Gelatin-Coated High-Sensitivity Microwave Sensor for Humidity-Sensing Applications
Abstract
:1. Introduction
2. Microwave Sensor Geometry and Characteristic
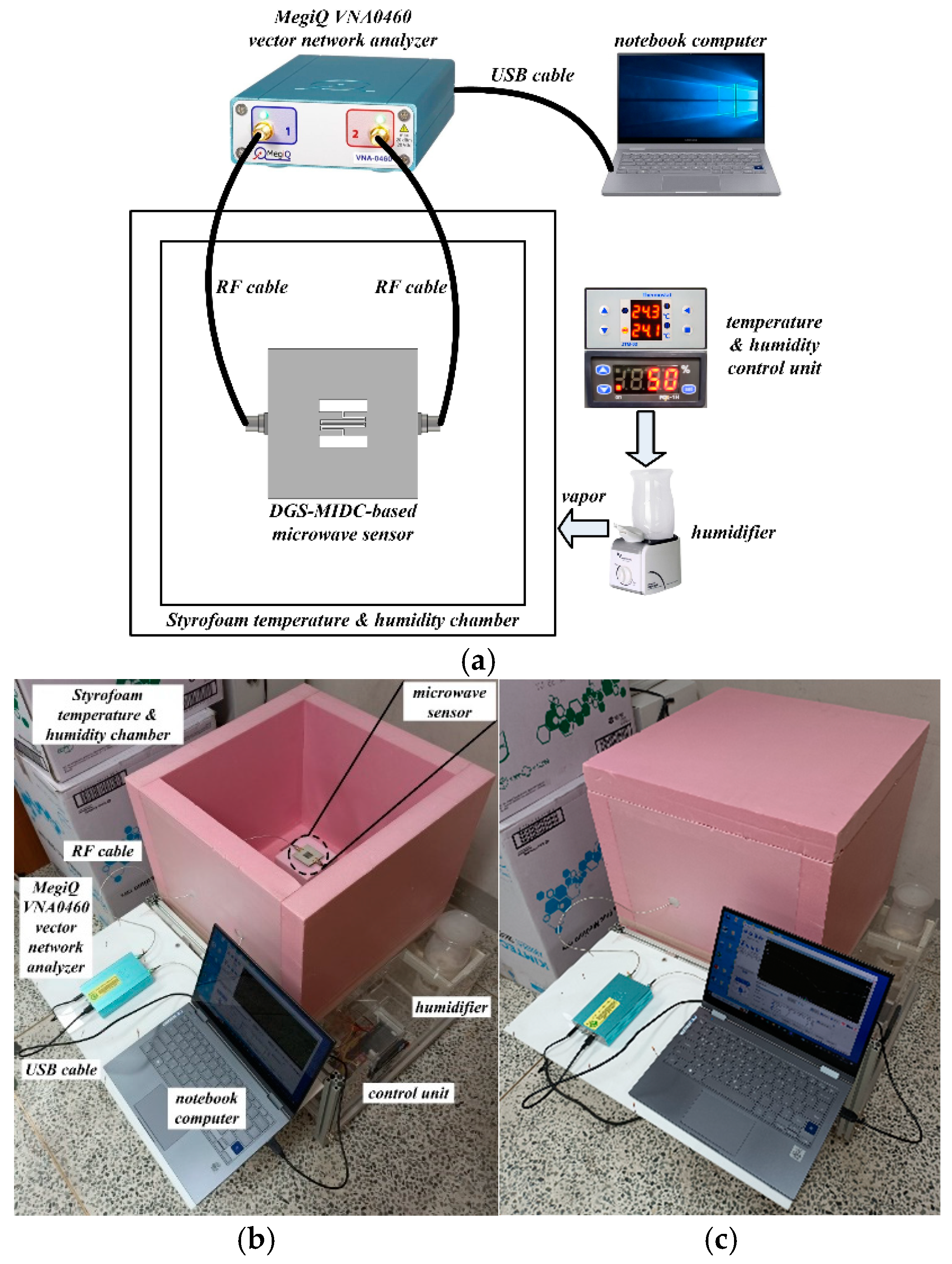
3. Experiment Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sajid, M.; Khattak, Z.; Rahman, K.; Hassan, G.; Choi, K.H. Progress and future of relative humidity sensors: A review from materials perspective. Bull. Mater. Sci. 2022, 45, 238. [Google Scholar] [CrossRef]
- Arman Kuzubasoglu, B. Recent studies on the humidity sensor: A mini review. ACS Appl. Electron. Mater. 2022, 4, 4797–4807. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lee, G.B. Humidity sensors: A review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Ali, S.; Hassan, A.; Hassan, G.; Bae, L.; Lee, C. All-printed humidity sensor based on graphene/methyl-red composite with high sensitivity. Carbon 2016, 105, 23–32. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Sekulić, D.L.; Ivetić, T.B. Characterization of an impedance-type humidity sensor based on porous SnO2/TiO2 composite ceramics modified with molybdenum and zinc. Sensors 2023, 23, 8261. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Gayathri, G.; Reshma, T.S.; Mangamma, G.; Prasad, A.K.; Das, A. A sensitive humidity sensor at low pressure with SnO2 QDs. Sens. Actuators A-Phys. 2022, 346, 113835. [Google Scholar] [CrossRef]
- Li, H.; Meng, B.; Jia, H.; Wang, D.; Wei, Z.; Li, R.; Chen, R. Optical humidity sensor based on ZnO nanomaterials. In Proceedings of the 2020 IEEE 5th Optoelectronics Global Conference (OGC), Shenzhen, China, 7–11 September 2020; pp. 169–172. [Google Scholar]
- Li, F.; Li, P.; Zhang, H. Preparation and research of a high-performance ZnO/SnO2 humidity sensor. Sensors 2022, 22, 293. [Google Scholar] [CrossRef]
- Sun, L.; Haidry, A.A.; Fatima, Q.; Li, Z.; Yao, Z. Improving the humidity sensing below 30% RH of TiO2 with GO modification. Mater. Res. Bull. 2018, 99, 124–131. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; Li, C.; Men, G.; Han, D.; Gu, F. Humidity-sensing performance of 3DOM WO3 with controllable structural modification. ACS Appl. Mater. Interfaces 2018, 10, 3776–3783. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Hassan, G.; Awais, M.; Bae, J. All printed full range humidity sensor based on Fe2O3. Sens. Actuators A Phys. 2022, 311, 112072. [Google Scholar] [CrossRef]
