Compact Laser-Induced Fluorescence Detector with Adjustable Laser Focal Spot for Multiple Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fluorescein Solution for LOD Analysis
2.2. LAMP Assay
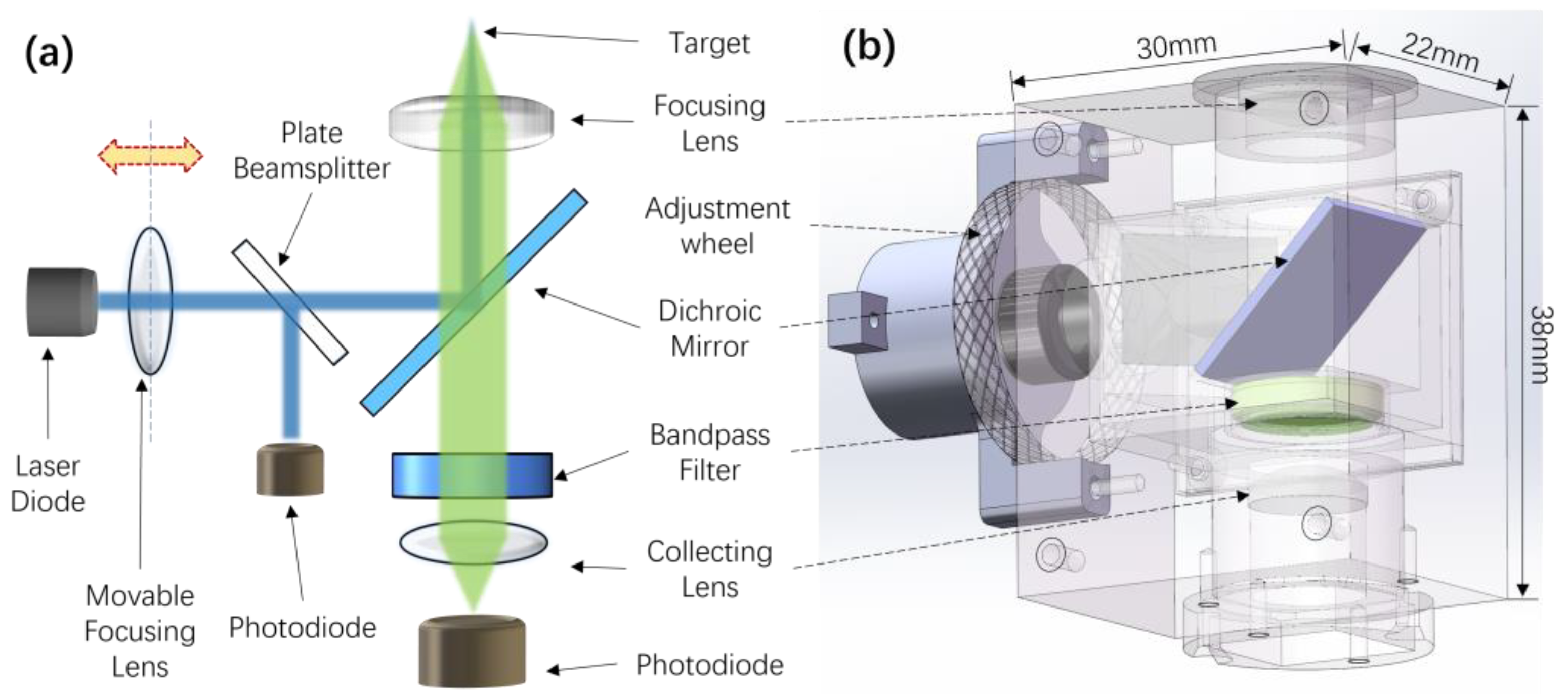
2.3. Building of the LIF Detection System
2.4. Positioning of the Detection System to the Target
2.5. Observation Process
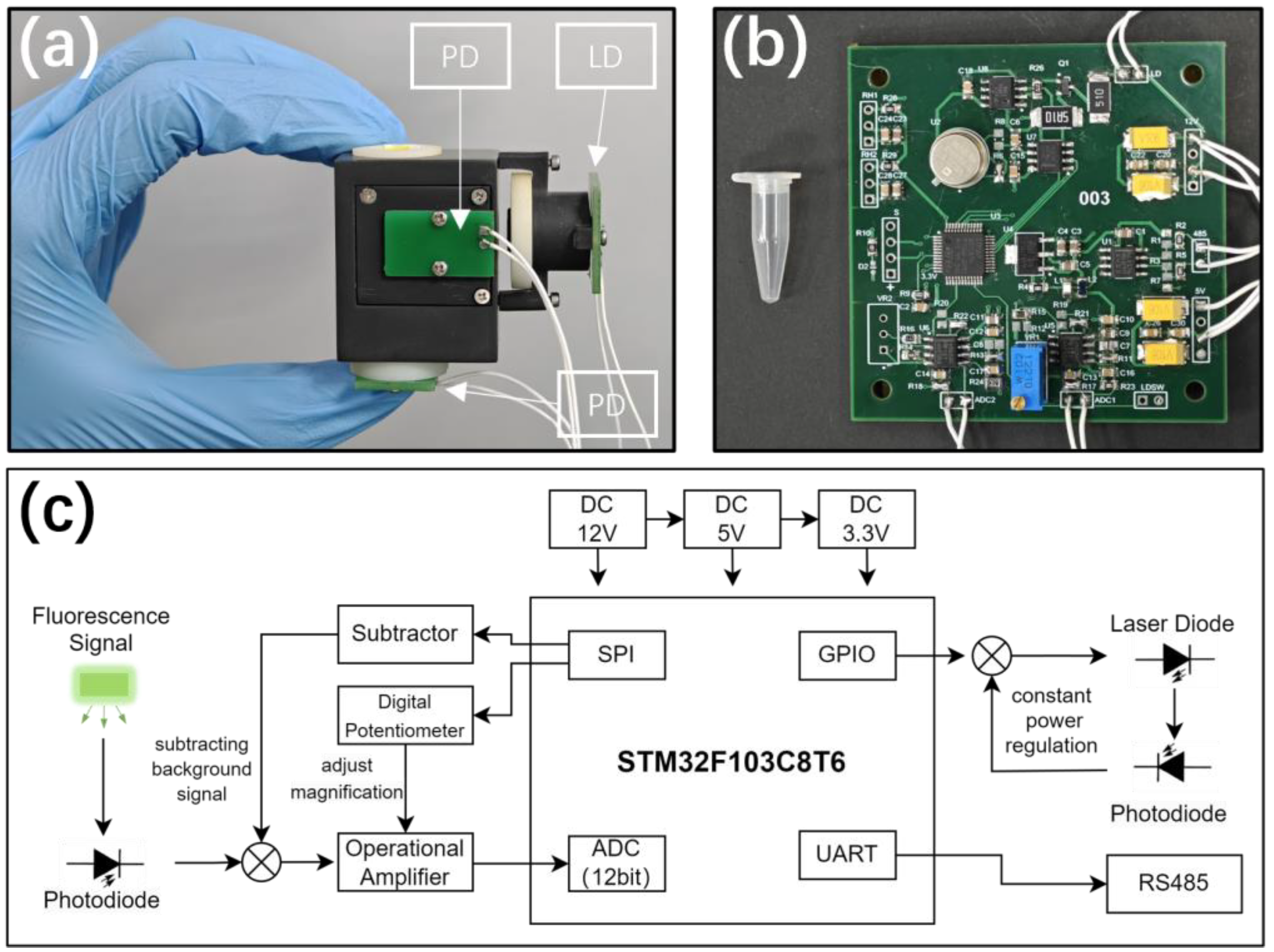
3. Construction of the Electronics System
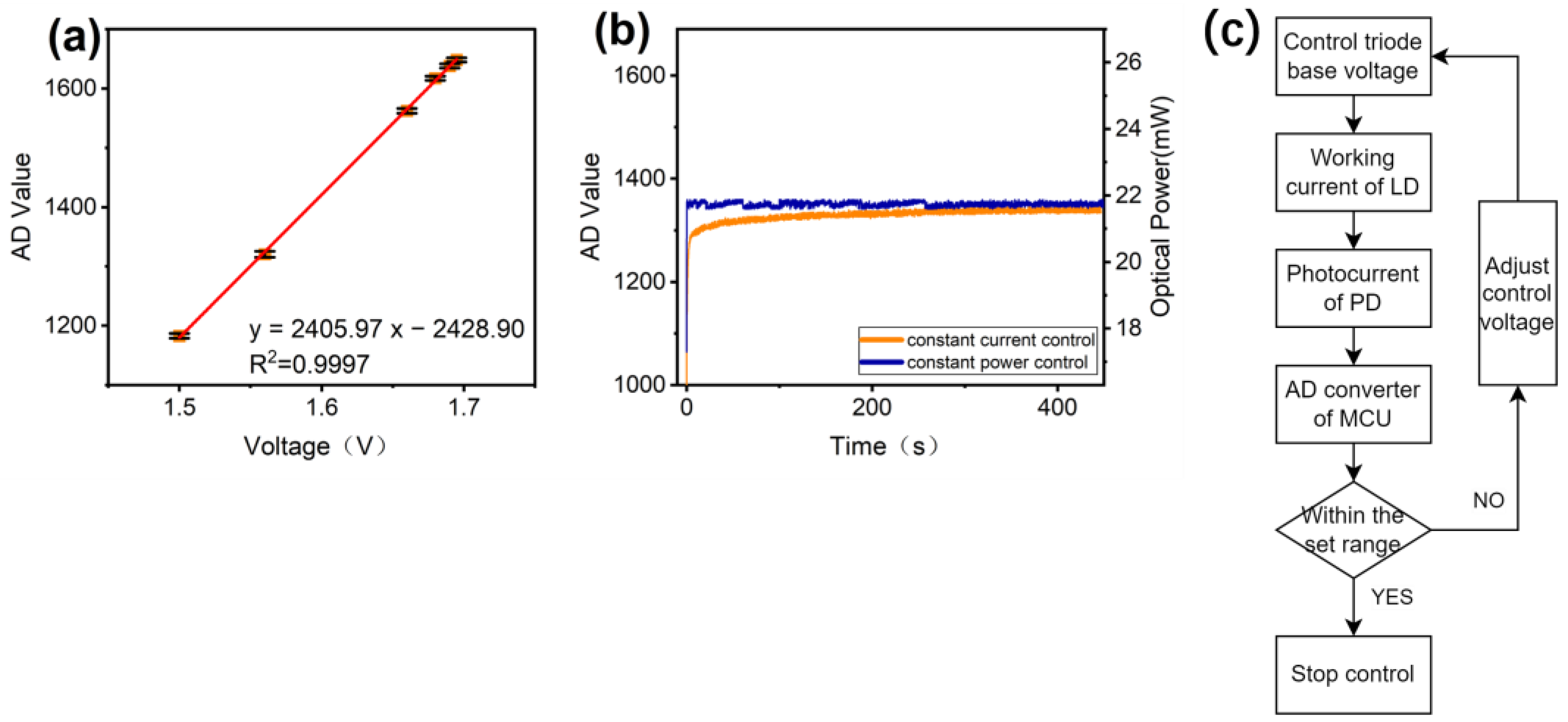
3.1. Adjustment of Dynamic Range and Background Correction
3.2. Constant Power Control of the Laser Source
4. Results and Discussion
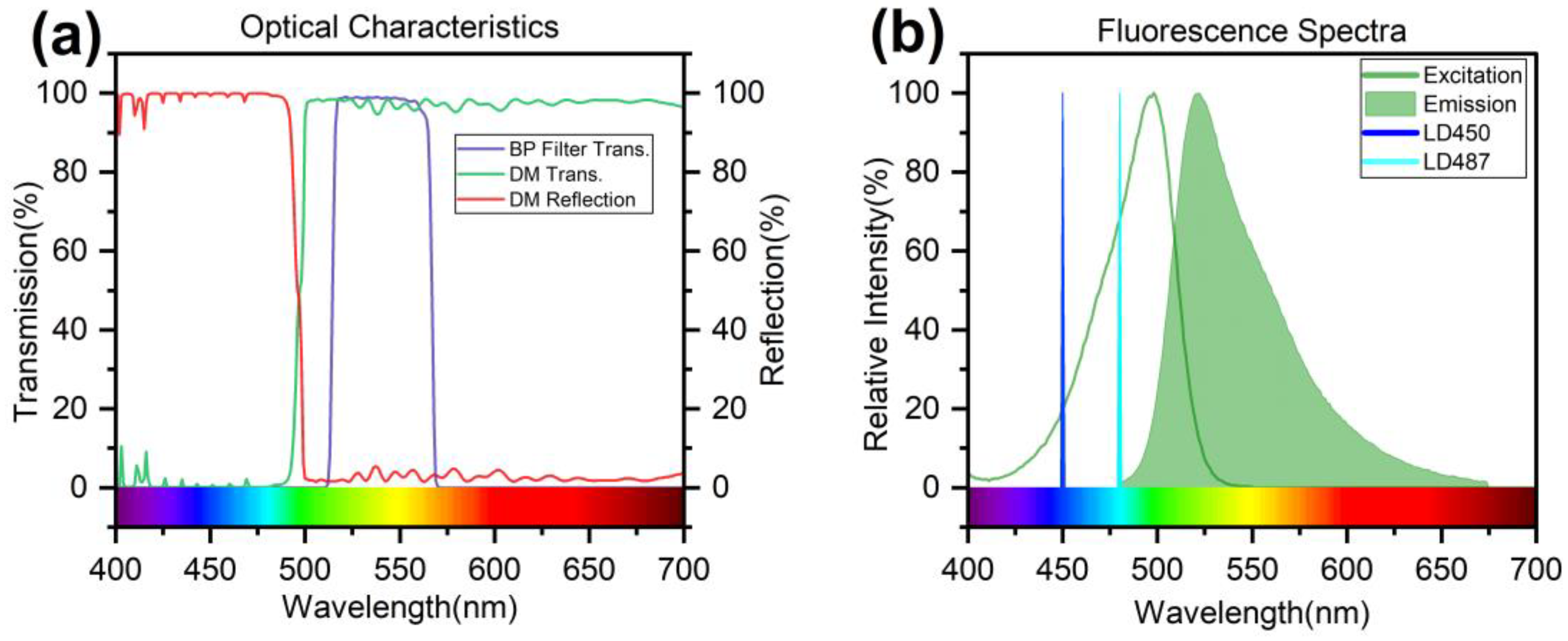
4.1. Optimization of Optical Components
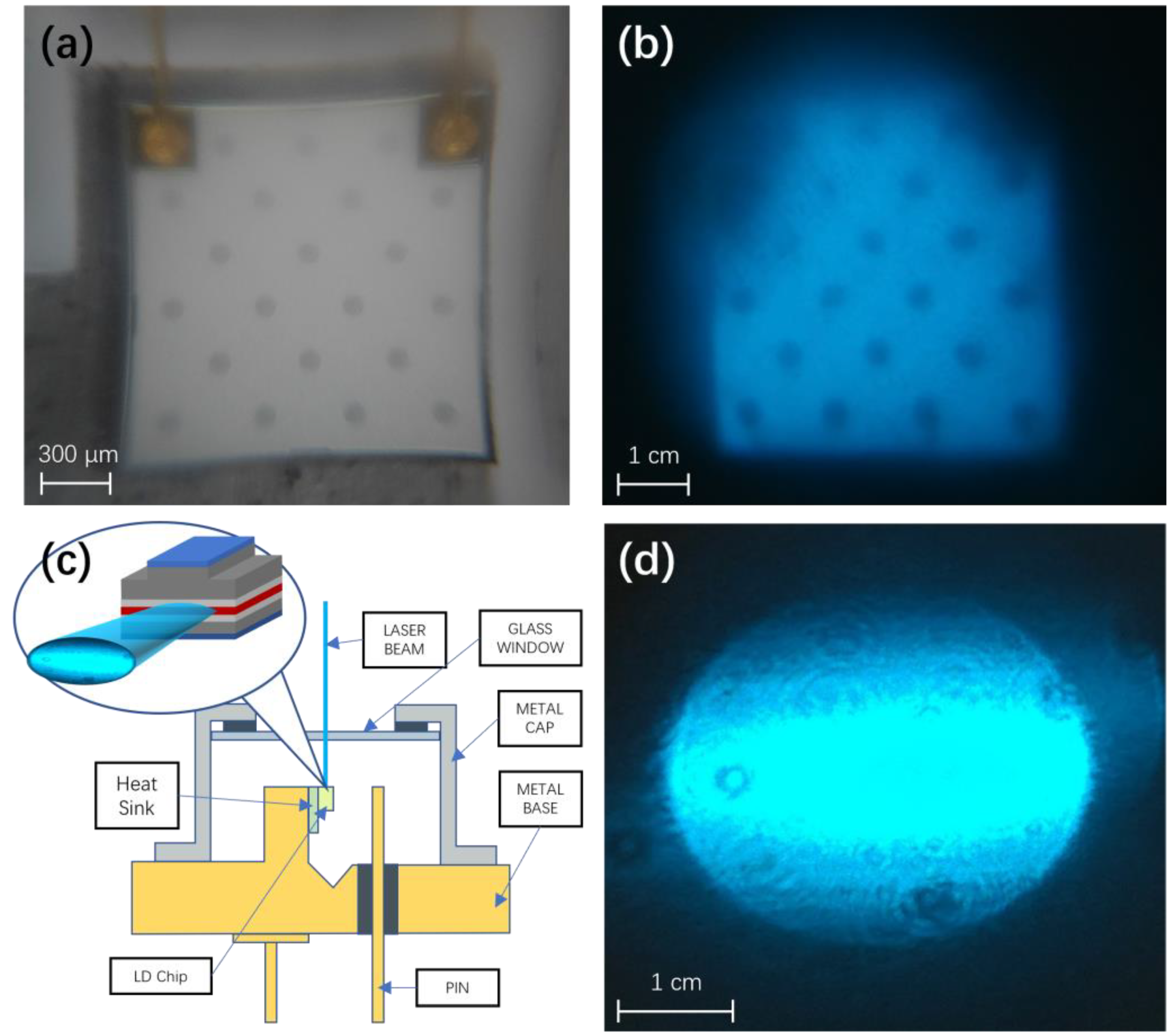
4.2. Optimization of the Excitation Source
4.3. Adaptive Adjustment of Dynamic Range
4.4. Constant Power Control of the Light Source
4.5. LOD Using 450/487 nm Excitation Source
4.6. Effect of the Adjustable Laser Focal Spot
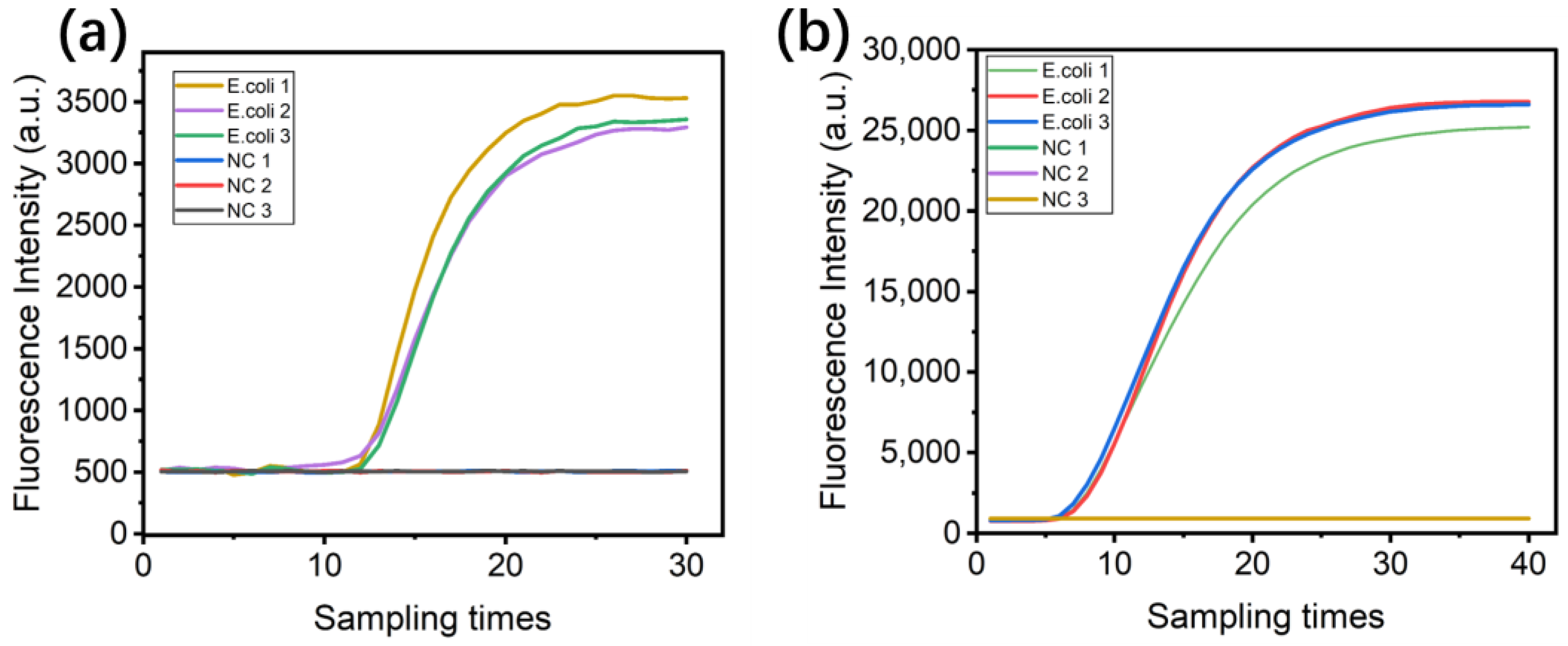
