Innovative Digital Phenotyping Method to Assess Body Representations in Autistic Adults: A Perspective on Multisensor Evaluation
Abstract
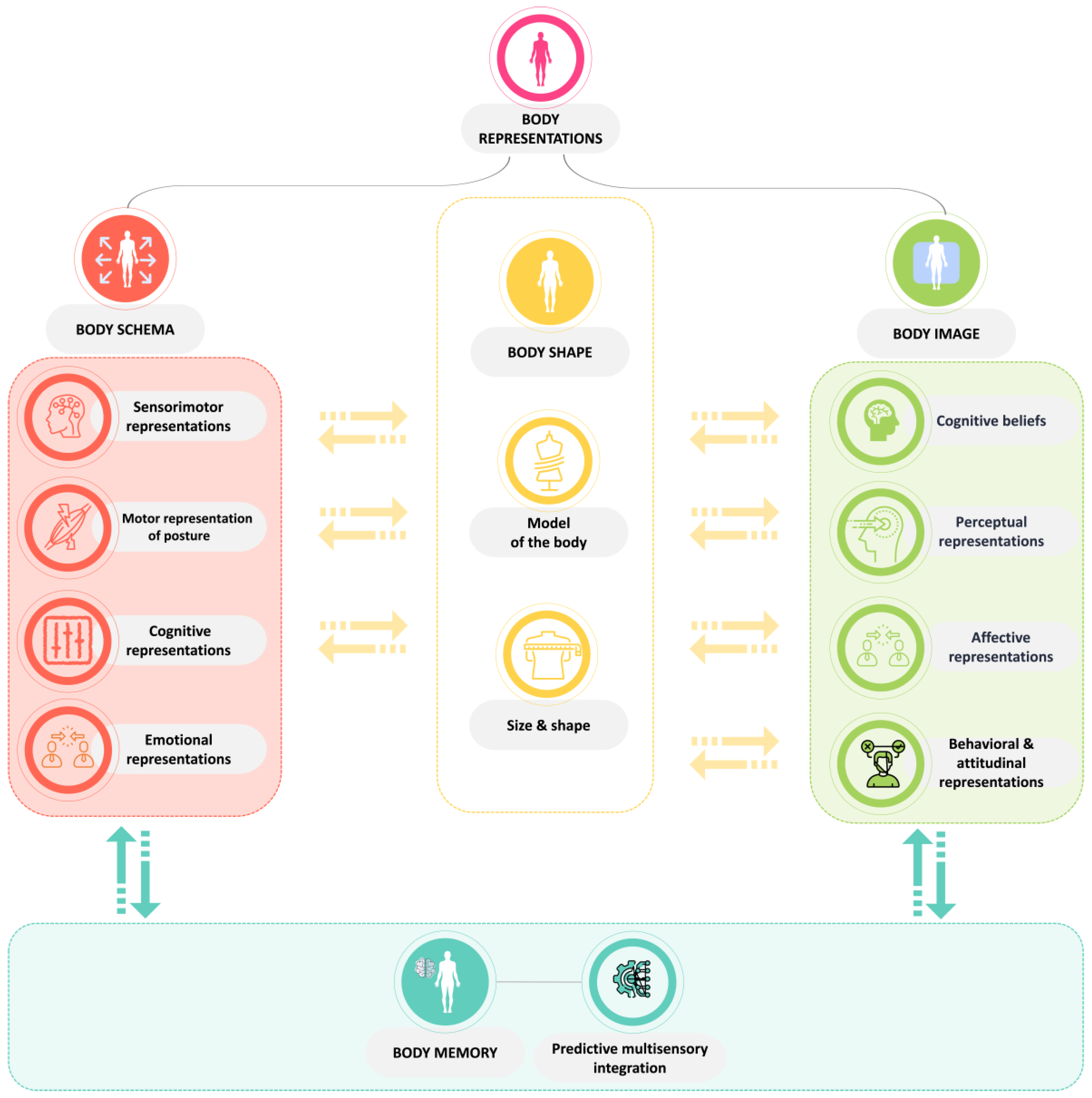
1. Context
2. New Paradigm Opportunity: The Digital Phenotyping Revolution
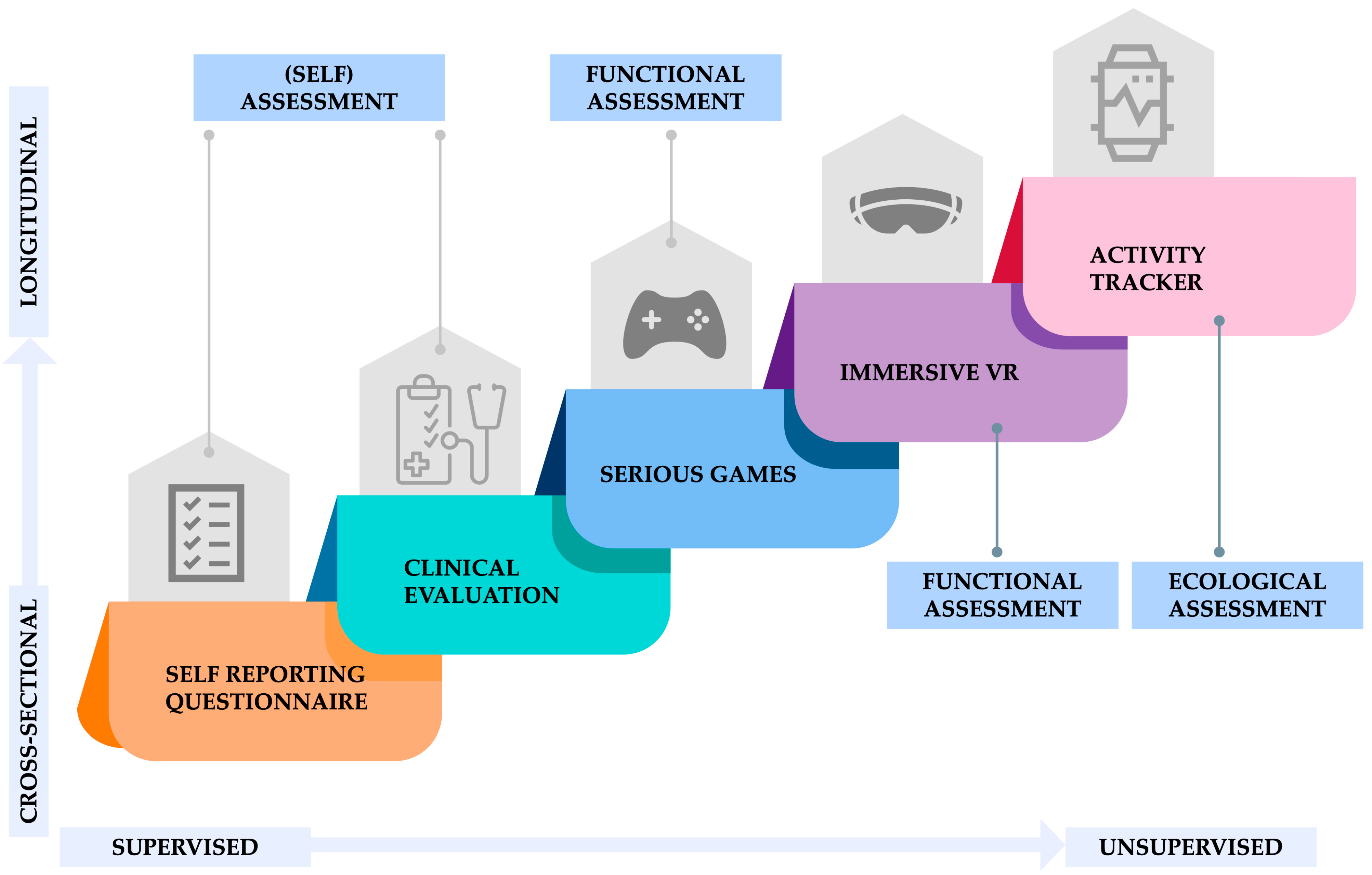
3. Data Collection Pipeline
3.1. Self-Reporting Questionnaire
3.2. Clinical Evaluation
3.3. Serious Games
3.4. Immersive Virtual Reality
3.5. Activity Trackers
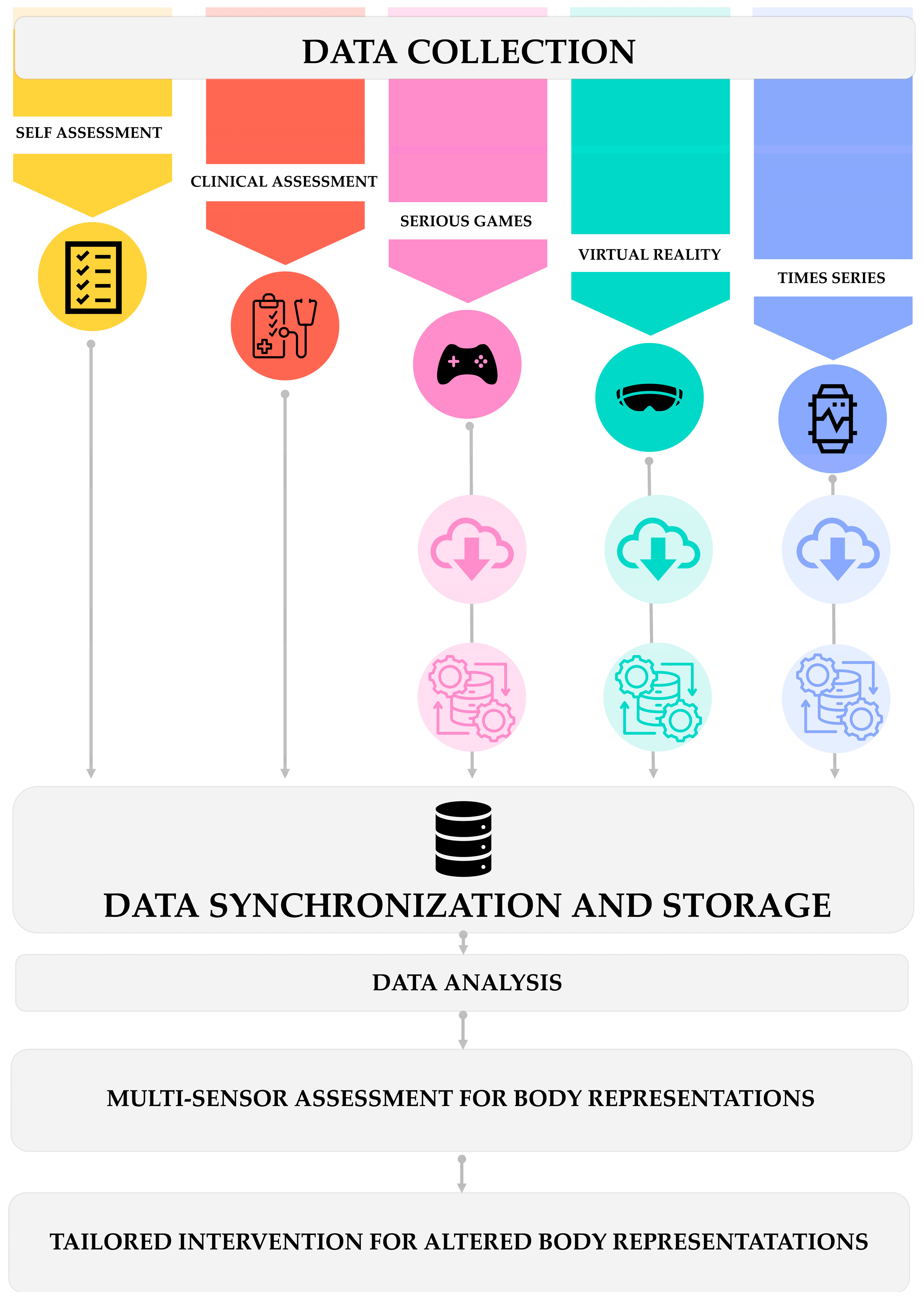
4. Data Management and Analysis Framework Development
4.1. Data Collection Standardization
4.2. Integration Architecture Design
4.3. Data Synchronization and Storage
4.4. Data Analysis
4.4.1. Exploratory Data Analysis
4.4.2. Statistical Modeling and Analysis
4.4.3. Artificial Intelligence: Between Machine Learning and Predictive Analytics
4.5. Feedback Mechanisms
4.6. Scalability and Flexibility
5. Challenges and Potential Pitfalls
5.1. Patients’ Perspectives
5.2. Technological Challenges
5.3. Data-Related Challenges
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. Off. J. Int. Soc. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Roman-Urrestarazu, A.; Dumas, G.; Warrier, V. Naming Autism in the Right Context. JAMA Pediatr. 2022, 176, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.J.; Boilson, M.; Rutherford, M.; Prior, S.; Johnston, L.; Maciver, D.; Forsyth, K. Neurodevelopmental Disorders and Neurodiversity: Definition of Terms from Scotland’s National Autism Implementation Team. Br. J. Psychiatry 2022, 221, 577–579. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Dietz, P.M.; Rose, C.E.; McArthur, D.; Maenner, M. National and State Estimates of Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 4258–4266. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-C.; Baron-Cohen, S. Identifying the Lost Generation of Adults with Autism Spectrum Conditions. Lancet Psychiatry 2015, 2, 1013–1027. [Google Scholar] [CrossRef]
- Hus Bal, V.; Kim, S.-H.; Cheong, D.; Lord, C. Daily Living Skills in Individuals with Autism Spectrum Disorder from 2 to 21 Years of Age. Autism Int. J. Res. Pract. 2015, 19, 774–784. [Google Scholar] [CrossRef]
- Auld, C.; Foley, K.; Cashin, A. Daily Living Skills of Autistic Adolescents and Young Adults: A Scoping Review. Aust. Occup. Ther. J. 2022, 69, 456–474. [Google Scholar] [CrossRef]
- Burling, J.M.; Kadambi, A.; Safari, T.; Lu, H. The Impact of Autistic Traits on Self-Recognition of Body Movements. Front. Psychol. 2019, 9, 2687. [Google Scholar] [CrossRef]
- Longhurst, P. Body Image and Autism: A Scoping Review. Res. Autism Spectr. Disord. 2023, 105, 102170. [Google Scholar] [CrossRef]
- Longhurst, P.; Aspell, J.; Todd, J.; Swami, V. “There’s No Separating My View of My Body from My Autism”: A Qualitative Study of Positive Body Image in Autistic Individuals. Body Image 2023, 48, 101655. [Google Scholar] [CrossRef]
- Sattin, D.; Parma, C.; Lunetta, C.; Zulueta, A.; Lanzone, J.; Giani, L.; Vassallo, M.; Picozzi, M.; Parati, E.A. An Overview of the Body Schema and Body Image: Theoretical Models, Methodological Settings and Pitfalls for Rehabilitation of Persons with Neurological Disorders. Brain Sci. 2023, 13, 1410. [Google Scholar] [CrossRef] [PubMed]
- Solano López, A.L.; Moore, S. Dimensions of Body-Awareness and Depressed Mood and Anxiety. West. J. Nurs. Res. 2019, 41, 834–853. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.F.; Laveway, K.; Campos, P.; de Carvalho, P.H.B. Body Image as a Global Mental Health Concern. Camb. Prisms Glob. Ment. Health 2023, 10, e9. [Google Scholar] [CrossRef] [PubMed]