- Abid, H.N.; Nayef, U.M.; Mutlak, F.A.H. Preparation and characterization Co3O4 nanoparticles on porous silicon for humidity sensor by photoluminescence. Optik 2019, 178, 379–383. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Zheng, W.; Wang, R.; Liu, X.; Xia, Y.; Zhao, J. Humidity sensing properties of BaTiO3 nanofiber prepared via electrospinning. Sens. Actuators B Chem. 2010, 146, 98–102. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Viola, G.; Afify, A.S.; Babeer, A.M.; Ferraris, M. Processing, structural and humidity sensing properties of PbTiO3 ceramic synthesized by solid state reaction. J. Porous Mater. 2020, 27, 947–958. [Google Scholar] [CrossRef]
- Chang, D.A.; Tseng, T.Y. Humidity-sensitivity characteristics of CaTiO3 porous ceramics. J. Mater. Sci. Lett. 1990, 9, 943–944. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Li, X.; Lu, G.; You, L.; Liang, X.; Liu, F.; Zhang, T.; Du, Y. Highly sensitive humidity sensor based on high surface area mesoporous LaFeO3 prepared by a nanocasting route. Sens. Actuators B Chem. 2013, 181, 802–809. [Google Scholar] [CrossRef]
- Bauskar, D.; Kale, B.B.; Patil, P. Synthesis and humidity sensing properties of ZnSnO3 cubic crystallites. Sens. Actuators B Chem. 2012, 161, 396–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhang, Y.; Cheng, X.; Feng, C.; Chen, L.; Zhou, J.; Ruan, S. A novel humidity sensor based on NaTaO3 nanocrystalline. Sens. Actuators B Chem. 2012, 174, 485–489. [Google Scholar] [CrossRef]
- Tambwe, K.; Ross, N.; Baker, P.; Bui, T.-T.; Goubard, F. Humidity sensing applications of lead-free halide perovskite nanomaterials. Materials 2022, 15, 4146. [Google Scholar] [CrossRef]
- Ahn, K.; Wessels, W.B.; Sampath, S. Spinel humidity sensors prepared by thermal spray direct writing. Sens. Actuators B Chem. 2005, 107, 342–346. [Google Scholar] [CrossRef]
- Shah, J.; Kotnala, R.K. Humidity sensing exclusively by physisorption of water vapors on magnesium ferrite. Sens. Actuators B Chem. 2012, 171–172, 832–837. [Google Scholar] [CrossRef]
- Saha, D.; Giri, R.; Mistry, K.K.; Sengupta, K. Magnesium chromate–TiO2 spinel tape cast thick film as humidity sensor. Sens. Actuators B Chem. 2005, 107, 323–331. [Google Scholar] [CrossRef]
- Bayhan, M.; Kavasoglu, N. A study on the humidity sensing properties of ZnCr2O4–K2CrO4 ionic conductive ceramic sensor. Sens. Actuators B Chem. 2006, 117, 261–265. [Google Scholar] [CrossRef]
- Urbiztondo, M.; Pellejero, I.; Rodriguez, A.; Pina, M.P.; Santamaria, J. Zeolite-coated interdigital capacitors for humidity sensing. Sens. Actuators B Chem. 2011, 157, 450–459. [Google Scholar] [CrossRef]
