Lure Monitoring for Mediterranean Fruit Fly Traps Using Air Quality Sensors
Abstract
1. Introduction
Sensors on Smart Traps for Mediterranean Fruit Fly
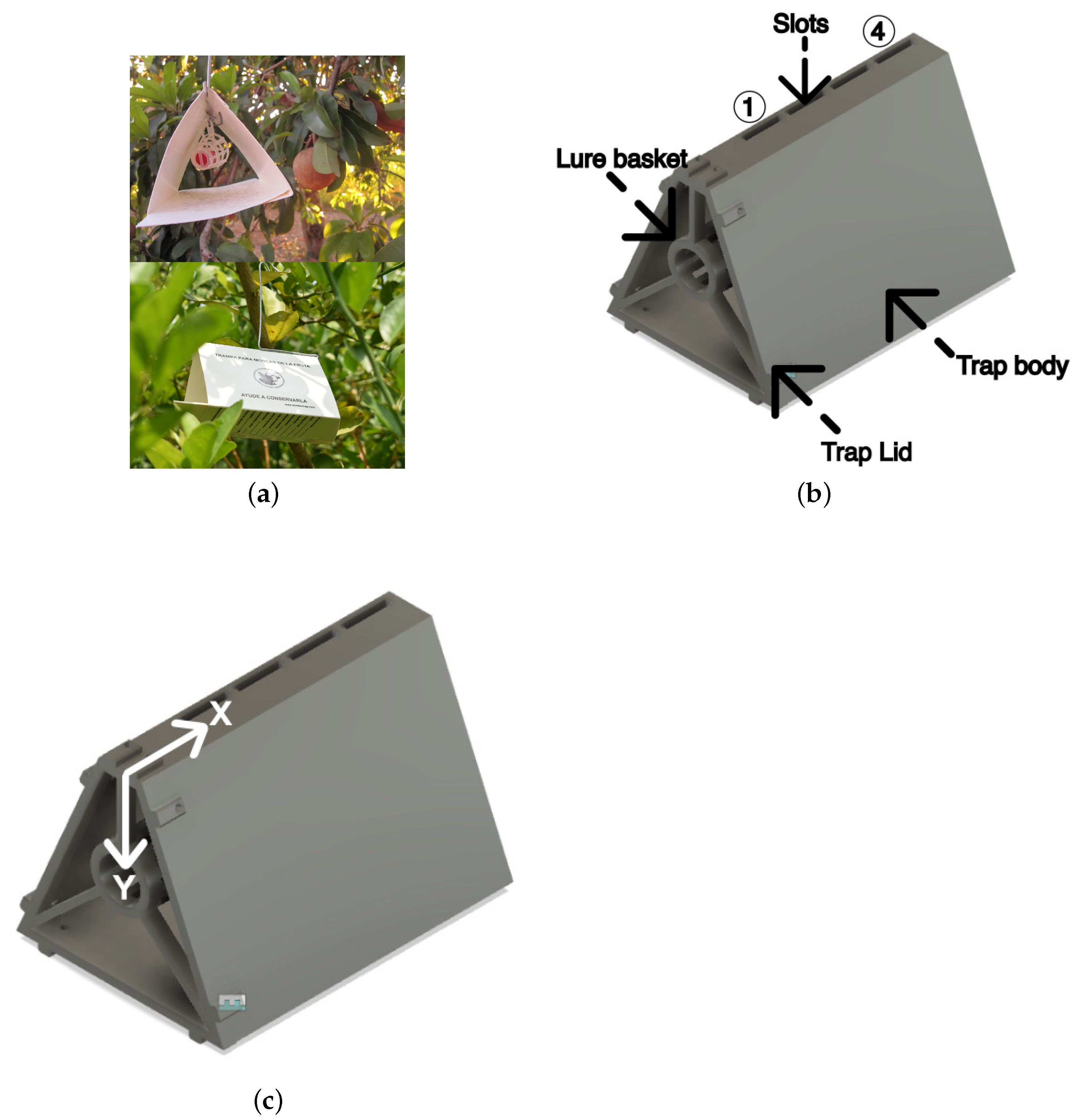
2. Materials and Methods
2.1. Sensors and Hardware Setup
2.1.1. Experimental Design
2.1.2. Sensor Placement and Distance Variation
2.1.3. Data Collection and Analysis
3. Results
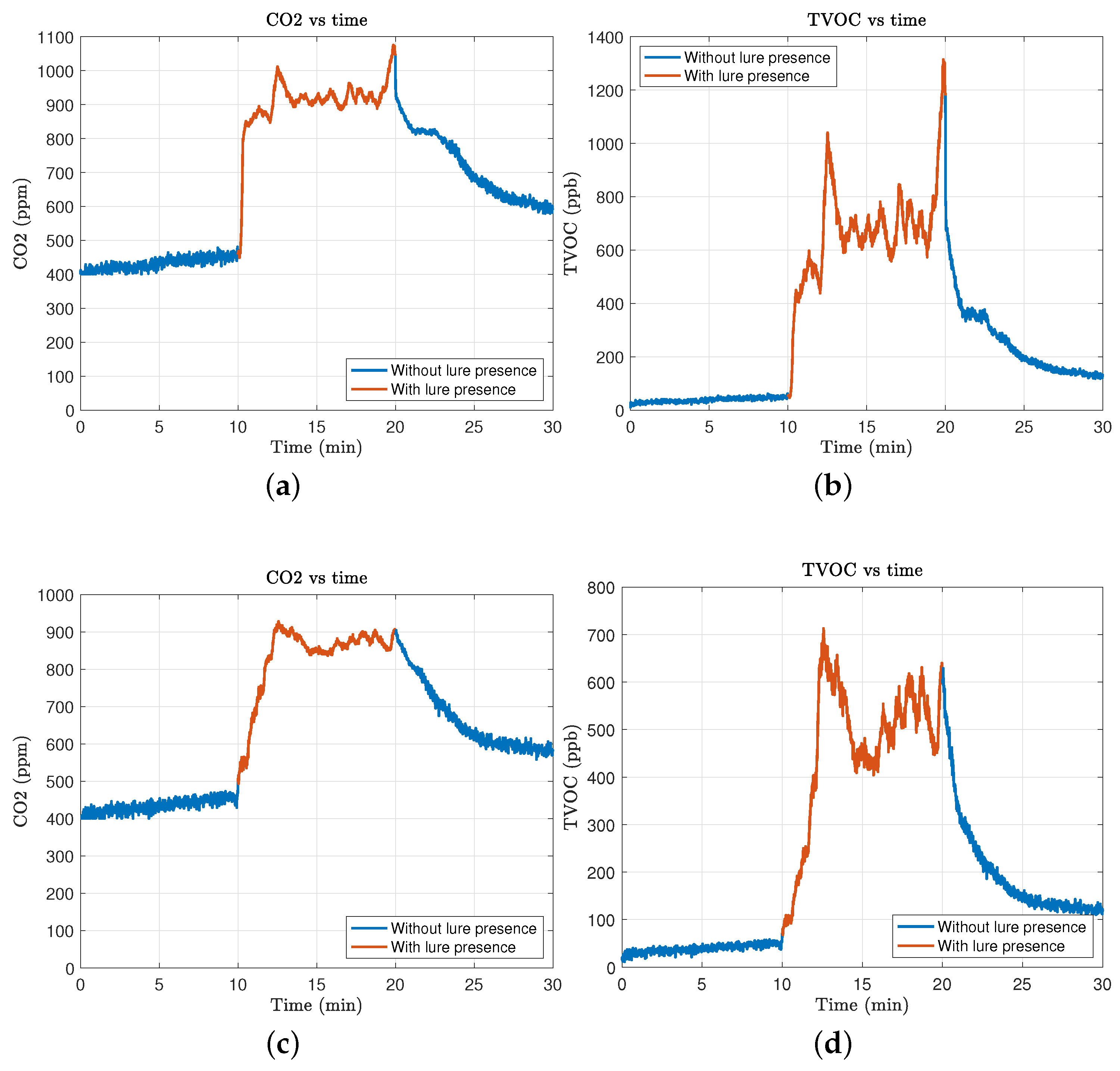
3.1. Sensor Performance Evaluation
- SGP30 Sensor Findings (Figure 2a,b):
- -
- eCO2 Detection: The sensor failed to show any significant change in eCO2 levels, remaining at baseline values around 500 ppm regardless of the lure presence.
- -
- TVOC Detection: The sensor responded to the lure presence with an increase in TVOC measurement, reaching up to 140 ppb. However, the response was unstable, fluctuating significantly with environmental changes.