- Möllmann, A.; Heinrichs, N.; Herwig, A. A Conceptual Framework on Body Representations and Their Relevance for Mental Disorders. Front. Psychol. 2024, 14, 1231640. [Google Scholar] [CrossRef]
- Gowen, E.; Earley, L.; Waheed, A.; Poliakoff, E. From “One Big Clumsy Mess” to “a Fundamental Part of My Character.” Autistic Adults’ Experiences of Motor Coordination. PLoS ONE 2023, 18, e0286753. [Google Scholar] [CrossRef]
- de Vignemont, F. Désenchanter le Corps: Aux Origines de la Conscience de Soi; Odile Jacob: Paris, France, 2023; ISBN 978-2-415-00472-9. [Google Scholar]
- Mandy, W.; Clarke, K.; McKenner, M.; Strydom, A.; Crabtree, J.; Lai, M.-C.; Allison, C.; Baron-Cohen, S.; Skuse, D. Assessing Autism in Adults: An Evaluation of the Developmental, Dimensional and Diagnostic Interview-Adult Version (3Di-Adult). J. Autism Dev. Disord. 2018, 48, 549–560. [Google Scholar] [CrossRef]
- Robins, D.L.; Casagrande, K.; Barton, M.; Chen, C.-M.A.; Dumont-Mathieu, T.; Fein, D. Validation of the Modified Checklist for Autism in Toddlers, Revised with Follow-up (M-CHAT-R/F). Pediatrics 2014, 133, 37–45. [Google Scholar] [CrossRef]
- Wetherby, A.M.; Brosnan-Maddox, S.; Peace, V.; Newton, L. Validation of the Infant-Toddler Checklist as a Broadband Screener for Autism Spectrum Disorders from 9 to 24 Months of Age. Autism Int. J. Res. Pract. 2008, 12, 487–511. [Google Scholar] [CrossRef]
- Stone, W.L.; Coonrod, E.E.; Turner, L.M.; Pozdol, S.L. Psychometric Properties of the STAT for Early Autism Screening. J. Autism Dev. Disord. 2004, 34, 691–701. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule-Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Lord, C.; Luyster, R.J.; Gotham, K.; Guthrie, W. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part II): Toddler Module; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Schopler, E.; Van Bourgondien, M.E.; Wellman, G.J.; Love, S.R. The Childhood Autism Rating Scale (2nd ed.) (CARS2). Los Angeles, CA Western Psychological Services. References-Scientific Research Publishing. 2010. Available online: https://scirp.org/reference/referencespapers?referenceid=1367654 (accessed on 16 February 2024).
- Kvig, E.I.; Nilssen, S. Does Method Matter? Assessing the Validity and Clinical Utility of Structured Diagnostic Interviews among a Clinical Sample of First-Admitted Patients with Psychosis: A Replication Study. Front. Psychiatry 2023, 14, 1076299. [Google Scholar] [CrossRef] [PubMed]
- Chahboun, S.; Stenseng, F.; Page, A.G. The Changing Faces of Autism: The Fluctuating International Diagnostic Criteria and the Resulting Inclusion and Exclusion—A Norwegian Perspective. Front. Psychiatry 2022, 13, 787893. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; McGrath, J. Autism Spectrum Disorders: Current Issues and Future Directions. Ir. J. Psychol. Med. 2022, 39, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Gabis, L.V.; Gross, R.; Barbaro, J. Editorial: Personalized Precision Medicine in Autism Spectrum-Related Disorders. Front. Neurol. 2021, 12, 730852. [Google Scholar] [CrossRef]
- The Digital Phenotype|Nature Biotechnology. Available online: https://www.nature.com/articles/nbt.3223 (accessed on 26 March 2024).
