; Wang, X.; Qiao, Y.; Huang, R.; Wu, W. Measurement of fractional exhaled nitric oxide and nasal nitric oxide in male patients with obstructive sleep apnea. Sleep Breath 2019, 23, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Stacewicz, T.; Bielecki, Z.; Wojtas, J.; Magryta, P.; Mikolajczyk, J.; Szabra, D. Detection of disease markers in human breath with laser absorption spectroscopy. Opto-Electron. Rev. 2016, 24, 82–94. [Google Scholar] [CrossRef]
- Wojtas, J.; Mikolajczyk, J.; Nowakowski, M.; Rutecka, B.; Medrzycki, R.; Bielecki, Z. Applying CEAS method to UV, VIS, and IR spectroscopy sensors. Bull. Pol. Acad. Sci. Tech. Sci. 2011, 59, 415–418. [Google Scholar] [CrossRef]
- American Thoracic Society Documents ATS / ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005. Crit. Care Med. 2005, 171, 912–930. [CrossRef]
- Myers, R.; Ruszkiewicz, D.M.; Meister, A.; Bartolomeu, C.; Atkar-Khattra, S.; Thomas, C.L.P.; Lam, S. Breath testing for SARS-CoV-2 infection. eBioMedicine 2023, 92, 104584. [Google Scholar] [CrossRef]
- Palaniappan, R.; Sundaraj, K.; Sundaraj, S. Adaptive neuro-fuzzy inference system for breath phase detection and breath cycle segmentation. Comput. Methods Programs Biomed. 2017, 145, 67–72. [Google Scholar] [CrossRef]
- Prokopiuk, A.; Bielecki, Z.; Wojtas, J. Improving the accuracy of the NDIR-based CO2 sensor for breath analysis. Metrol. Meas. Syst. 2021, 28, 803–812. [Google Scholar] [CrossRef]
- Wojtas, J.; Bielecki, Z. Signal processing system in cavity enhanced spectroscopy. Opto-Electron. Rev. 2008, 16, 420–427. [Google Scholar] [CrossRef]
- Beauchamp, J.D.; Miekisch, W. Breath sampling and standardization. In Breathborne Biomarkers and the Human Volatilome, 2nd ed.; Beauchamp, J., Davis, C., Pleil, J., Eds.; Elsevier: Boston, MA, USA, 2020; pp. 23–41. [Google Scholar] [CrossRef]
- Czippelová, B.; Nováková, S.; Šarlinová, M.; Baranovičová, E.; Urbanová, A.; Turianiková, Z.; Krohová, J.Č.; Halašová, E.; Škovierová, H. Impact of breath sample collection method and length of storage of breath samples in Tedlar bags on the level of selected volatiles assessed using gas chromatography-ion mobility spectrometry (GC-IMS). J. Breath Res. 2024, 18, 036004. [Google Scholar] [CrossRef] [PubMed]
- Siobal, M.S. Monitoring Exhaled Carbon Dioxide. Respir. Care 2016, 61, 1397–1416. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Howe, T.A.; Malarvili, M.B. Real-time human respiration carbon dioxide measurement device for cardiorespiratory assessment. J. Breath Res. 2018, 12, 026003. [Google Scholar] [CrossRef]
- MBW Phase-III Slope Analysis. Available online: https://nddmed.com/pulmonary-resources/download/application-notes (accessed on 21 September 2024).