- Prasad, N.V.K.; Prasad, K.V.; Ramesh, S.; Phanidhar, S.V.; Ratnam, K.V.; Janardhan, S.; Manjunatha, H.; Sarma, M.S.S.R.K.N.; Srinivas, K. Ceramic sensors: A mini-review of their applications. Front. Mater. 2020, 7, 593342. [Google Scholar]
- Tulliani, J.M.; Inserra, B.; Ziegler, D. Carbon-based materials for humidity sensing: A short review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.L.; Hu, C.G.; Fang, L.; Wang, S.X.; Tian, Y.S.; Pan, C.Y. Humidity sensor based on multi-walled carbon nanotube thin films. J. Nanomater. 2011, 2011, 707303. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wu, F.; Hu, R.; Deng, C. Humidity response of single carbon nanocoil and its temperature sensor independent of humidity and strain. Appl. Surf. Sci. 2022, 605, 154745. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Lee, C.-W.; Rhee, H.-W.; Gong, M.-S. Humidity sensor using epoxy resin containing quaternary ammonium salts. Sens. Actuators B Chem. 2001, 73, 124–129. [Google Scholar] [CrossRef]
- Sakai, Y.; Matsuguchi, M.; Yonesato, N. Humidity sensor based on alkali salts of poly(2-acrylamido-2-methylpropane sulfonic acid). Electrochim. Acta 2001, 46, 1509–1514. [Google Scholar] [CrossRef]
- Gong, M.-S.; Lee, C.-W.; Joo, S.-W.; Choi, B.-K. Humidity-sensitive properties of phosphonium salt-containing polyelectrolytes. J. Mater. Sci. 2002, 37, 4615–4620. [Google Scholar] [CrossRef]
- Nechtschein, M.; Santier, C.; Travers, J.P.; Chroboczek, J.; Alix, A.; Ripert, M. Water effects in polyaniline: NMR and transport properties. Synth. Met. 1987, 18, 311–316. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Zhan, X.; Ling, M. A novel resistive-type humidity sensor based on poly(p-diethynylbenzene). J. Appl. Polym. Sci. 1999, 74, 2010–2015. [Google Scholar] [CrossRef]
- Liu, J.; Agarwal, M.; Varahramyan, K.; Berney, E.S.; Hodo, W.D. Polymer-based microsensor for soil moisture measurement. Sens. Actuators B Chem. 2008, 129, 599–604. [Google Scholar] [CrossRef]
- Skabara, P.J.; Li, L.; Vilela, F.; Uttamchandani, D.; Forgie, J. Miniature humidity micro-sensor based on organic conductive polymer—Poly(3,4-ethylenedioxythiophene). Micro Nano Lett. 2009, 4, 84–87. [Google Scholar]
- Roman, C.; Bodea, O.; Prodan, N.; Levi, A.; Cordos, E.; Manoviciu, I. A capacitive-type humidity sensor using crosslinked poly(methylmethacrylate-co-(2 hydroxypropyl)-methacrylate). Sens Actuators B Chem. 1995, 25, 710–713. [Google Scholar] [CrossRef]