- Torous, J.; Kiang, M.V.; Lorme, J.; Onnela, J.-P. New Tools for New Research in Psychiatry: A Scalable and Customizable Platform to Empower Data Driven Smartphone Research. JMIR Ment. Health 2016, 3, e5165. [Google Scholar] [CrossRef] [PubMed]
- Daniels, K.; Vonck, S.; Robijns, J.; Spooren, A.; Hansen, D.; Bonnechère, B. Unveiling the Digital Phenotype: A Protocol for a Prospective Study on Physical Activity Behavior in Community-Dwelling Older Adults; Research Square Platform LLC: Durham, NC, USA, 2024. [Google Scholar]
- Bodenstein, K.C.; Paquin, V.; Sekhon, K.; Lesage, M.; Cinalioglu, K.; Rej, S.; Vahia, I.; Sekhon, H. Digital Markers of Mental Health Problems: Phenotyping Across Biological, Psychological, and Environmental Dimensions. In Biomarkers in Neuropsychiatry: A Primer; Teixeira, A.L., Rocha, N.P., Berk, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 105–122. ISBN 978-3-031-43356-6. [Google Scholar]
- Carmi, L.; Abbas, A.; Schultebraucks, K.; Galatzer-Levy, I.R. 10-Digital Phenotyping. In Mental Health in a Digital World; Stein, D.J., Fineberg, N.A., Chamberlain, S.R., Eds.; Global Mental Health in Practice; Academic Press: Cambridge, MA, USA, 2022; pp. 207–222. ISBN 978-0-12-822201-0. [Google Scholar]
- De La Fabián, R.; Jiménez-Molina, Á.; Pizarro Obaid, F. A Critical Analysis of Digital Phenotyping and the Neuro-Digital Complex in Psychiatry. Big Data Soc. 2023, 10, 20539517221149097. [Google Scholar] [CrossRef]
- Engle, R.L.; Mohr, D.C.; Holmes, S.K.; Seibert, M.N.; Afable, M.; Leyson, J.; Meterko, M. Evidence-Based Practice and Patient-Centered Care: Doing Both Well. Health Care Manage Rev. 2021, 46, 174–184. [Google Scholar] [CrossRef]
- Warmerdam, E.; Hausdorff, J.M.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-Term Unsupervised Mobility Assessment in Movement Disorders. Lancet Neurol. 2020, 19, 462–470. [Google Scholar] [CrossRef]
- Malik-Soni, N.; Shaker, A.; Luck, H.; Mullin, A.E.; Wiley, R.E.; Lewis, M.E.S.; Fuentes, J.; Frazier, T.W. Tackling Healthcare Access Barriers for Individuals with Autism from Diagnosis to Adulthood. Pediatr. Res. 2022, 91, 1028–1035. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism Spectrum Disorder. Lancet Lond. Engl. 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Rutherford, M.; Johnston, L. Perspective Chapter: Rethinking Autism Assessment, Diagnosis, and Intervention within a Neurodevelopmental Pathway Framework. In Autism Spectrum Disorders—Recent Advances and New Perspectives; Carotenuto, M., Ed.; IntechOpen: London, UK, 2023; ISBN 978-1-83768-342-0. [Google Scholar]
- Ali, D.; O’Brien, S.; Hull, L.; Kenny, L.; Mandy, W. “The Key to This Is Not so Much the Technology. It’s the Individual Who Is Using the Technology”: Perspectives on Telehealth Delivery for Autistic Adults during the COVID-19 Pandemic. Autism Int. J. Res. Pract. 2023, 27, 552–564. [Google Scholar] [CrossRef]
- Lee, K.; Lee, T.C.; Yefimova, M.; Kumar, S.; Puga, F.; Azuero, A.; Kamal, A.; Bakitas, M.A.; Wright, A.A.; Demiris, G.; et al. Using Digital Phenotyping to Understand Health-Related Outcomes: A Scoping Review. Int. J. Med. Inf. 2023, 174, 105061. [Google Scholar] [CrossRef]
- Irvine, B.; Elise, F.; Brinkert, J.; Poole, D.; Farran, E.K.; Milne, E.; Scerif, G.; Crane, L.; Remington, A. ‘A Storm of Post-It Notes’: Experiences of Perceptual Capacity in Autism and ADHD. Neurodiversity 2024, 2, 27546330241229004. [Google Scholar] [CrossRef]
