An Electrically Small Patch Antenna Sensor for Salt Concentration Measurement of NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dielectric Properties of Saline Solution
2.2. Design of the Patch Antenna Sensor
3. Results
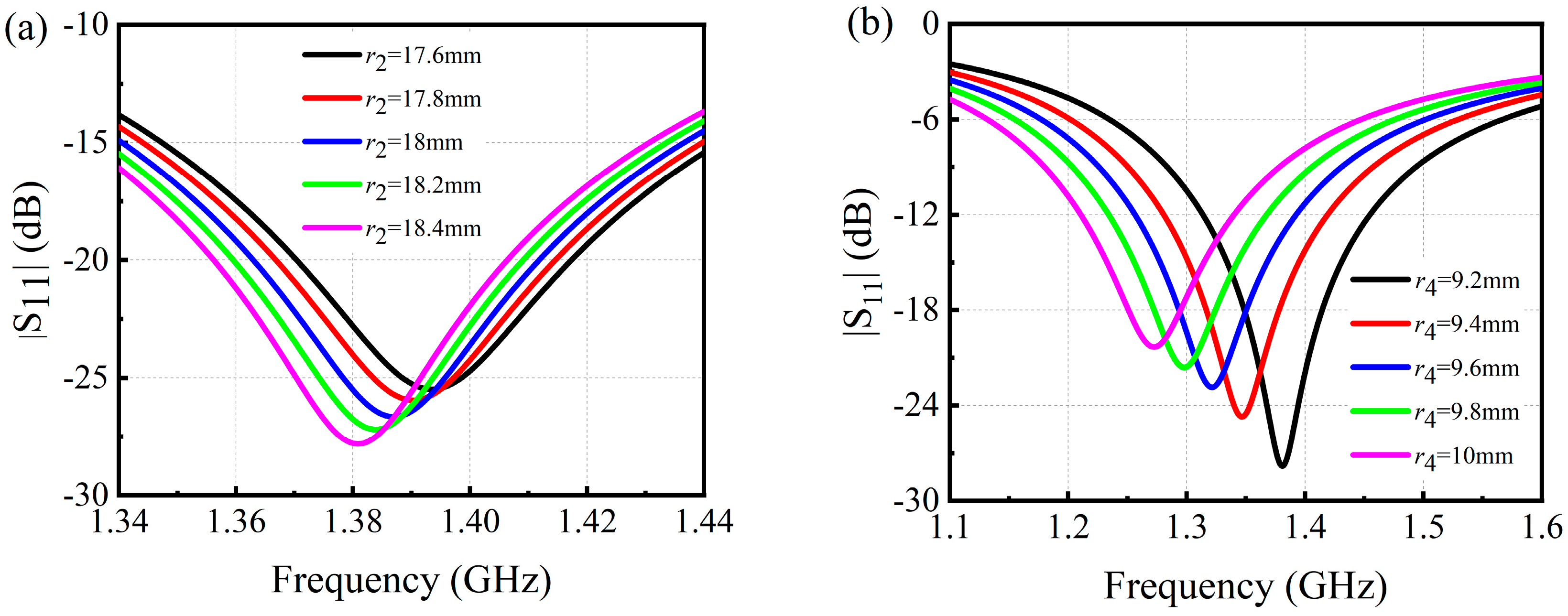
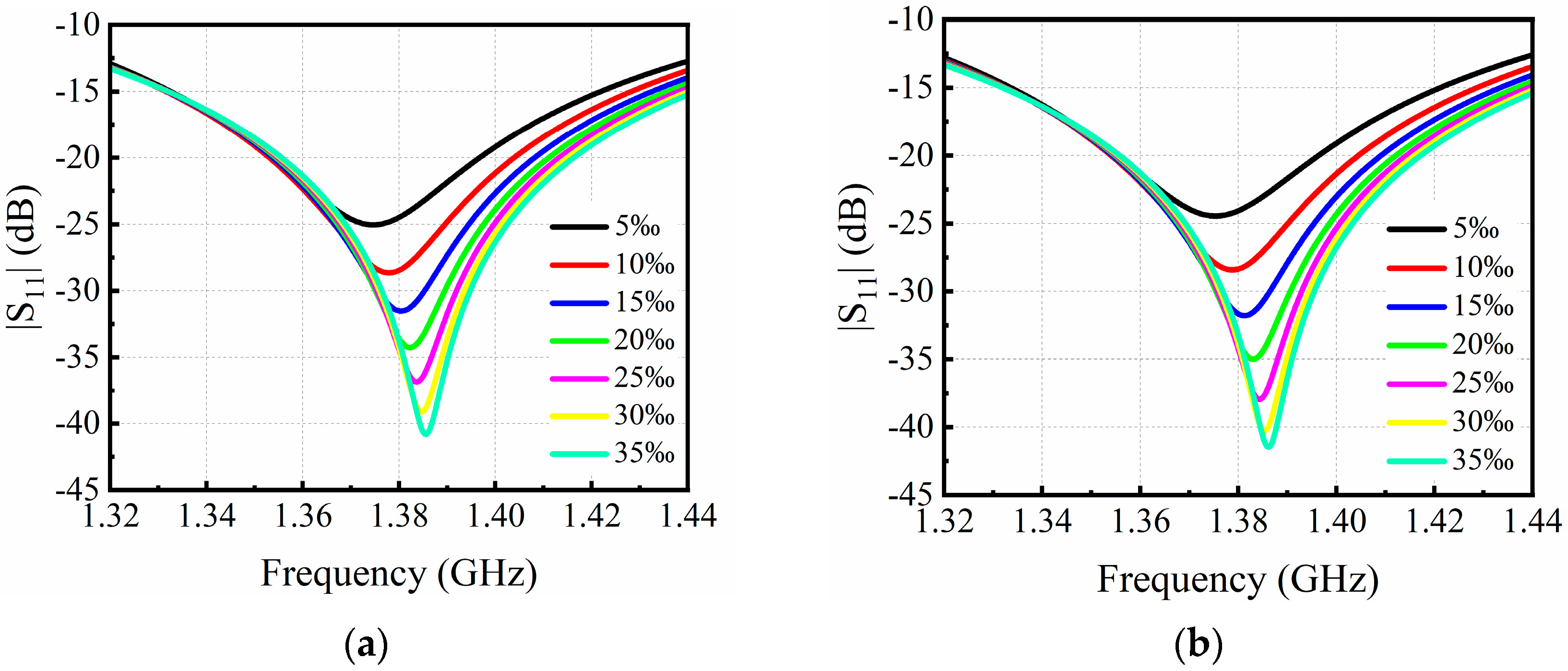
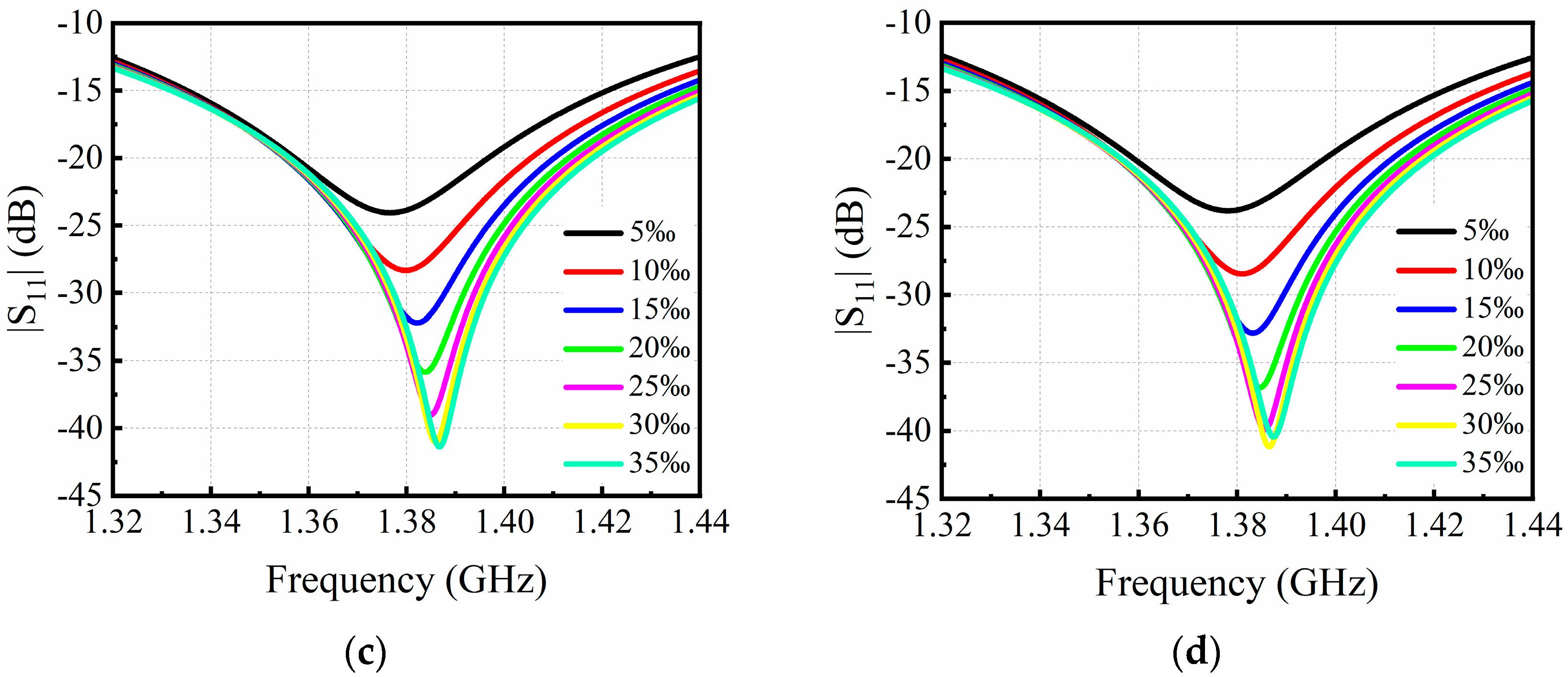
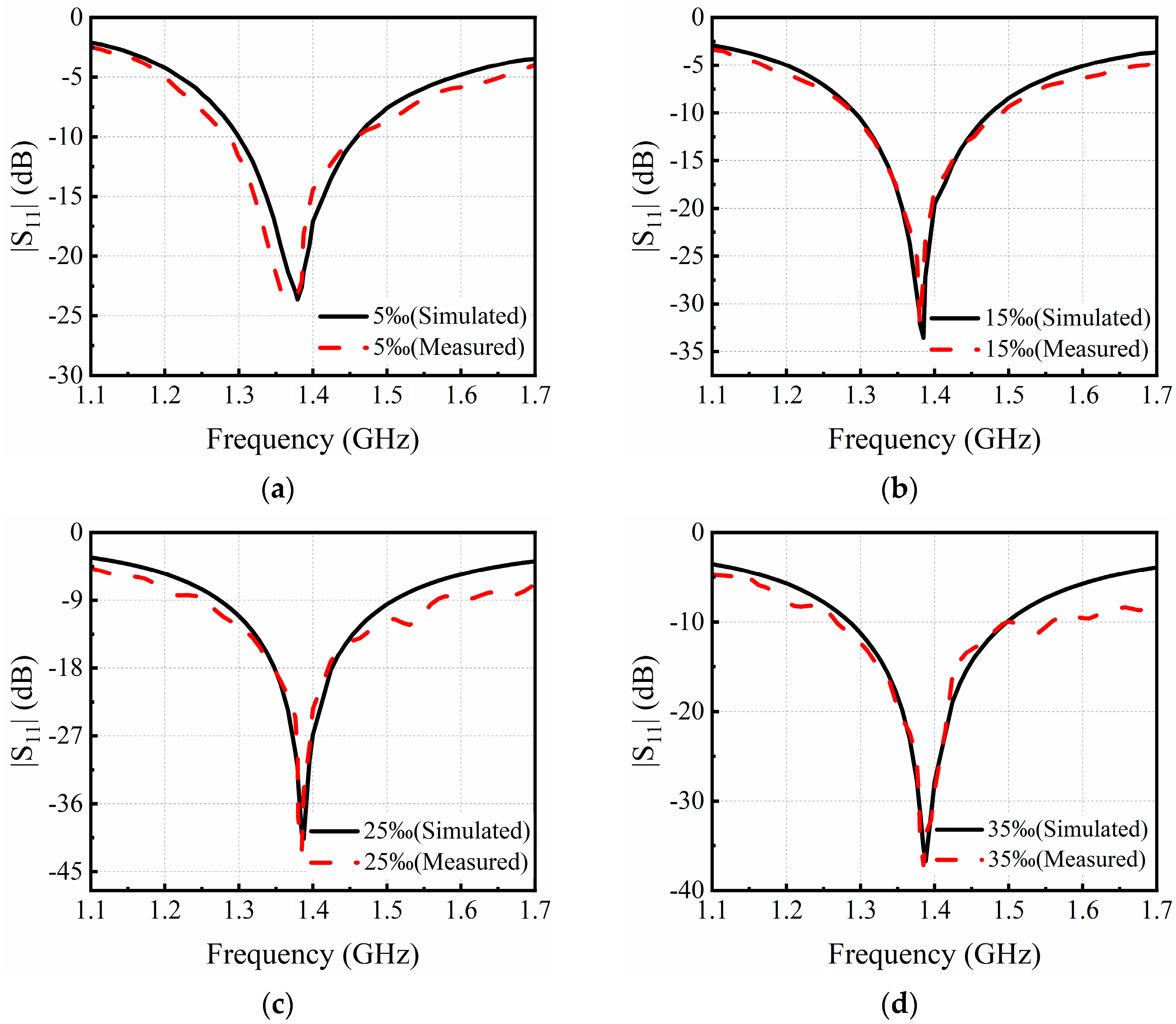
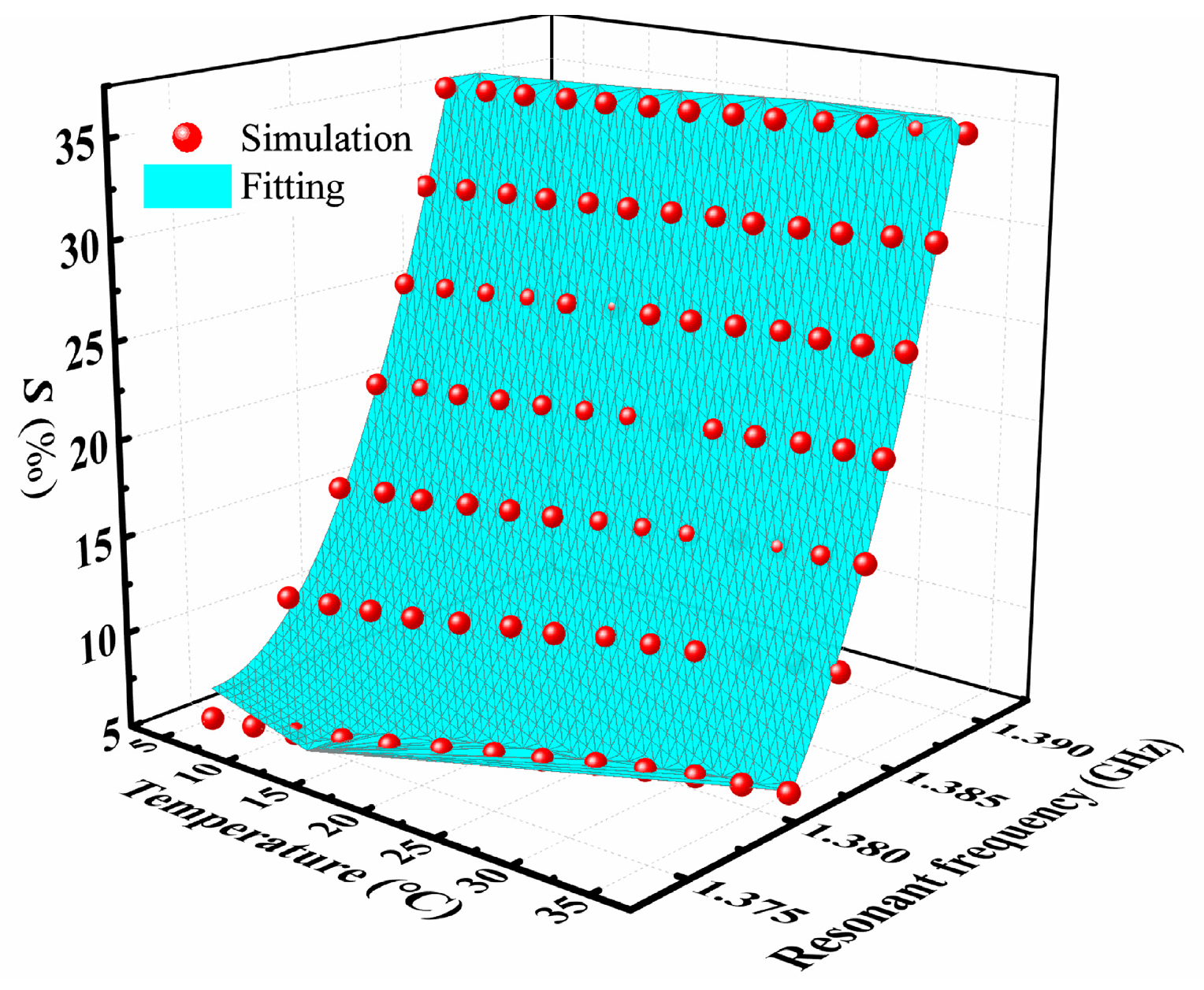
3.1. Simulation Results
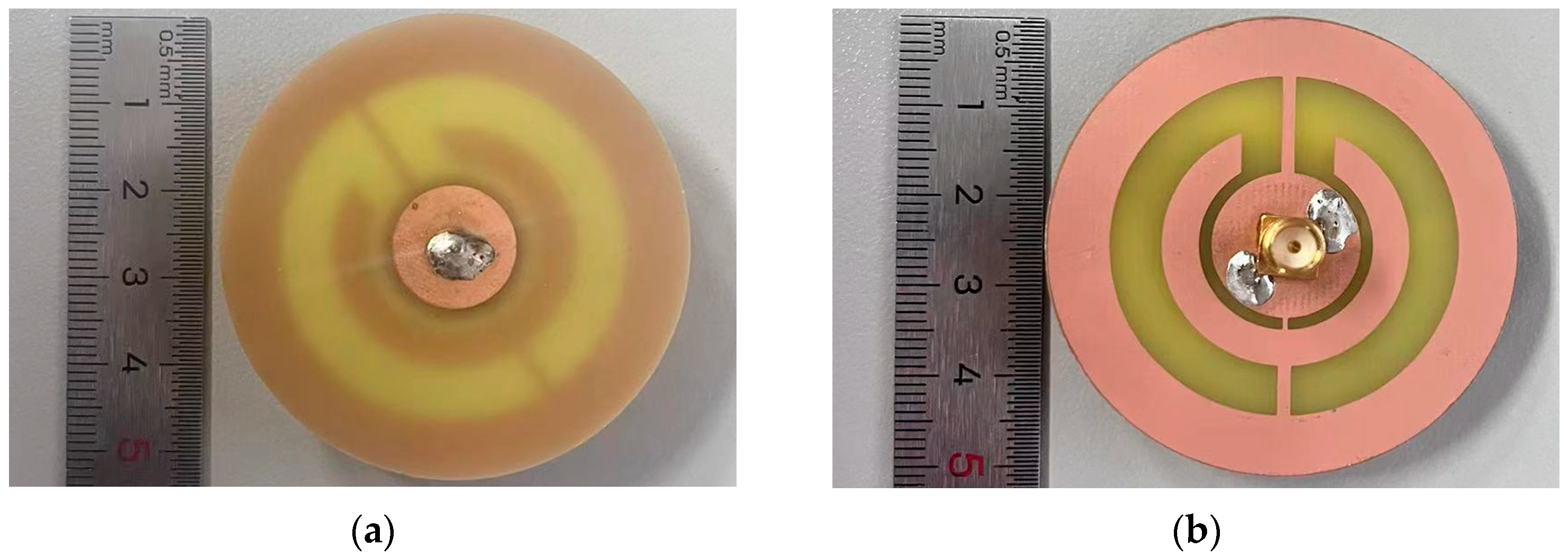
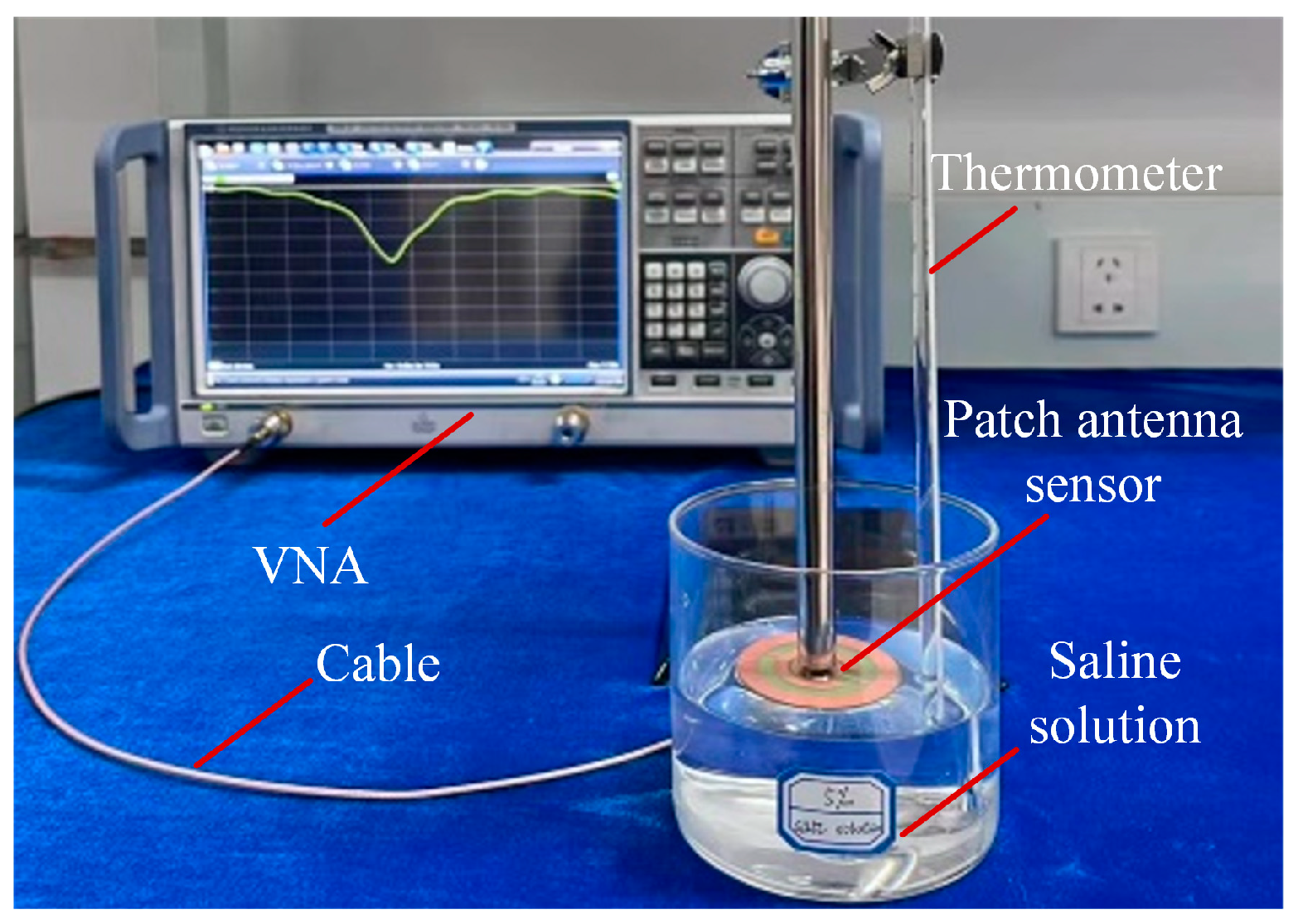
3.2. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, R.W.; Dhaun, N.; Bailey, M.A. The impact of excessive salt intake on human health. Nat. Rev. Nephrol. 2022, 18, 321–335. [Google Scholar] [CrossRef]
- Ruttanakorn, K.; Phadungcharoen, N.; Laiwattanapaisal, W.; Chinsriwongkul, A.; Rojanarata, T. Smartphone-based technique for the determination of a titration equivalence point from an RGB linear-segment curve with an example application to miniaturized titration of sodium chloride injections. Talanta 2021, 233, 122602. [Google Scholar] [CrossRef]
- Sappati, K.K.; Bhadra, S. Printed acoustic sensor for low concentration volatile organic compound monitoring. IEEE Sens. J. 2021, 21, 9808–9818. [Google Scholar] [CrossRef]
- Bottomley, P.A.; Hardy, C.J.; Roemer, P.B. Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn. Reson. 1990, 14, 425–434. [Google Scholar] [CrossRef]