4.7. LAMP Fluorescence Amplification Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ban, E.; Song, E.J. Recent developments and applications of capillary electrophoresis with laser-induced fluorescence detection in biological samples. J. Chromatogr. B 2013, 929, 180–186. [Google Scholar] [CrossRef]
- Wu, C.; Sun, Y.; Wang, Y.; Duan, W.; Hu, J.; Zhou, L.; Pu, Q. 7-(Diethylamino)coumarin-3-carboxylic acid as derivatization reagent for 405 nm laser-induced fluorescence detection: A case study for the analysis of sulfonamides by capillary electrophoresis. Talanta 2019, 201, 16–22. [Google Scholar] [CrossRef]
- Dada, O.O.; Ramsay, L.M.; Dickerson, J.A.; Cermak, N.; Jiang, R.; Zhu, C.; Dovichi, N.J. Capillary array isoelectric focusing with laser-induced fluorescence detection: Milli-pH unit resolution and yoctomole mass detection limits in a 32-channel system. Anal. Bioanal. Chem. 2010, 397, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wei, X.; Men, X.; Zhang, X.; Yu, Y.-L.; Xu, Z.-R.; Chen, M.-L.; Wang, J.-H. Two-Dimensional Cytometry Platform for Single-Particle/Cell Analysis with Laser-Induced Fluorescence and ICP–MS. Anal. Chem. 2021, 93, 8203–8209. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wei, X.; Men, X.; Xu, Y.; Bai, J.; Wang, Y.; Zhou, L.; Yu, Y.-L.; Xu, Z.-R.; Chen, M.-L.; et al. Open flow cytometer with the combination of 3D hydrodynamic single cell focusing and confocal laser-induced fluorescence detection. Talanta 2023, 258, 124424. [Google Scholar] [CrossRef]
- Hart, S.J.; Hall, G.J.; Kenny, J.E. A laser-induced fluorescence dual-fiber optic array detector applied to the rapid HPLC separation of polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem. 2002, 372, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Patil, A.; Rai, L.; Kartha, V.B.; Chidangil, S. Application of HPLC Combined with Laser Induced Fluorescence for Protein Profile Analysis of Tissue Homogenates in Cervical Cancer. Sci. World J. 2012, 2012, 976421. [Google Scholar] [CrossRef] [PubMed]
- Berner, M.; Hilbig, U.; Schubert, M.B.; Gauglitz, G. Laser-induced fluorescence detection platform for point-of-care testing. Meas. Sci. Technol. 2017, 28, 085701. [Google Scholar] [CrossRef]
- Kamei, T. Laser-induced fluorescence detection modules for point-of- care microfluidic biochemical analysis. Procedia Eng. 2011, 25, 709–712. [Google Scholar] [CrossRef]
- Li, R.; Zou, H.; Xiao, H.; Wu, R. Carbon Nanotubes as Intracellular Carriers for Multidrug Resistant Cells Studied by Capillary Electrophoresis–Laser–Induced Fluorescence. In Carbon Nanotubes: Methods and Protocols; Balasubramanian, K., Burghard, M., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 153–168. [Google Scholar]
- Gibbons, S.E.; Stayton, I.; Ma, Y. Optimization of urinary pteridine analysis conditions by CE-LIF for clinical use in early cancer detection. Electrophoresis 2009, 30, 3591–3597. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, S.; Lin, L.; Yao, S. A CIEF-LIF method for simultaneous analysis of multiple protein kinases and screening of inhibitors. Electrophoresis 2016, 37, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Weaver, M.T.; Lynch, K.B.; Zhu, Z.; Chen, H.; Lu, J.J.; Pu, Q.; Liu, S. Confocal