- Schmalbach, I.; Schmalbach, B.; Zenger, M.; Berth, H.; Albani, C.; Petrowski, K.; Brähler, E. A Brief Assessment of Body Image Perception: Norm Values and Factorial Structure of the Short Version of the FKB-20. Front. Psychol. 2020, 11, 579783. [Google Scholar] [CrossRef]
- Williams, S.E.; Cumming, J.; Ntoumanis, N.; Nordin-Bates, S.M.; Ramsey, R.; Hall, C. Further Validation and Development of the Movement Imagery Questionnaire. J. Sport Exerc. Psychol. 2012, 34, 621–646. [Google Scholar] [CrossRef]
- Puyjarinet, F.; Jean-François, C.; Maury, L.; Régis, S. Développement et Validation d’un Questionnaire d’imagerie Motrice Sur Une Population Développementale Française: Le QUIMOT. ANAE—Approch. Neuropsychol. Apprentiss. Chez Enfant 2023, 183, 215–228. [Google Scholar]
- Cash, T.F.; Phillips, K.A.; Santos, M.T.; Hrabosky, J.I. Measuring “Negative Body Image”: Validation of the Body Image Disturbance Questionnaire in a Nonclinical Population. Body Image 2004, 1, 363–372. [Google Scholar] [CrossRef]
- Wilson, P.H.; Maruff, P.; Ives, S.; Currie, J. Abnormalities of Motor and Praxis Imagery in Children with DCD. Hum. Mov. Sci. 2001, 20, 135–159. [Google Scholar] [CrossRef]
- Kushnir, A.; Kachmar, O.; Bonnechère, B. STASISM: A Versatile Serious Gaming Multi-Sensor Platform for Personalized Telerehabilitation and Telemonitoring. Sensors 2024, 24, 351. [Google Scholar] [CrossRef]
- Alcañiz Raya, M.; Marín-Morales, J.; Minissi, M.E.; Teruel Garcia, G.; Abad, L.; Chicchi Giglioli, I.A. Machine Learning and Virtual Reality on Body Movements’ Behaviors to Classify Children with Autism Spectrum Disorder. J. Clin. Med. 2020, 9, 1260. [Google Scholar] [CrossRef]
- Mittal, P.; Bhadania, M.; Tondak, N.; Ajmera, P.; Yadav, S.; Kukreti, A.; Kalra, S.; Ajmera, P. Effect of Immersive Virtual Reality-Based Training on Cognitive, Social, and Emotional Skills in Children and Adolescents with Autism Spectrum Disorder: A Meta-Analysis of Randomized Controlled Trials. Res. Dev. Disabil. 2024, 151, 104771. [Google Scholar] [CrossRef]
- Chiappini, M.; Dei, C.; Micheletti, E.; Biffi, E.; Storm, F.A. High-Functioning Autism and Virtual Reality Applications: A Scoping Review. Appl. Sci. 2024, 14, 3132. [Google Scholar] [CrossRef]
- Carnett, A.; Neely, L.; Gardiner, S.; Kirkpatrick, M.; Quarles, J.; Christopher, K. Systematic Review of Virtual Reality in Behavioral Interventions for Individuals with Autism. Adv. Neurodev. Disord. 2023, 7, 426–442. [Google Scholar] [CrossRef]
- Stone, A.A.; Shiffman, S. Ecological Momentary Assessment (EMA) in Behavorial Medicine. Ann. Behav. Med. 1994, 16, 199–202. [Google Scholar] [CrossRef]
- Bonnechère, B. Serious Games in Physical Rehabilitation: From Theory to Practice; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-66121-6. [Google Scholar]
- Bonnechère, B.; Jansen, B.; Haack, I.; Omelina, L.; Feipel, V.; Van Sint Jan, S.; Pandolfo, M. Automated Functional Upper Limb Evaluation of Patients with Friedreich Ataxia Using Serious Games Rehabilitation Exercises. J. Neuroeng. Rehabil. 2018, 15, 87. [Google Scholar] [CrossRef]
- Bonnechère, B.; Bier, J.-C.; Van Hove, O.; Sheldon, S.; Samadoulougou, S.; Kirakoya-Samadoulougou, F.; Klass, M. Age-Associated Capacity to Progress When Playing Cognitive Mobile Games: Ecological Retrospective Observational Study. JMIR Serious Games 2020, 8, e17121. [Google Scholar] [CrossRef]