- ENS160 Sensor Findings (Figure 2c,d):
- -
- eCO2 Detection: The ENS160 showed a clear response to the lure with eCO2 levels increasing by an average of 200 ppm above the ambient baseline, providing a reliable indicator of lure presence.
- -
- TVOC Detection: This sensor detected TVOC concentrations consistently above the baseline, with an average increase of 300 ppb when exposed to the lure. The measurements were stable across multiple tests, with a low variance.
3.2. Distance-Based Sensor Performance
- 1 cm distance: At this proximity, both sensors showed heightened sensitivity, but the ENS160 readings exhibited a tendency towards saturation, suggesting that too close a placement may lead to overestimation of lure concentrations (Figure 3c,d).
- 2 cm distance: This distance was found to be optimal, offering a balance between sensitivity and accuracy, with clear differentiation between baseline and lure-present states (Figure 3a,b).
- 3 cm distance: At this range, the effectiveness of the sensors decreased slightly, with lower but still detectable increases in both eCO2 and TVOC levels compared to closer ranges (Figure 2c,d).
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Analog-to-Digital Converter |
| ARM | Advanced RISC Machine |
| CAD | Computer-Aided Design |
| CDFA | California Department of Food and Agriculture |
| CNN | Convolutional Neural Network |
| FFT | Fast Fourier Transform |
| FID | Flame Ionization Detector |
| FMCW | Frequency Modulated Continuous Wave |
| GPS | Global Positioning System |
| GSM | Global System for Mobile Communications |
| HAL | Hardware Abstraction Layer |
| HCP | Host Control Platform |
| KB | Kilobytes |
| IDE | Integrated Development Environment |
| PA | Precision Agriculture |
| PID | Photoionization Detector |
| PLA | Polylactic Acid |
| MH | MegaHertz |
| MOX | Metal Oxide |
| NDIR | Non-Dispersive Infrared |
| LED | Light-Emitting Diode |
| VOC | Volatile Organic Compounds |
| SQL | Structured Query Language |
| RAM | Random Access Memory |
References
- Manrakhan, A.; Daneel, J.H.; Beck, R.; Love, C.N.; Gilbert, M.J.; Virgilio, M.; Meyer, M.D. Effects of Male Lure Dispensers and Trap Types For Monitoring of Ceratitis Capitata and Bactrocera dorsalis (Diptera: Tephritidae). Pest Manag. Sci. 2021, 77, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Cardim Ferreira Lima, M.; Damascena de Almeida Leandro, M.E.; Valero, C.; Pereira Coronel, L.C.; Gonçalves Bazzo, C.O. Automatic Detection and Monitoring of Insect Pests—A Review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
- Huang, R.; Yao, T.; Zhan, C.; Zhang, G.; Zheng, Y. A Motor-Driven and Computer Vision-Based Intelligent E-Trap for Monitoring Citrus Flies. Agriculture 2021, 11, 460. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D. Vertical-Looking Radar: A New Tool for Monitoring High-Altitude Insect Migration. Bioscience 2003, 53, 503–511. [Google Scholar] [CrossRef]
- Diller, Y.; Shamsian, A.; Shaked, B.; Altman, Y.; Danziger, B.C.; Manrakhan, A.; Serfontein, L.; Bali, E.; Wernicke, M.; Egartner, A.; et al. A real-time remote surveillance system for fruit flies of economic importance: Sensitivity and image analysis. J. Pest Sci. 2022, 96, 611–622. [Google Scholar] [CrossRef]
- Guarnieri, A.; Maini, S.; Molari, G.; Rondelli, V. Automatic trap for moth detection in integrated pest management. Bull. Insectol. 2011, 64, 247–251. [Google Scholar]
- Martin, B.; Shaby, S.M.; Premi, M. Studies on Acoustic Activity of Red Palm Weevil the Deadly Pest on Coconut Crops. Procedia Mater. Sci. 2015, 10, 455–466. [Google Scholar] [CrossRef]