laser-induced fluorescence detector for narrow capillary system with yoctomole limit of detection. Talanta 2017, 165, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-X.; Li, H.-Y.; Fang, P.; Pan, J.-Z.; Fang, Q. A handheld laser-induced fluorescence detector for multiple applications. Talanta 2016, 150, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Shi, M.; Ning, H.; Feng, C.; Guan, Y. A compact and low-cost laser induced fluorescence detector with silicon based photodetector assembly for capillary flow systems. Talanta 2018, 182, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-Z.; Fang, P.; Fang, X.-X.; Hu, T.-T.; Fang, J.; Fang, Q. A Low-Cost Palmtop High-Speed Capillary Electrophoresis Bioanalyzer with Laser Induced Fluorescence Detection. Sci. Rep. 2018, 8, 1791. [Google Scholar] [CrossRef]
- Peng, Y.-M.; Pan, J.-Z.; Fang, Q. Handheld laser-induced fluorescence detection systems with different optical configurations. Talanta 2021, 230, 122329. [Google Scholar] [CrossRef] [PubMed]
- Oborny, N.J.; Kehl, F.; Cretu, V.; Noell, A.C.; Willis, P.A. A radiation tolerant laser-induced fluorescence detection system for a potential Europa Lander mission. Acta Astronaut. 2021, 186, 465–472. [Google Scholar] [CrossRef]
- Zhu, Z.; Lies, M.; Silzel, J. Native fluorescence detection with a laser driven light source for protein analysis in capillary electrophoresis. Anal. Chim. Acta 2021, 1183, 338936. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Meade, J.D.; Shester, B.R.; Walden, J.C.; Guo, Z.; Liang, P. Linear dynamic range for signal detection in fluorescent differential display. Biotechnol. Lett. 2010, 32, 1593–1597. [Google Scholar] [CrossRef]
- Whitmore, C.D.; Essaka, D.; Dovichi, N.J. Six orders of magnitude dynamic range in capillary electrophoresis with ultrasensitive laser-induced fluorescence detection. Talanta 2009, 80, 744–748. [Google Scholar] [CrossRef]
- de Jong, E.P.; Lucy, C.A. Low-picomolar limits of detection using high-power light-emitting diodes for fluorescence. Analyst 2006, 131, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Galievsky, V.A.; Stasheuski, A.S.; Krylov, S.N. “Getting the best sensitivity from on-capillary fluorescence detection in capillary electrophoresis”—A tutorial. Anal. Chim. Acta 2016, 935, 58–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zheng, W.; Tong, G.; Zheng, W.; Yuan, Y.; Tian, Q.; Xu, D.; Jia, C.; Zhang, T. Experimental system of life ecological science on China Space Station. In Proceedings of the Earth and Space: From Infrared to Terahertz (ESIT 2022), Nantong, China, 17–19 September 2022; Volume 12505. [Google Scholar]
- Xu, Z.; Liu, F.; Zhang, X.; Tian, Q.; Zhang, T. Development of the Microbial Online Monitoring Module (MOMM) for the Chinese Space Station. Microgravity Sci. Technol. 2024, 36, 39. [Google Scholar] [CrossRef]



| Size | Cost (USD) | Optical Configuration | Excitation Source | Photodetector | Analyte | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| 9.1 × 6.2 × 4.1 cm3 | 2000 | Quasi-confocal | 450 nm LD | PMT | Fluorescein | 0.42 nM | [14] |
| 29 × 25 × 15.5 cm3 | Over 500 | Confocal | 450 nm LD | PD (AccuOpt2000) | Fluorescein | 3 pM | [15] |