- Wagner, A.K. A Rehabilomics Framework for Personalized and Translational Rehabilitation Research and Care for Individuals with Disabilities: Perspectives and Considerations for Spinal Cord Injury. J. Spinal Cord Med. 2014, 37, 493. [Google Scholar] [CrossRef]
- Biocca, F.; Delaney, B. Immersive Virtual Reality Technology; Communication in the age of virtual reality; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1995; p. 124. ISBN 978-0-8058-1549-8. [Google Scholar]
- Huygelier, H.; Mattheus, E.; Abeele, V.V.; van Ee, R.; Gillebert, C.R. The Use of the Term Virtual Reality in Post-Stroke Rehabilitation: A Scoping Review and Commentary. Psychol. Belg. 2021, 61, 145–162. [Google Scholar] [CrossRef]
- Riva, G. Virtual Reality in Clinical Psychology. Compr. Clin. Psychol. 2022, 10, 91–105. [Google Scholar] [CrossRef]
- Pieri, L.; Tosi, G.; Romano, D. Virtual Reality Technology in Neuropsychological Testing: A Systematic Review. J. Neuropsychol. 2023, 17, 382–399. [Google Scholar] [CrossRef]
- Bourgeois, A.; Schnider, A.; Turri, F.; Ptak, R. Virtual Reality in the Rehabilitation of Cognitive Impairment after Stroke. Clin. Transl. Neurosci. 2023, 7, 3. [Google Scholar] [CrossRef]
- Feitosa, J.A.; Fernandes, C.A.; Casseb, R.F.; Castellano, G. Effects of Virtual Reality-Based Motor Rehabilitation: A Systematic Review of fMRI Studies. J. Neural Eng. 2022, 19, 011002. [Google Scholar] [CrossRef]
- Glaser, N.; Schmidt, M. Systematic Literature Review of Virtual Reality Intervention Design Patterns for Individuals with Autism Spectrum Disorders. Int. J. Hum. Comput. Interact. 2022, 38, 753–788. [Google Scholar] [CrossRef]
- Trincado-Alonso, F.; Dimbwadyo-Terrer, I.; de los Reyes-Guzmán, A.; López-Monteagudo, P.; Bernal-Sahún, A.; Gil-Agudo, Á. Kinematic Metrics Based on the Virtual Reality System Toyra as an Assessment of the Upper Limb Rehabilitation in People with Spinal Cord Injury. BioMed Res. Int. 2014, 2014, 904985. [Google Scholar] [CrossRef][Green Version]
- Kim, W.-S.; Cho, S.; Ku, J.; Kim, Y.; Lee, K.; Hwang, H.-J.; Paik, N.-J. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J. Clin. Med. 2020, 9, 3369. [Google Scholar] [CrossRef]
- Pielage, H.; Zekveld, A.A.; van de Ven, S.; Kramer, S.E.; Naber, M. The Pupil near Response Is Short Lasting and Intact in Virtual Reality Head Mounted Displays. J. Eye Mov. Res. 2022, 15. [Google Scholar] [CrossRef]
- Felix, R.B.; Rao, A.; Khalid, M.; Wang, Y.; Colloca, L.; Murthi, S.B.; Morris, N.A. Adjunctive Virtual Reality Pain Relief Following Traumatic Injury: Protocol for a Randomised within-Subjects Clinical Trial. BMJ Open 2021, 11, e056030. [Google Scholar] [CrossRef]
- Nyholm, B.; Obling, L.; Hassager, C.; Grand, J.; Møller, J.; Othman, M.; Kondziella, D.; Kjaergaard, J. Superior Reproducibility and Repeatability in Automated Quantitative Pupillometry Compared to Standard Manual Assessment, and Quantitative Pupillary Response Parameters Present High Reliability in Critically Ill Cardiac Patients. PLoS ONE 2022, 17, e0272303. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic Control of the Eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef] [PubMed]
- Okutucu, S.; Civelekler, M.; Aparci, M.; Sabanoglu, C.; Dikmetas, O.; Aksoy, H.; Yetis Sayin, B.; Oto, A. Computerized Dynamic Pupillometry Indices Mirrors the Heart Rate Variability Parameters. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2099–2105. [Google Scholar] [PubMed]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Zhang, J.; Morley, J.; Gallifant, J.; Oddy, C.; Teo, J.T.; Ashrafian, H.; Delaney, B.; Darzi, A. Mapping and Evaluating National Data Flows: Transparency, Privacy, and Guiding Infrastructural Transformation. Lancet Digit. Health 2023, 5, e737–e748. [Google Scholar] [CrossRef] [PubMed]