- Petibois, C.; Melin, A.-M.; Perromat, A.; Cazorla, G.; Déléris, G. Glucose and lactate concentration determination on single microsamples by Fourier-transform infrared spectroscopy. J. Lab. Clin. Med. 2000, 135, 210–215. [Google Scholar] [CrossRef]
- Hui, S.K.; Jang, H.; Gum, C.K.; Song, K.H.; Yong, H.K. A new design of inductive conductivity sensor for measuring electrolyte concentration in industrial field. Sens. Actuators A Phys. 2019, 301, 111761. [Google Scholar]
- Thomason, S.J.; Bialkowski, K.S. Dielectric spectroscopy based determination of sugar content in solution. IEEE Sens. Lett. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Mondal, D.; Tiwari, N.K.; Akhtar, M.J. Microwave assisted non-invasive microfluidic biosensor for monitoring glucose concentration. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2019; pp. 1–4. [Google Scholar]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Aboott, D. High-sensitivity metamaterial-inspired sensor for microfluidic dielectric characterization. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Microwave reflective biosensor for glucose level detection in aqueous solutions. Sens. Actuators A Phys. 2020, 301, 111662. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Complementary split-ring resonator-loaded microfluidic ethanol chemical sensor. Sensors 2016, 16, 1802. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Wu, J.K.; Wang, X.; Kim, N.Y.; Gu, X.F.; Liang, J.G. Specific detection of organic and inorganic solution based on microwave resonator array. IEEE Sens. J. 2022, 22, 10532–10540. [Google Scholar] [CrossRef]
- Hosseini, A.; Niksan, O.; Kazemi, K.K.; Jain, M.C.; Adhikari, K.K.; Zarifi, M.H. Planar sensing platform based on split ring resonators and microstrip Yagi-Uda antennas. IEEE Sens. J. 2023, 23, 24428–24437. [Google Scholar] [CrossRef]
- Nitika; Kaur, J.; Khanna, R. Novel monkey-wrench-shaped microstrip patch sensor for food evaluation and analysis. J. Sci. Food Agric. 2022, 102, 1443–1456. [Google Scholar] [CrossRef]
- Bhatti, M.H.; Jabbar, M.A.; Khan, M.A.; Massoud, Y. Low-cost microwave sensor for characterization and adulteration detection in edible oil. Appl. Sci. 2022, 12, 8665. [Google Scholar] [CrossRef]
- Gennarelli, G.; Romeo, S.; Scarfi, M.R.; Soldovieri, F. A microwave resonant sensor for concentration measurements of liquid solutions. IEEE Sens. J. 2013, 13, 1857–1864. [Google Scholar] [CrossRef]
- Chudpooti, N.; Duangrit, N.; Sangpet, P.; Akkaraekthalin, P.; Imberg, B.U.; Robertson, I.D.; Somjit, N. In-situ self-aligned NaCl- solution fluidic-integrated microwave sensors for industrial and biomedical applications. IEEE Access 2020, 8, 188897–188907. [Google Scholar] [CrossRef]