- Boudaden, J.; Steinmaßl, M.; Endres, H.E.; Drost, A.; Eisele, I.; Kutter, C.; Müller-Buschbaum, P. Polyimide-based capacitive humidity sensor. Sensors 2018, 18, 1516. [Google Scholar] [CrossRef]
- Reddy, A.S.G.; Narakathu, B.B.; Atashbar, M.Z.; Rebros, M.; Rebrosova, E.; Joyce, M.K. Fully printed flexible humidity sensor. Procedia Eng. 2011, 25, 120–123. [Google Scholar] [CrossRef]
- Amin, E.M.; Karmakar, N.C.; Winther-Jensen, B. Polyvinyl-alcohol (PVA)-based RF humidity sensor in microwave frequency. Prog. Electromagn. Res. B 2013, 54, 149–166. [Google Scholar] [CrossRef]
- Yeo, J.; Kwon, Y. Humidity sensing performance of defected ground structure-based microwave sensors coated with PMMA, PHEMA, and PVA. Microw. Opt. Technol. Lett. 2021, 63, 1194–1200. [Google Scholar] [CrossRef]
- Eyebe, G.A.; Bideau, B.; Boubekeur, N.; Loranger, É.; Domingue, F. Environmentally-friendly cellulose nanofibre sheets for humidity sensing in microwave frequencies. Sens. Actuators B Chem. 2017, 245, 484–492. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Khan, M.; Rehman, M.M.; Khan, S.A.; Saqib, M.; Kim, W.Y. Characterization and performance evaluation of fully biocompatible gelatin-based humidity sensor for health and environmental monitoring. Front. Mater. 2023, 10, 1233136. [Google Scholar] [CrossRef]
- Shapardanis, S.; Hudpeth, M.; Kaya, T. Gelatin as a new humidity sensing material: Characterization and limitations. AIP Adv. 2014, 4, 127132. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Burlaka, A.; Paz, J.; Salavagione, H.J.; Gonzalez, J.C.; Hernandez, R. Compact polyelectrolyte hydrogels of gelatin and chondroitin sulfate as ion’s mobile media in sustainable all-solid state electrochemical devices. Mater. Adv. 2020, 1, 2526–2535. [Google Scholar] [CrossRef]
- Vatanpour, V.; Teber, O.O.; Mehrabi, M.; Koyuncu, I. Polyvinyl alcohol-based separation membranes: A comprehensive review on fabrication techniques, applications and future prospective. Mater. Today Chem. 2023, 28, 101381. [Google Scholar] [CrossRef]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin nanoparticles and their biofunctionalization. In Nanotechnologies for the Life Sciences: Online; Wiley-VCH: Weinheim, Germany, 2007; pp. 330–352. [Google Scholar] [CrossRef]
- Gelatin Handbook, Gelatin Manufacturers Institute of America Members 2019. Available online: http://www.gelatin-gmia.com/uploads/1/1/8/4/118450438/gmia_gelatin_manual_2019.pdf (accessed on 23 August 2024).