- Stuart-Andrews, C.R.; Kelly, V.J.; Sands, S.A.; Lewis, A.J.; Ellis, M.J.; Thompson, B.R. Automated detection of the phase III slope during inert gas washout testing. J. Appl. Physiol. 2012, 112, 1073–1081. [Google Scholar] [CrossRef]
- Francescato, M.P.; Thieschäfer, L.; Cettolo, V.; Hoffmann, U. Comparison of different breath-by-breath gas exchange algorithms using a gas exchange simulation system. Respir. Physiol. Neurobiol. 2019, 266, 171–178. [Google Scholar] [CrossRef]
- Song, C.; Wang, J.; Zhao, P.; Zhang, Y.; Zhang, Q. A novel method for chronic obstructive pulmonary disease detection using comprehensive respiratory impedance and electrocardiogram-derived respiration. Measurement 2024, 236, 114978. [Google Scholar] [CrossRef]
- Oestreich, M.A.; Doswald, I.; Salem, Y.; Künstle, N.; Wyler, F.; Frauchiger, B.S.; Kentgens, A.-C.; Latzin, P.; Yammine, S. A computerized tool for the systematic visual quality assessment of infant multiple-breath washout measurements. Front. Pediatr. 2024, 12, 1393291. [Google Scholar] [CrossRef]
- Xu, M.; Xu, Y.; Tao, J.; Wen, L.; Zheng, C.; Yu, Z.; He, S. Development of a compact NDIR CO2 gas sensor for harsh environments. Infrared Phys. Technol. 2024, 136, 105035. [Google Scholar] [CrossRef]
- McRae, G.J. A Simple Procedure for Calculating Atmospheric Water Vapor Concentration. J. Air Pollut. Control Assoc. 1980, 30, 394. [Google Scholar] [CrossRef]
- Bake, B.; Larsson, P.; Ljungkvist, G.; Ljungström, E.; Olin, A.C. Exhaled particles and small airways. Respir. Res. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Bouza, M.; Gonzalez-Soto, J.; Pereiro, R.; De Vicente, J.C.; Sanz-Medel, A. Exhaled breath and oral cavity VOCs as potential biomarkers in oral cancer patients. J. Breath Res. 2017, 11, 016015. [Google Scholar] [CrossRef]
- Alkhouri, N.; Singh, T.; Alsabbagh, E.; Guirguis, J.; Chami, T.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Isoprene in the exhaled breath is a novel biomarker for advanced fibrosis in patients with chronic liver disease: A pilot study. Clin. Transl. Gastroenterol. 2015, 6, e112. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.R. Diagnostic utility of serum procalcitonin in ventilator-associated pneumonia in the pediatric intensive care unit. Minia J. Med. Res. 2024. [Google Scholar] [CrossRef]
- Wojtas, J. Application of cavity enhanced absorption spectroscopy to the detection of nitric oxide, carbonyl sulphide, and ethane—Breath biomarkers of serious diseases. Sensors 2015, 15, 14356–14369. [Google Scholar] [CrossRef]
- Bodaghi, A.; Fattahi, N.; Ramazani, A. Biomarkers: Promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon 2023, 9, e13323. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, A.; Chludziński, T.; Saidi, T.; Welearegay, T.G.; Jaimes-Mogollón, A.L.; El Bari, N.; Borys, S.; Bouchikhi, B.; Smulko, J.; Ionescu, R. Assessment of electronic sensing techniques for the rapid identification of alveolar echinococcosis through exhaled breath analysis. Sensors 2020, 20, 2666. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef]
- Geiger, K.; Guttmann, J.; Braun, G.; Schubert, J.K.; Spittler, K.-H. CO2-controlled sampling of alveolar gas in mechanically ventilated patients. J. Appl. Physiol. 2017, 90, 486–492. [Google Scholar] [CrossRef]
- Ganta, R.; Raj, T.D.; Capnography, I. Data Interpretation in Anesthesia: A Clinical Guide; Raj, T.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 35–38. [Google Scholar] [CrossRef]
- Kazemi, M.; Krishnan, M.B.; Howe, T.A. Frequency analysis of capnogram signals to differentiate asthmatic and non-asthmatic conditions using radial basis function neural networks. Iran. J. Allergy Asthma Immunol. 2013, 12, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Bhavani-Shankar, K.; Philip, J.H. Defining segments and phases of a time capnogram. Anesth. Analg. 2000, 91, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Sipmann, F.; Villar, J.; Ferrando, C.; Sánchez-Giralt, J.A.; Tusman, G. Monitoring Expired CO2 Kinetics to Individualize Lung-Protective Ventilation in Patients with the Acute Respiratory Distress Syndrome. Front. Physiol. 2021, 12, 785014. [Google Scholar] [CrossRef]
- Verscheure, S.; Massion, P.B.; Verschuren, F.; Damas, P.; Magder, S. Volumetric capnography: Lessons from the past and current clinical applications. Crit. Care 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Böhm, S.H.; Maisch, S.; Von Sandersleben, A.; Thamm, O.; Passoni, I.; Arca, J.M.; Tusman, G. The effects of lung recruitment on the phase III slope of volumetric capnography in morbidly obese patients. Anesth. Analg. 2009, 109, 151–159. [Google Scholar] [CrossRef]
- Jaffe, M.B. Carbon dioxide measurement. In Capnography; Paulus, D.A., Gravenstein, J.S., Jaffe, M.B., Gravenstein, N., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 381–396. [Google Scholar]
- Golja, P.; Cettolo, V.; Francescato, M.P. Calculation algorithms for breath-by-breath alveolar gas exchange: The unknowns! Eur. J. Appl. Physiol. 2018, 118, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- How to Read and Interpret End-Tidal Capnography Waveforms. Available online: https://www.jems.com/patient-care/how-to-read-and-interpret-end-tidal-capnography-waveforms/ (accessed on 21 September 2024).