- Mao, Y.J.; Zhang, Y.J.; Chen, Z.R.; Tong, M.S. A noncontact microwave sensor based on cylindrical resonator for detecting Concentration of Liquid Solutions. IEEE Sens. J. 2020, 21, 1208–1214. [Google Scholar] [CrossRef]
- Jha, A.K.; Akhter, Z.; Tiwari, N.; Shafi, K.T.M.; Samant, H.; Akhtar, M.J.; Cifra, M. Broadband wireless sensing system for non-invasive testing of biological samples. IEEE J. Emer. Select. Top. Circuits Syst. 2018, 8, 251–259. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. A non-contact planar microwave sensor for detection of high-salinity water containing NaCl, KCl, CaCl2, MgCl2 and Na2CO3. Sens. Actuators B Chem. 2021, 331, 129355. [Google Scholar] [CrossRef]
- Rezki, Y.P.; Syahputra, R.F.; Ginting, D. Design and testing of circular metamaterial-based salinity sensors. Sci. Technol. Commun. J. 2023, 3, 79–84. [Google Scholar]
- Vélez, P.; Muñoz-Enano, J.; Gil, M.; Mata-Contreras, J.; Martín, F. Differential microfluidic sensors based on dumbbell-shaped defect ground structures in microstrip technology: Analysis, optimization, and applications. Sensors 2019, 19, 3189. [Google Scholar] [CrossRef] [PubMed]