- Su, L.; Vélez, P.; Casacuberta, P.; Muñoz-Enano, J.; Martín, F. Microwave humidity sensor for early detection of sweat and urine leakage. Electronics 2023, 12, 2276. [Google Scholar] [CrossRef]
- Velez, P.; Su, L.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martin, F. Microwave microfluidic sensor based on a microstrip splitter/combiner configuration and split ring resonators (SRRs) for dielectric characterization of liquids. IEEE Sens. J. 2017, 17, 6589–6598. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.-I. High-sensitivity microwave sensor based on an interdigital-capacitor-shaped defected ground structure for permittivity characterization. Sensors 2019, 19, 498. [Google Scholar] [CrossRef] [PubMed]
- Electromagnetic Simulation Solvers, CST Studio Suite. Available online: https://www.3ds.com/products-services/simulia/products/cst-studio-suite/solvers/ (accessed on 23 August 2024).
- Hong, J.S.; Karyamapudi, B.M. A general circuit model for defected ground structures in planar transmission lines. IEEE Microw. Wirel. Compon. Lett. 2005, 15, 706–708. [Google Scholar] [CrossRef]
- Saber, O.; Abu-Abdeen, M.; Aljaafari, A.; Mazher, J.; Ahmed, M.M.; BoNagma, O. New preparation approach, electrical and mechanical properties of poly(vinyl alcohol)-loaded graphene films. J. Thermoplast. Compos. Mater. 2021, 34, 1504–1522. [Google Scholar] [CrossRef]
- Gareev, K.G.; Bagrets, V.S.; Golubkov, V.A.; Ivanitsa, M.G.; Khmelnitskiy, I.K.; Luchinin, V.V.; Mikhailova, O.N.; Testov, D.O. Synthesis and characterization of polyaniline-based composites for electromagnetic compatibility of electronic devices. Electronics 2020, 9, 734. [Google Scholar] [CrossRef]
- Lu, D.; Maasch, M.; Penirschke, A.; Zheng, Y.; Damm, C.; Jakoby, R. Broadband permittivity characterization of polyvinyl-alcohol film for humidity sensing applications. IEEE Trans. Microw. Theory Tech. 2016, 64, 3255–3263. [Google Scholar] [CrossRef]