- Min, K.; Wada, S. A mathematical model for the first derivative wave analysis of the volumetric capnogram from the perspective of erythrocyte motion profiles. Heliyon 2019, 5, e01824. [Google Scholar] [CrossRef]
- Singh, O.P.; Palaniappan, R.; Malarvili, M. Automatic Quantitative Analysis of Human Respired Carbon Dioxide Waveform for Asthma and Non-Asthma Classification Using Support Vector Machine. IEEE Access 2018, 6, 55245–55256. [Google Scholar] [CrossRef]
- Hirabayashi, G.; Yokose, Y.; Oshika, H.; Saito, M.; Maruyama, K.; Andoh, T. Effects of volume-targeted pressure-controlled inverse ratio ventilation on functional residual capacity and dead space in obese patients undergoing robot-assisted laparoscopic radical prostatectomy. BJA Open 2022, 3, 100020. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 2016, 121, 2020–2027. [Google Scholar] [CrossRef]
- Akter, S.; Khan, F.; Ullah, M.A. Development of Algorithm to Extract Features from Capnograph Signal. In Proceedings of the 12th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 21–23 December 2022. [Google Scholar] [CrossRef]
- Mikolajczyk, J.; Wojtas, J.; Bielecki, Z.; Stacewicz, T.; Szabra, D.; Nowakowski, M.; Magryta, P.; Prokopiuk, A.; Mędrzycki, R. System of Optoelectronic Sensors for Breath Analysis. Metrol. Meas. Syst. 2016, 23, 481–489. [Google Scholar] [CrossRef]
- Singh, O.P.; El-Badawy, I.M.; Malarvili, M.B. Design and validation of a handheld capnography device for cardiopulmonary assessment based on the Arduino platform. J. Innov. Opt. Health Sci. 2021, 14, 2150015. [Google Scholar] [CrossRef]
- Thompson, J.E.; Jaffe, M.B. Capnographic waveforms in the mechanically ventilated patient. Respir. Care 2005, 50, 100–108. [Google Scholar] [PubMed]
- Soleimanpour, H. Capnography in the Emergency Department. Emerg. Med. Open Access 2012, 02, 7–10. [Google Scholar] [CrossRef]
- Farish, S.E.; Garcia, P.S. Capnography primer for oral and maxillofacial surgery: Review and technical considerations. J. Anesth. Clin. Res. 2013, 4, 295. [Google Scholar] [CrossRef]
- Griffis, C.A. End-tidal CO2 monitoring during anesthesia. J. Am. Assoc. Nurse Anesth. 1986, 54, 4. [Google Scholar]
- Ni, W.; Wang, T.; Wu, Y.; Liu, X.; Li, Z.; Yang, R.; Zhang, K.; Yang, J.; Zeng, M.; Hu, N.; et al. Multi-task deep learning model for quantitative volatile organic compounds analysis by feature fusion of electronic nose sensing. Sens. Actuators B Chem. 2024, 417, 136206. [Google Scholar] [CrossRef]
- Walker, H.J.; Burrell, M.M. Could breath analysis by MS could be a solution to rapid, non-invasive testing for COVID-19? Bioanalysis 2020, 2, 1213–1217. [Google Scholar] [CrossRef]
- Kwiatkowski, A.; Borys, S.; Sikorska, K.; Drozdowska, K.; Smulko, J.M. Clinical studies of detecting COVID-19 from exhaled breath with electronic nose. Sci. Rep. 2022, 12, 15990. [Google Scholar] [CrossRef]
- Gaude, E.; Nakhleh, M.K.; Patassini, S.; Boschmans, J.; Allsworth, M.; Boyle, B.; Van Der Schee, M.P. Targeted breath analysis: Exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J. Breath Res. 2019, 13, 032001. [Google Scholar] [CrossRef]
- Filipiak, W.; Ager, C.; Troppmair, J. Predicting the future from the past: Volatile markers for respiratory infections. Eur. Respir. J. 2017, 49, 1700264. [Google Scholar] [CrossRef] [PubMed]
- Musiałek, F.; Szabra, D.; Wojtas, J. Time-Efficient SNR Optimization of WMS-Based Gas Sensor Using a Genetic Algorithm. Sensors 2024, 24, 1842. [Google Scholar] [CrossRef] [PubMed]



| Principle of Operation | Disadvantages | Literature |
|---|---|---|