- Nangai, V.L.; Martin, B. Interpreting the Acoustic Characteristics of Rpw Towards Its Detection—A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012178. [Google Scholar] [CrossRef]
- Dosunmu, O.G.; Herrick, N.J.; Haseeb, M.; Hix, R.L.; Mankin, R.W. Acoustic Detectability of Rhynchophorus cruentatus (Coleoptera: Dryophthoridae). Fla. Entomol. 2014, 97, 431–438. [Google Scholar] [CrossRef]
- Liu, B.; Hu, Z.; Zhao, Y.; Bai, Y.; Wang, Y. Recognition of Pyralidae Insects Using Intelligent Monitoring Autonomous Robot Vehicle in Natural Farm Scene. arXiv 2019, arXiv:1903.10827. [Google Scholar]
- Wang, R.; Hu, C.; Fu, X.; Long, T.; Zeng, T. Micro-Doppler measurement of insect wing-beat frequencies with W-band coherent radar. Sci. Rep. 2017, 7, 1396. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.S.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Moraes, F.S.; Nava, D.E.; Scheunemann, T.; Rosa, V. Development of an Optoelectronic Sensor for Detecting and Classifying Fruit Fly (Diptera: Tephritidae) for Use in Real-Time Intelligent Traps. Sensors 2019, 19, 1254. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Gao, J.; Lei, Q.; Zhou, Y. A Vision-Based Counting and Recognition System for Flying Insects in Intelligent Agriculture. Sensors 2018, 18, 1489. [Google Scholar] [CrossRef]
- Liao, M.S.; Chuang, C.L.; Lin, T.S.; Chen, C.P.; Zheng, X.Y.; Chen, P.; Liao, K.C.; Jiang, J. Development of an autonomous early warning system for Bactrocera dorsalis (Hendel) outbreaks in remote fruit orchards. Comput. Electron. Agric. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Shaked, B.; Amore, A.; Ioannou, C.; Valdés, F.; Alorda, B.; Papanastasiou, S.; Goldshtein, E.; Shenderey, C.; Leza, M.; Pontikakos, C.; et al. Electronic traps for detection and population monitoring of adult fruit flies (Diptera: Tephritidae). J. Appl. Entomol. 2018, 142, 43–51. [Google Scholar] [CrossRef]
- Doitsidis, L.; Fouskitakis, G.N.; Varikou, K.N.; Rigakis, I.I.; Chatzichristofis, S.A.; Papafilippaki, A.K.; Birouraki, A.E. Remote monitoring of the Bactrocera oleae (Gmelin) (Diptera: Tephritidae) population using an automated McPhail trap. Comput. Electron. Agric. 2017, 137, 69–78. [Google Scholar] [CrossRef]
- Goldshtein, E.; Cohen, Y.; Hetzroni, A.; Gazit, Y.; Timar, D.; Rosenfeld, L.; Grinshpon, Y.; Hoffman, A.; Mizrach, A. Development of an automatic monitoring trap for Mediterranean fruit fly (Ceratitis capitata) to optimize control applications frequency. Comput. Electron. Agric. 2017, 139, 115–125. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.I.; Tatlas, N.A. Automated Surveillance of Fruit Flies. Sensors 2017, 17, 110. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.I.; Vidakis, N.; Petousis, M.; Weber, M. Affordable Bimodal Optical Sensors to Spread the Use of Automated Insect Monitoring. J. Sens. 2018, 2018, 3949415. [Google Scholar] [CrossRef]
- Haff, R.P.; Saranwong, S.; Thanapase, W.; Janhiran, A.; Kasemsumran, S.; Kawano, S. Automatic image analysis and spot classification for detection of fruit fly infestation in hyperspectral images of mangoes. Postharvest Biol. Technol. 2013, 86, 23–28. [Google Scholar] [CrossRef]
- Uzun, Y.; Tolun, M.R.; Eyyuboğlu, H.T.; Sari, F. An ıntelligent system for detecting Mediterranean fruit fly [Medfly; Ceratitis Capitata (Wiedemann)]. J. Agric. Eng. 2022, 53. [Google Scholar] [CrossRef]
- Kalamatianos, R.; Karydis, I.; Doukakis, D.; Avlonitis, M. DIRT: The Dacus Image Recognition Toolkit. J. Imaging 2018, 4, 129. [Google Scholar] [CrossRef]