- Stausberg, J.; Harkener, S.; Jenetzky, E.; Jersch, P.; Martin, D.; Rupp, R.; Schönthaler, M. FAIR and Quality Assured Data—The Use Case of Trueness. Stud. Health Technol. Inform. 2022, 289, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Child, A.W.; Hinds, J.; Sheneman, L.; Buerki, S. Centralized Project-Specific Metadata Platforms: Toolkit Provides New Perspectives on Open Data Management within Multi-Institution and Multidisciplinary Research Projects. BMC Res. Notes 2022, 15, 106. [Google Scholar] [CrossRef]
- Hegselmann, S.; Storck, M.; Gessner, S.; Neuhaus, P.; Varghese, J.; Bruland, P.; Meidt, A.; Mertens, C.; Riepenhausen, S.; Baier, S.; et al. Pragmatic MDR: A Metadata Repository with Bottom-up Standardization of Medical Metadata through Reuse. BMC Med. Inform. Decis. Mak. 2021, 21, 160. [Google Scholar] [CrossRef]
- McManus, M.L.; França, U.L. Visualizing Patterns in Pediatric and Adult Hospital Care. Hosp. Pediatr. 2019, 9, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Rijnhart, J.J.M.; Lamp, S.J.; Valente, M.J.; MacKinnon, D.P.; Twisk, J.W.R.; Heymans, M.W. Mediation Analysis Methods Used in Observational Research: A Scoping Review and Recommendations. BMC Med. Res. Methodol. 2021, 21, 226. [Google Scholar] [CrossRef]
- Wagner, B.; Cleland, K. Using Autoregressive Integrated Moving Average Models for Time Series Analysis of Observational Data. BMJ 2023, 383, 2739. [Google Scholar] [CrossRef]
- Moreira, J.; Silva, B.; Faria, H.; Santos, R.; Sousa, A.S.P. Systematic Review on the Applicability of Principal Component Analysis for the Study of Movement in the Older Adult Population. Sensors 2022, 23, 205. [Google Scholar] [CrossRef]
- Le Glaz, A.; Haralambous, Y.; Kim-Dufor, D.-H.; Lenca, P.; Billot, R.; Ryan, T.C.; Marsh, J.; DeVylder, J.; Walter, M.; Berrouiguet, S.; et al. Machine Learning and Natural Language Processing in Mental Health: Systematic Review. J. Med. Internet Res. 2021, 23, e15708. [Google Scholar] [CrossRef]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Kringle, E.A.; Knutson, E.C.; Engstrom, C.; Terhorst, L. Iterative Processes: A Review of Semi-Supervised Machine Learning in Rehabilitation Science. Disabil. Rehabil. Assist. Technol. 2020, 15, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Demanse, D.; Saxer, F.; Lustenberger, P.; Tankó, L.B.; Nikolaus, P.; Rasin, I.; Brennan, D.F.; Roubenoff, R.; Premji, S.; Conaghan, P.G.; et al. Unsupervised Machine-Learning Algorithms for the Identification of Clinical Phenotypes in the Osteoarthritis Initiative Database. Semin. Arthritis Rheum. 2023, 58, 152140. [Google Scholar] [CrossRef]
- Denizdurduran, B.; Markram, H.; Gewaltig, M.-O. Optimum Trajectory Learning in Musculoskeletal Systems with Model Predictive Control and Deep Reinforcement Learning. Biol. Cybern. 2022, 116, 711–726. [Google Scholar] [CrossRef]
- McGonigal, M.; Bauer, M.; Post, C. Physician Engagement: A Key Concept in the Journey for Quality Improvement. Crit. Care Nurs. Q. 2019, 42, 215. [Google Scholar] [CrossRef] [PubMed]