- Velez, P.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Highly-sensitive microwave sensors based on open complementary split ring resonators (OCSRRs) for dielectric characterization and solute concentration measurement in liquids. IEEE Access 2018, 6, 48324–48338. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Xu, L. A transmission line sensor with sensitivity improved for detection of ionic concentration in microfluidic channel. IEEE Sens. J. 2021, 21, 24066–24074. [Google Scholar] [CrossRef]
- Banerjee, A.; Kumar, A.; Tiwari, N.K.; Akhtar, M.J. Design of bridge type C-band planar RF sensor for detection of solute concentration level in water. IEEE Sens. J. 2021, 21, 22670–22678. [Google Scholar] [CrossRef]
- Kálovics, M.; Iván, K.; Szabó, Z. Microfluidic mixing device with integrated dual band microwave sensor. IEEE Sens. J. 2023, 23, 15350–15360. [Google Scholar] [CrossRef]
- Lee, K.; Hassan, A.; Lee, C.H.; Bae, J. Microstrip patch sensor for salinity determination. Sensors 2017, 17, 2941. [Google Scholar] [CrossRef] [PubMed]
- Pechrkool, T.; Chudpooti, N.; Duangrit, N. Zeroth-order resonator based on mushroom-like structure for liquid mixture concentration sensing of sodium chloride solution. In Proceedings of the IEEE Conference on Antenna Measurements & Applications (CAMA), Kuta, Bali, Indonesia, 23–25 October 2019; pp. 222–224. [Google Scholar]
- Islam, M.T.; Rahman, M.N.; Singh, M.S.J.; Samsuzzaman, M. Detection of salt and sugar contents in water on the basis of dielectric properties using microstrip antenna-based sensor. IEEE Access 2018, 6, 4118–4126. [Google Scholar] [CrossRef]