| 90 × 75 × 77 mm3 | 500 | Orthogonal | 450 nm LD | PD | Fluorescamine-labeled proteins | 1 nM | [16] |
| 50 × 20 × 46 mm3 | 380 | Orthogonal | 450 nm LD | PD | Fluorescein | 10 pM | [17] |
| Not available | Not available | Confocal | 405/488 nm LD | PMT | Amino acids | 1 nM | [18] |
| 40 × 22 × 38 mm3 | 320 | Epifluorescence | 487 nm LD | PD | Fluorescein | 0.4 nM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Chen, X.; Liu, F. Compact Laser-Induced Fluorescence Detector with Adjustable Laser Focal Spot for Multiple Purposes. Sensors 2024, 24, 6224. https://doi.org/10.3390/s24196224
Xu Z, Chen X, Liu F. Compact Laser-Induced Fluorescence Detector with Adjustable Laser Focal Spot for Multiple Purposes. Sensors. 2024; 24(19):6224. https://doi.org/10.3390/s24196224
Chicago/Turabian StyleXu, Zihe, Xi Chen, and Fangwu Liu. 2024. "Compact Laser-Induced Fluorescence Detector with Adjustable Laser Focal Spot for Multiple Purposes" Sensors 24, no. 19: 6224. https://doi.org/10.3390/s24196224