- Micai, M.; Ciaramella, A.; Salvitti, T.; Fulceri, F.; Fatta, L.M.; Poustka, L.; Diehm, R.; Iskrov, G.; Stefanov, R.; Guillon, Q.; et al. Autistic Adult Health and Professional Perceptions of It: Evidence From the ASDEU Project. Front. Psychiatry 2021, 12, 614102. [Google Scholar] [CrossRef]
- Povey, C. What Should Services for People with Autism Look Like? Adv. Autism 2015, 1, 41–46. [Google Scholar] [CrossRef]
- Chien, L.J.; Slade, D.; Dahm, M.R.; Brady, B.; Roberts, E.; Goncharov, L.; Taylor, J.; Eggins, S.; Thornton, A. Improving Patient-Centred Care through a Tailored Intervention Addressing Nursing Clinical Handover Communication in Its Organizational and Cultural Context. J. Adv. Nurs. 2022, 78, 1413–1430. [Google Scholar] [CrossRef]
- Bonnechère, B.; Timmermans, A.; Michiels, S. Current Technology Developments Can Improve the Quality of Research and Level of Evidence for Rehabilitation Interventions: A Narrative Review. Sensors 2023, 23, 875. [Google Scholar] [CrossRef]
- Deschrijver, E.; Wiersema, J.R.; Brass, M. Action-Based Touch Observation in Adults with High Functioning Autism: Can Compromised Self-Other Distinction Abilities Link Social and Sensory Everyday Problems? Soc. Cogn. Affect. Neurosci. 2016, 12, 273–282. [Google Scholar] [CrossRef]
- Tarantino, L.; Attanasio, M.; Di Mascio, T.; De Gasperis, G.; Valenti, M.; Mazza, M. On the Evaluation of Engagement in Immersive Applications When Users Are on the Autism Spectrum. Sensors 2023, 23, 2192. [Google Scholar] [CrossRef] [PubMed]
- Dallman, A.R.; Bailliard, A.; Harrop, C. Identifying Predictors of Momentary Negative Affect and Depression Severity in Adolescents with Autism: An Exploratory Ecological Momentary Assessment Study. J. Autism Dev. Disord. 2022, 52, 291–303. [Google Scholar] [CrossRef]
- Clare, J.D.J.; Townsend, P.A.; Anhalt-Depies, C.; Locke, C.; Stenglein, J.L.; Frett, S.; Martin, K.J.; Singh, A.; Van Deelen, T.R.; Zuckerberg, B. Making Inference with Messy (Citizen Science) Data: When Are Data Accurate Enough and How Can They Be Improved? Ecol. Appl. Publ. Ecol. Soc. Am. 2019, 29, e01849. [Google Scholar] [CrossRef]
- Ridzuan, F.; Wan Zainon, W.M.N. A Review on Data Cleansing Methods for Big Data. Procedia Comput. Sci. 2019, 161, 731–738. [Google Scholar] [CrossRef]
- Movsessian, T.; Osoba, T.A. Association between Therapeutic Interventions and Quality of Life in People with Autism. J. Soc. Behav. Health Sci. 2022, 16, 284–305. [Google Scholar] [CrossRef]
- Tarsha, M.S.; Park, S.; Tortora, S. Body-Centered Interventions for Psychopathological Conditions: A Review. Front. Psychol. 2020, 10, 2907. [Google Scholar] [CrossRef]
- Skulmowski, A. Ethical Issues of Educational Virtual Reality. Comput. Educ. X Real. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for Scientific Data Management and Stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.; Agrifoglio, R.; Lepore, L.; Landriani, L. Explaing Users’ Technology Acceptance through National Cultural Values in the Hospital Context. BMC Health Serv. Res. 2022, 22, 84. [Google Scholar] [CrossRef]
- Cascio, C.J.; Foss-Feig, J.H.; Burnette, C.P.; Heacock, J.L.; Cosby, A.A. The Rubber Hand Illusion in Children with Autism Spectrum Disorders: Delayed Influence of Combined Tactile and Visual Input on Proprioception. Autism 2012, 16, 406–419. [Google Scholar] [CrossRef]
- Hirvikoski, T.; Mittendorfer-Rutz, E.; Boman, M.; Larsson, H.; Lichtenstein, P.; Bölte, S. Premature Mortality in Autism Spectrum Disorder. Br. J. Psychiatry 2016, 208, 232–238. [Google Scholar] [CrossRef] [PubMed]