- Jiang, J.; Tseng, C.L.; Lu, F.M.; Yang, E.C.; Wu, Z.S.; Chen, C.P.; Lin, S.H.; Lin, K.; Liao, C.S. A GSM-based remote wireless automatic monitoring system for field information: A case study for ecological monitoring of the oriental fruit fly, Bactrocera dorsalis (Hendel). Comput. Electron. Agric. 2008, 62, 243–259. [Google Scholar] [CrossRef]
- Hernández Rosas, M.; Espinosa Flores-Verdad, G.; Peregrina Barreto, H.; Liedo, P.; Altamirano Robles, L. Shadow Effect for Small Insect Detection by W-Band Pulsed Radar. Sensors 2023, 23, 9169. [Google Scholar] [CrossRef]
- Mcgovern, T.P.; Cunningham, R.T.; Leonhardt, B.A. Attractiveness of trans-Trimedlure and Its Four Isomers in Field Tests with the Mediterranean Fruit Fly (Diptera: Tephritidae). J. Econ. Entomol. 1987, 80, 617–620. [Google Scholar] [CrossRef]
- Jang, E.B.; Light, D.M.; Dickens, J.C.; Mcgovern, T.P.; Nagata, J.T. Electroantennogram responses of mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae) to trimedlure and itstrans isomers. J. Chem. Ecol. 1989, 15, 2219–2231. [Google Scholar] [CrossRef]
- Khatib, M.; Haick, H. Sensors for volatile organic compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar] [CrossRef]
- Dervieux, E.; Théron, M.; Uhring, W. Carbon dioxide sensing—Biomedical applications to human subjects. Sensors 2021, 22, 188. [Google Scholar] [CrossRef]
- Price, D.J. Carbon Dioxide. In Hamilton & Hardy’s Industrial Toxicology; Wiley: Hoboken, NJ, USA, 2015; pp. 305–308. [Google Scholar]
- Montoya, P.; Toledo, J.; Hernández, E. (Eds.) Mosca de la Fruta: Fundamentos y Procedimientos para su Manejo. 2010; S y G Editores, 2019. Available online: https://www.researchgate.net/publication/332446868_Montoya_P_J_Toledo_E_Hernandez_Eds_Mosca_de_la_Fruta_Fundamentos_y_Procedimientos_para_su_Manejo_2010 (accessed on 16 August 2024).
- Lewis, T.; Macaulay, E.D.M. Design and elevation of sex-attractant traps for pea moth, Cydia nigricana (Steph.) and the effect of plume shape on catches. Ecol. Entomol. 1976, 1, 175–187. [Google Scholar] [CrossRef]

| Sensor Measures | 1 cm | 2 cm | 3 cm |
|---|---|---|---|
| CO2 | |||
| Mean (ppm) | 785.5 ± 86.1 | 905.7 ± 82.3 | 831.5 ± 97.4 |
| Max/Min (ppm) | 943/449 | 1075/449 | 928/493 |
| Range | 494 | 626 | 435 |
| 95% CI | 6.8 | 6.5 | 7.7 |
| TVOC | |||
| Mean (ppb) | 336.2 ± 133.6 | 665.5 ± 188.7 | 458.5 ± 153.4 |
| Max/Min (ppb) | 769/46 | 1314/46 | 713/68 |
| Range | 723 | 1268 | 645 |
| 95% CI | 10.6 | 14.9 | 12.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández Rosas, M.; Espinosa Flores-Verdad, G.; Peregrina Barreto, H.; Liedo, P.; Altamirano Robles, L. Lure Monitoring for Mediterranean Fruit Fly Traps Using Air Quality Sensors. Sensors 2024, 24, 6348. https://doi.org/10.3390/s24196348
Hernández Rosas M, Espinosa Flores-Verdad G, Peregrina Barreto H, Liedo P, Altamirano Robles L. Lure Monitoring for Mediterranean Fruit Fly Traps Using Air Quality Sensors. Sensors. 2024; 24(19):6348. https://doi.org/10.3390/s24196348
Chicago/Turabian StyleHernández Rosas, Miguel, Guillermo Espinosa Flores-Verdad, Hayde Peregrina Barreto, Pablo Liedo, and Leopoldo Altamirano Robles. 2024. "Lure Monitoring for Mediterranean Fruit Fly Traps Using Air Quality Sensors" Sensors 24, no. 19: 6348. https://doi.org/10.3390/s24196348
APA StyleHernández Rosas, M., Espinosa Flores-Verdad, G., Peregrina Barreto, H., Liedo, P., & Altamirano Robles, L. (2024). Lure Monitoring for Mediterranean Fruit Fly Traps Using Air Quality Sensors. Sensors, 24(19), 6348. https://doi.org/10.3390/s24196348