- Rahman, M.N.; Islam, M.T.; Sobuz, M.S. Microwave measurement system to detect salt and sugar concentration. Microw. Opt. Technol. Lett. 2018, 60, 1772–1774. [Google Scholar] [CrossRef]
- Rahman, N.; Hassan, S.A.; Samsuzzaman; Singh, M.S.J.; Islam, M.T. Determination of salinity and sugar concentration using microwave sensor. Microw. Opt. Technol. Lett. 2018, 61, 361–364. [Google Scholar] [CrossRef]
- Njokweni, S.N.; Kumar, P. Salt and sugar detection system using a compact microstrip patch antenna. Int. J. Smart Sens. Intell. Syst. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- El Gharbi, M.; Martinez-Estrada, M.; Fernandez-Garcia, R.; Gil, I. Determination of salinity and sugar concentration by means of a circular-ring monopole textile antenna-based sensor. IEEE Sens. J. 2021, 21, 23751–23760. [Google Scholar] [CrossRef]
- Stogryn, A. Equations for calculating the dielectric constant of saline water. IEEE Trans. Microw. Theory Tech. 1971, 19, 733–736. [Google Scholar] [CrossRef]
- Klein, L.; Swift, C.T. An improved model for the dielectric constant of sea water at microwave frequencies. IEEE Trans. Antennas Propag. 1977, 25, 104–111. [Google Scholar] [CrossRef]