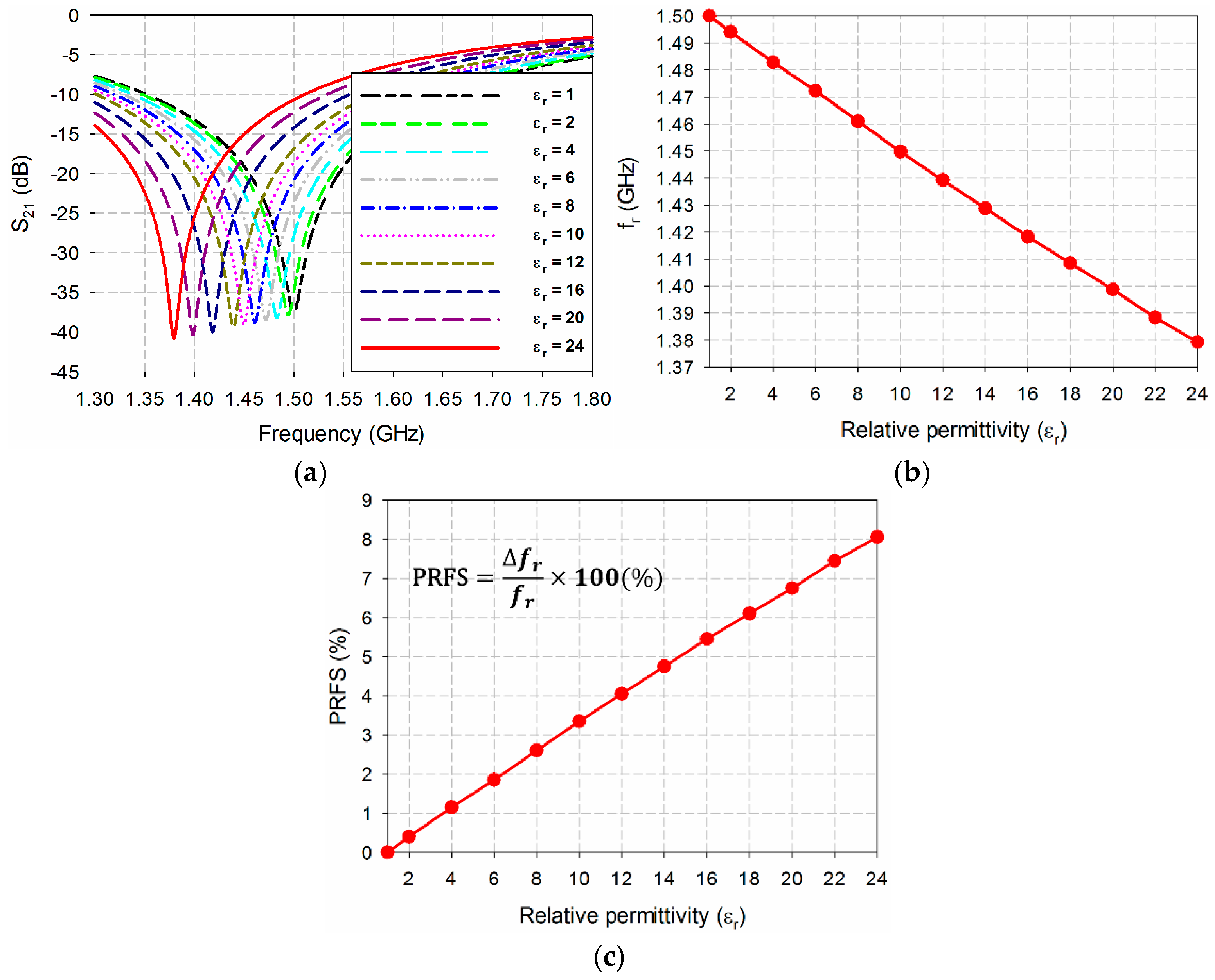



| εr = 1 | εr = 2 | εr = 4 | εr = 6 | εr = 8 | εr = 10 | εr = 12 | εr = 14 | εr = 16 | εr = 18 | εr = 20 | εr = 22 | εr = 24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fr (GHz) | 1.5 | 1.4940 | 1.4828 | 1.4723 | 1.4610 | 1.4497 | 1.4393 | 1.4288 | 1.4183 | 1.4085 | 1.3987 | 1.3883 | 1.3793 |
| PRFS (%) | 0 | 0.40 | 1.15 | 1.85 | 2.60 | 3.35 | 4.05 | 4.75 | 5.45 | 6.10 | 6.75 | 7.45 | 8.05 |
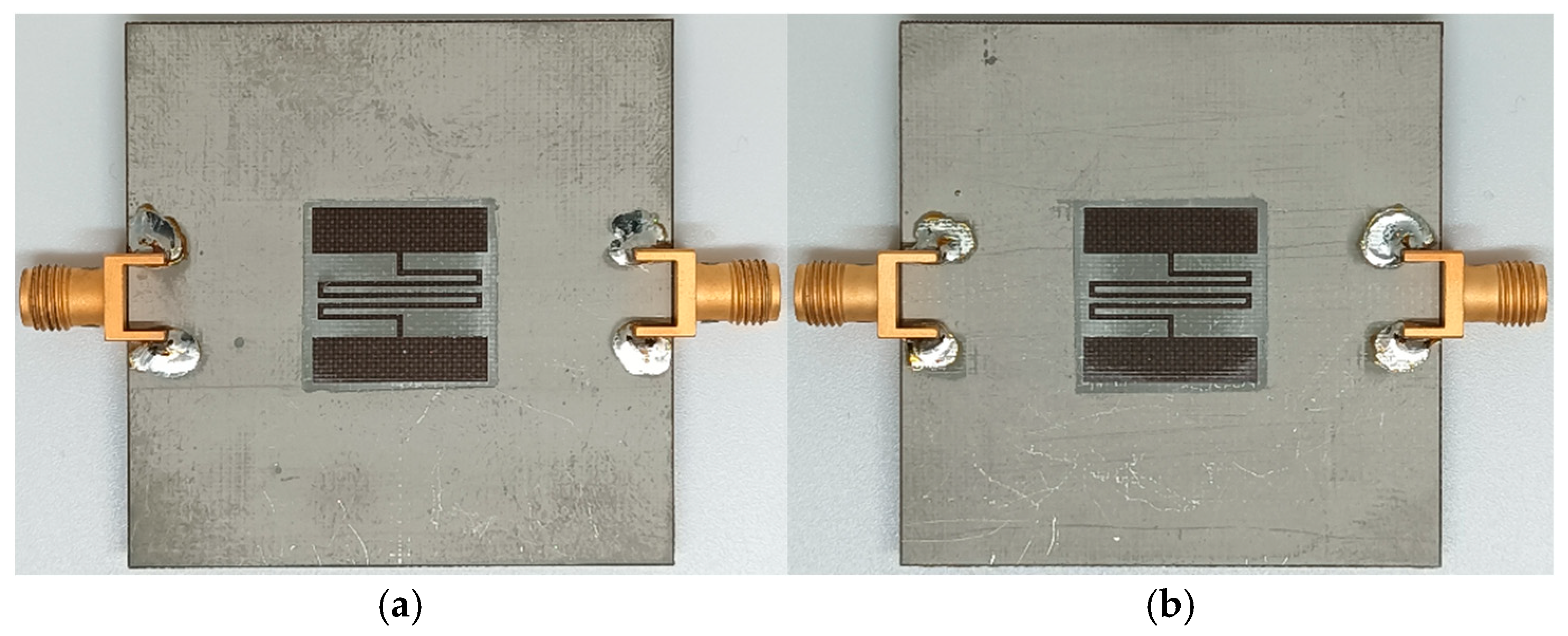
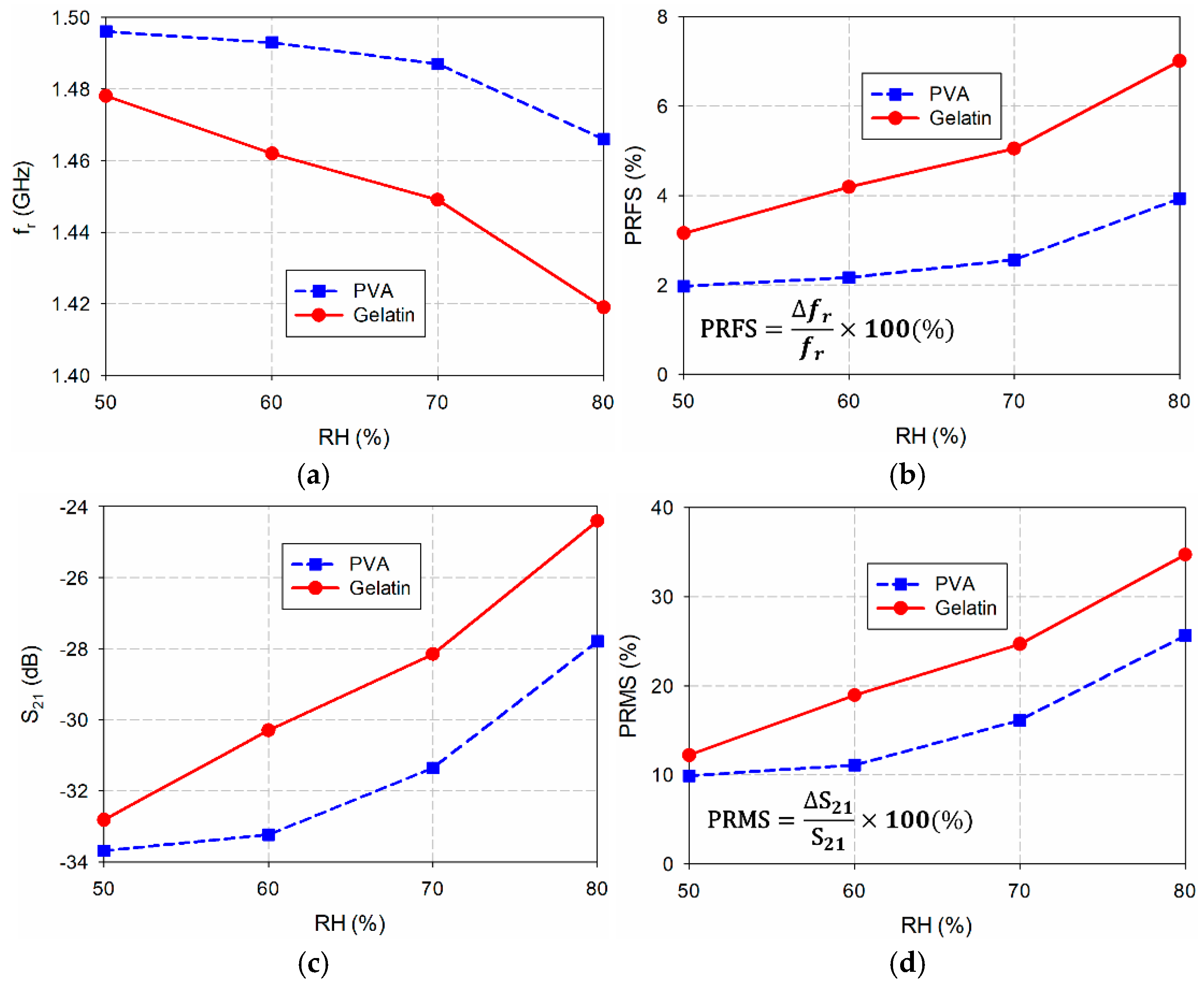
| PVA | Unloaded | RH50 | RH60 | RH70 | RH80 |
|---|---|---|---|---|---|
| fr (GHz) | 1.526 | 1.496 | 1.493 | 1.487 | 1.466 |
| PRFS (%) | 0 | 1.97 | 2.16 | 2.56 | 3.93 |
| S21 (dB) | −37.38 | −33.69 | −33.24 | −31.36 | −27.79 |
| PRMS (%) | 0 | 9.89 | 11.09 | 16.12 | 25.66 |
| Gelatin | Unloaded | RH50 | RH60 | RH70 | RH80 |