| Variables | Description | Type | Target | Added Value |
|---|---|---|---|---|
| Self-Reporting Questionnaire | Administered to evaluate self-representation and perception of BRs, it includes different types of representation of body schema and image and understanding the complex interplay of BRs in autistic adults. | Self-reported short sentences questionnaire |
| Accommodates attention span variability and neurodivergence-related difficulties; Utilizes randomized question sequence; Provides additional data through neuropsychological assessment [42] |
| Clinical Evaluation | Involves structured tasks and activities and a clinical BR assessment by experienced therapists and psychologists in order to have a comprehensive assessment of motor skills, cognitive functioning, body image, and body schema | Body Representation Assessment (clinical assessment) |
| Utilizes gold-standard questionnaires; Provides a holistic understanding of individual’s body awareness [43,44,45,46,47] |
| Serious Games | Participants will be assessed in a clinical, unsupervised manner to understand their BR performance | STASISM |
| Offers engagement, customization, and real-time feedback; Utilizes advanced analytics for precise motion analysis; Incorporates wearable sensors for unsupervised daily mobility assessments [48] |
| Immersive VR | Gain insights into the psychomotor profile and holistic understanding of abilities and needs by applying VR systems to assess BRs in autistic individuals. Monitors upper limb mobility and physiological data. | PICO Neo3 |
| Offers customization of environments; Provides ecological validity to simulated situations; Collects physiological data for deeper understanding; Analyzes autonomic measures through pupillometry and heart rate analysis [49,50,51,52] |
| Activity Tracker | Uses smartwatches for unsupervised assessment of daily activities. Incorporates ecological momentary assessment (EMA) in order to understand the impact of BRs on daily living activities and gain insights into emotional states and habits | Garmin Vivosmart 5 |
| Provides continuous monitoring of various parameters; Offers real-time insights into behavior and cognition; Aims to understand BR effects on needs and behaviors [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourad, J.; Daniels, K.; Bogaerts, K.; Desseilles, M.; Bonnechère, B. Innovative Digital Phenotyping Method to Assess Body Representations in Autistic Adults: A Perspective on Multisensor Evaluation. Sensors 2024, 24, 6523. https://doi.org/10.3390/s24206523
Mourad J, Daniels K, Bogaerts K, Desseilles M, Bonnechère B. Innovative Digital Phenotyping Method to Assess Body Representations in Autistic Adults: A Perspective on Multisensor Evaluation. Sensors. 2024; 24(20):6523. https://doi.org/10.3390/s24206523
Chicago/Turabian StyleMourad, Joanna, Kim Daniels, Katleen Bogaerts, Martin Desseilles, and Bruno Bonnechère. 2024. "Innovative Digital Phenotyping Method to Assess Body Representations in Autistic Adults: A Perspective on Multisensor Evaluation" Sensors 24, no. 20: 6523. https://doi.org/10.3390/s24206523
APA StyleMourad, J., Daniels, K., Bogaerts, K., Desseilles, M., & Bonnechère, B. (2024). Innovative Digital Phenotyping Method to Assess Body Representations in Autistic Adults: A Perspective on Multisensor Evaluation. Sensors, 24(20), 6523. https://doi.org/10.3390/s24206523