- Gadani, D.; Rana, V.; Bhatnagar, S.; Prajapati, A.; Vyas, A. Effect of salinity on the dielectric properties of water. Indian J. Pure Appl. Phys. 2012, 50, 405–410. [Google Scholar]
- Chu, L.J. Physical limitations of omni-directional antennas. J. Appl. Phys. 1948, 19, 1163. [Google Scholar] [CrossRef]
- Wen, G.Y. A new derivation of the upper bounds for the ratio of gain to Q. IEEE Trans. Antennas Propag. 2012, 60, 3488–3490. [Google Scholar]
- Wen, G.Y. Physical limitations of antenna. IEEE Trans. Antennas Propag. 2003, 51, 2116–2123. [Google Scholar]
- Grimes, D.M.; Grimes, C.A. Bandwidth and Q of antennas radiating TE and TM modes. IEEE Trans. Electromag. Compat. 1995, 37, 217–226. [Google Scholar] [CrossRef]
- Hansen, R.C.; Collin, R.E. A new Chu formula for Q. IEEE Antennas Propag. Mag. 2009, 51, 38–41. [Google Scholar] [CrossRef]
- Thiele, G.A.; Detweiler, P.L.; Penno, R.P. On the lower bound of the radiation Q for electrically small antennas. IEEE Trans. Antennas Propag. 2003, 51, 1263–1269. [Google Scholar] [CrossRef]
- McLean, J.S. The radiative properties of electrically-small antennas. In Proceedings of the IEEE Symposium on Electromagnetic Compatibility, Chicago, IL, USA, 22–26 August 1994; pp. 320–324. [Google Scholar]
- Tang, M.C.; Ziolkowski, R.W. A study of low-profile, broadside radiation, efficient, electrically small antennas based on complementary split ring resonators. IEEE Trans. Antennas Propag. 2013, 61, 4419–4430. [Google Scholar] [CrossRef]