| Analysis of the sounds of air flowing through the trachea or in the lungs | Relatively complex signal analysis (spectral analysis, phase-shift information, neuro-fuzzy systems) | [13] |
| Assumed constant volume or duration of phases | The phases’ duration and volumes vary from patient to patient and depend on gender, age, etc. | [14] |
| Determined by the operator | An experienced operator is required, and identification is subjective | [15] |
| Analysis of pressure changes in the patient’s exhalation | Identification results depend on many important factors resulting from the patient’s health condition | [16] |
| Constant threshold of selected gas concentration | The threshold may be different for each patient | [17] |
| Analysis of dynamics of gas concentration changes | Complex procedures that require a lot of computing power and time, usually performed after exhalation | [18] |
| Breath Phase | Description |
|---|---|
| I | Beginning of exhalation. Air from the mouth and trachea (known as dead space) that is not involved in gas exchange. Low CO2 concentration, is usually used as a reference in the analysis. |
| II | Mixed air from dead space and alveoli. A rapid increase in CO2 concentration to approximately 3–5%. |
| III | Air from the alveoli. Slow increase of the CO2 concentration to the maximum level known as the end-tidal point (EtCO2). |
| IV | Very fast decrease in CO2 concentration to the ambient level and the beginning of the following breath process. |
| Definition | Literature |
|---|---|
| The point is generally placed between the expiratory stroke and the alveolar plateau. | [34] |
| The beginning of the flat part of CO2 changes as a function of time (time-resolved capnography) | [35] |
| The beginning of the flat part of CO2 changes as a function of volume (volume-resolved capnography) | [36] |
| The point of intersection of both slopes is determined by the linear regression method. | [37] |
| Dependencies between specific constant breath volumes | [38] |
| Constant CO2 concentration threshold about 3% ÷ 3.8% | [39] |
| Analysis of dynamics of gas concentration changes | [16] |
| Methods | Literature |
|---|---|
| Finite impulse response (FIR) 10 Hz low pass filter and moving average filters to limit signal bandwidth, remove noise, and smooth raw CO2 signal | [16] |
| Averaging data for removing fluctuations in ventilation | [45] |
| Dedicated filtering methods | [46] |
| Sex (M—Male, F—Female) | Age (Years) | Weight (kg) | Height (cm) | Smokers (Y—yes, N—No) | Number of Air Samples Collected |
|---|---|---|---|---|---|
| M | 46 | 85 | 176 | N | 30 |
| F | 35 | 60 | 164 | N | 40 |
| M | 26 | 63 | 166 | N | 50 |
| F | 42 | 52 | 164 | N | 30 |
| M | 45 | 98 | 186 | N | 30 |
| M | 47 | 70 | 170 | N | 30 |
| F | 33 | 58 | 169 | N | 40 |
| M | 46 | 77 | 180 | N | 30 |
| M | 48 | 87 | 172 | N | 10 |
| M | 36 | 76 | 169 | N | 10 |
| F | 52 | 55 | 164 | N | 10 |
| F | 49 | 58 | 165 | N | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokopiuk, A.; Wojtas, J. Accelerating the Diagnosis of Pandemic Infection Based on Rapid Sampling Algorithm for Fast-Response Breath Gas Analyzers. Sensors 2024, 24, 6164. https://doi.org/10.3390/s24196164
Prokopiuk A, Wojtas J. Accelerating the Diagnosis of Pandemic Infection Based on Rapid Sampling Algorithm for Fast-Response Breath Gas Analyzers. Sensors. 2024; 24(19):6164. https://doi.org/10.3390/s24196164
Chicago/Turabian StyleProkopiuk, Artur, and Jacek Wojtas. 2024. "Accelerating the Diagnosis of Pandemic Infection Based on Rapid Sampling Algorithm for Fast-Response Breath Gas Analyzers" Sensors 24, no. 19: 6164. https://doi.org/10.3390/s24196164