| fr (GHz) | 1.526 | 1.478 | 1.462 | 1.449 | 1.419 |
| PRFS (%) | 0 | 3.15 | 4.19 | 5.05 | 7.01 |
| S21 (dB) | −37.38 | −32.82 | −30.30 | −28.15 | −24.41 |
| PRMS (%) | 0 | 12.21 | 18.95 | 24.69 | 34.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, J.; Kwon, Y. Gelatin-Coated High-Sensitivity Microwave Sensor for Humidity-Sensing Applications. Sensors 2024, 24, 6286. https://doi.org/10.3390/s24196286
Yeo J, Kwon Y. Gelatin-Coated High-Sensitivity Microwave Sensor for Humidity-Sensing Applications. Sensors. 2024; 24(19):6286. https://doi.org/10.3390/s24196286
Chicago/Turabian StyleYeo, Junho, and Younghwan Kwon. 2024. "Gelatin-Coated High-Sensitivity Microwave Sensor for Humidity-Sensing Applications" Sensors 24, no. 19: 6286. https://doi.org/10.3390/s24196286
APA StyleYeo, J., & Kwon, Y. (2024). Gelatin-Coated High-Sensitivity Microwave Sensor for Humidity-Sensing Applications. Sensors, 24(19), 6286. https://doi.org/10.3390/s24196286