| Parameter | Value (mm) | Parameter | Value (mm) |
|---|---|---|---|
| r1 | 25 | w1 | 1.4 |
| r2 | 18.4 | w2 | 0.53 |
| r3 | 13.1 | w3 | 4.2 |
| r4 | 9.2 | w1 | 1.6 |
| r5 | 7.9 | w2 | 0.1 |
| r6 | 6.6 | h1 | 1.6 |
| Ref. No. | f0 (GHz) | Sensing Parameter | Max. Sensitivity | Electrical Size |
|---|---|---|---|---|
| [16] | 1.91 | S21 | 0.015 (kHz/(mg/L)) | 0.7 |
| [19] | 2.93 | S21 | 0.122 (kHz/(mg/L)) | 1.89 |
| [20] | 0.65 | S11 | 0.063 (kHz/(mg/L)) | 0.16 |
| [21] | 0.25 | S11 | 0.0183 (kHz/(mg/L)) | 0.14 |
| [28] | 5.65 | S11 | N/A | 0.57 |
| This work | 1.37 | S11 | 0.367 (kHz/(mg/L)) | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Chen, C.; Sun, X.; Ding, G.; Wang, S. An Electrically Small Patch Antenna Sensor for Salt Concentration Measurement of NaCl Solution. Sensors 2024, 24, 6389. https://doi.org/10.3390/s24196389
Zhu J, Chen C, Sun X, Ding G, Wang S. An Electrically Small Patch Antenna Sensor for Salt Concentration Measurement of NaCl Solution. Sensors. 2024; 24(19):6389. https://doi.org/10.3390/s24196389
Chicago/Turabian StyleZhu, Jinfeng, Cheng Chen, Xiao Sun, Guowen Ding, and Shenyun Wang. 2024. "An Electrically Small Patch Antenna Sensor for Salt Concentration Measurement of NaCl Solution" Sensors 24, no. 19: 6389. https://doi.org/10.3390/s24196389







