Inkjet-Printed Localized Surface Plasmon Resonance Subpixel Gas Sensor Array for Enhanced Identification and Visualization of Gas Spatial Distributions from Multiple Odor Sources
Abstract
1. Introduction
2. Related Work
3. Materials and Methods
3.1. Fabrication of Subpixel Gas Sensor Array
3.1.1. Materials
3.1.2. Fabrication of the Subpixel Pattern
3.2. Detection of the Gas
3.3. Visualization of Gas Spatial Distribution
3.4. Distinguishing Multiple Gases with PCA Methods
4. Results and Discussion
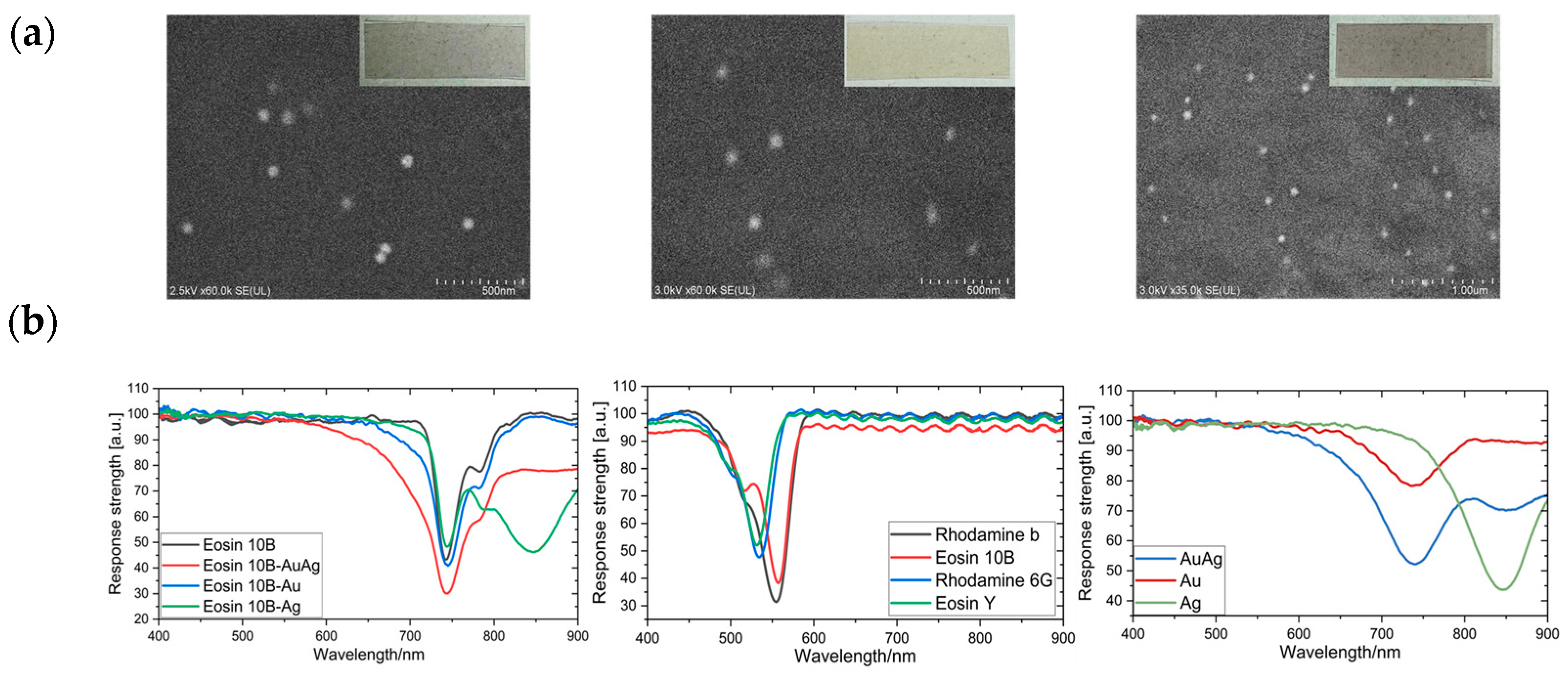
4.1. Surface Morphology of the Printed PET Substrate with Nanoparticles
4.2. Comparison of the LSPR Signals for Different Gases
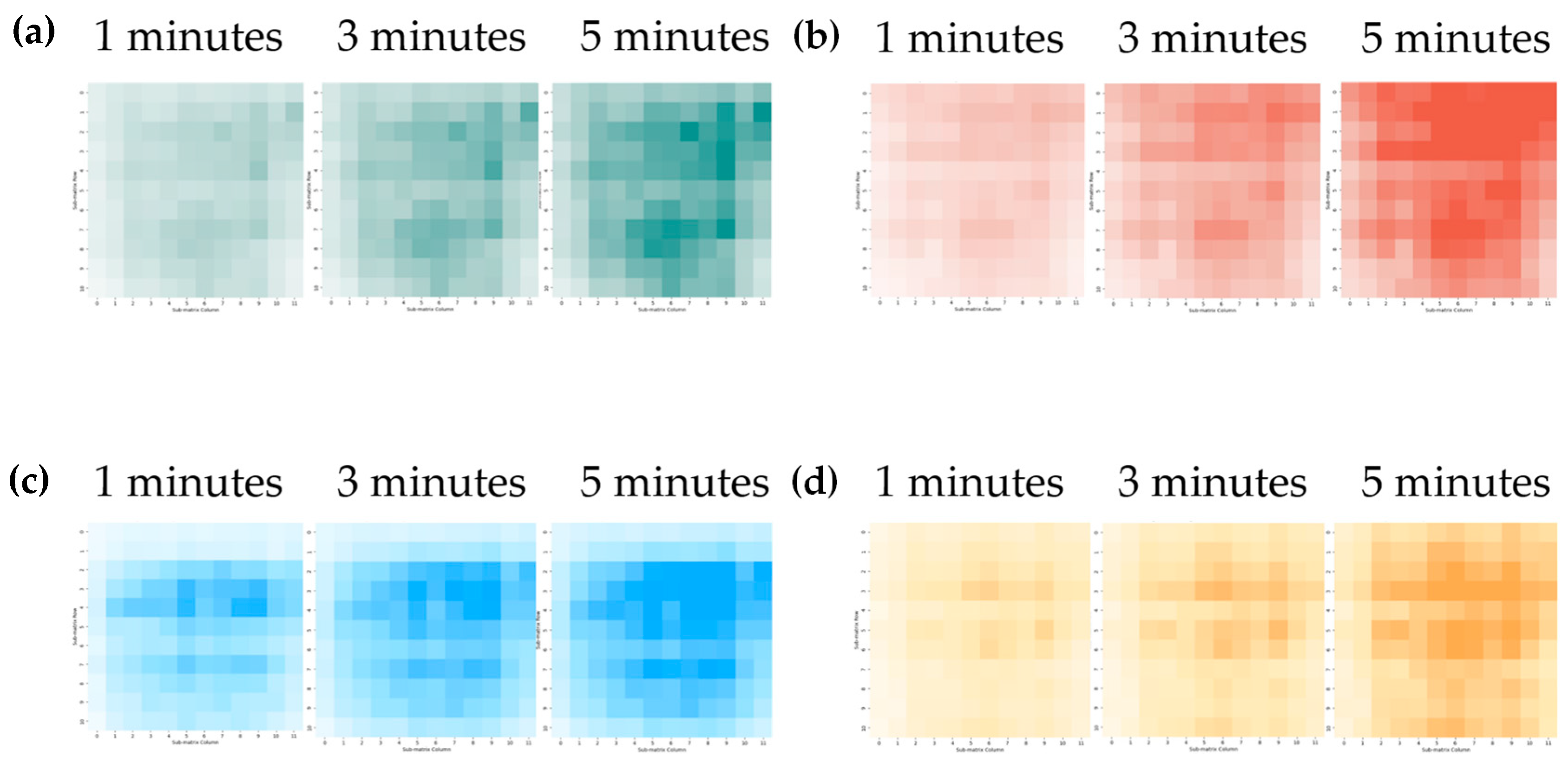
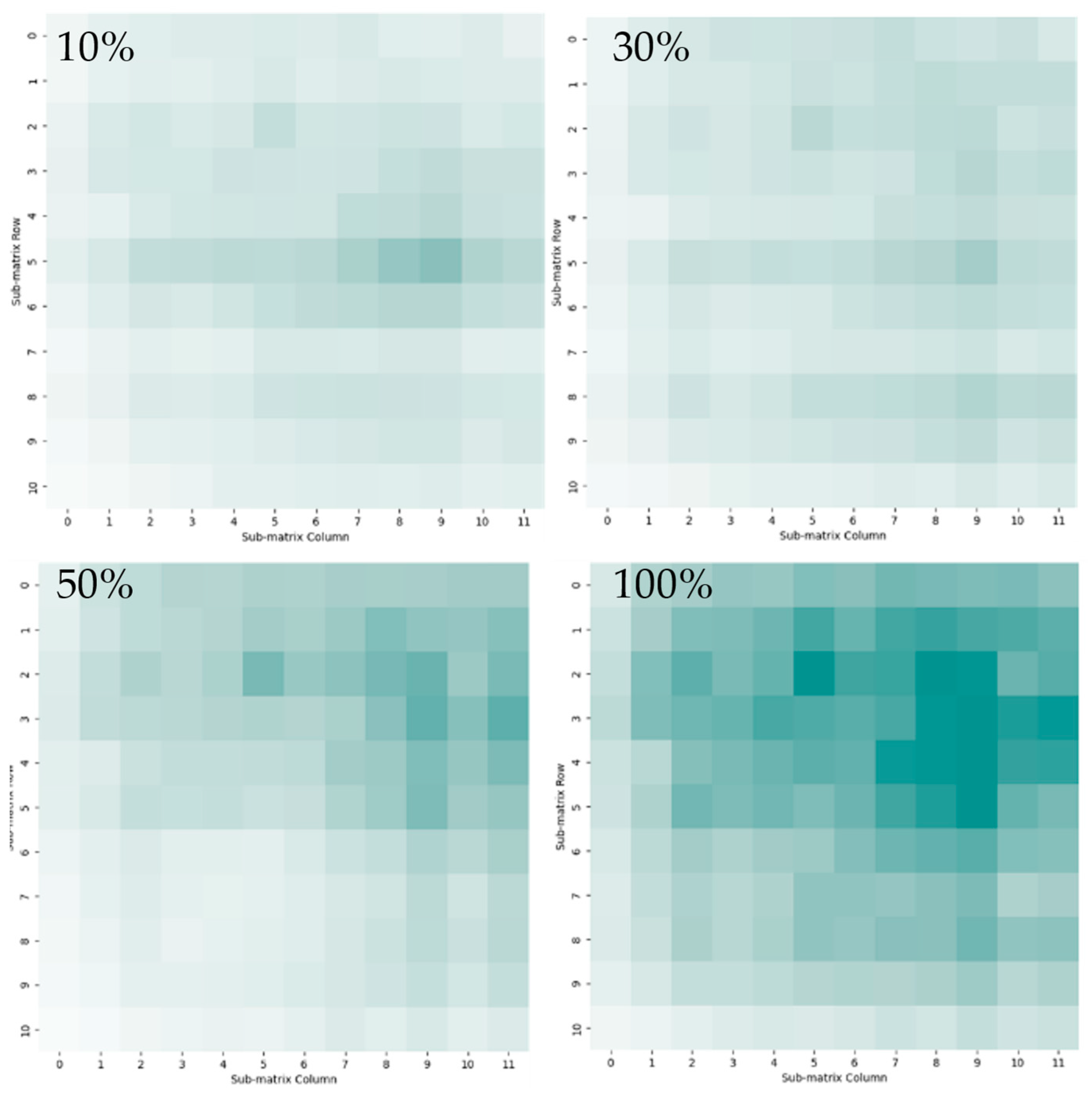
4.3. Visualization of Gas Spatial and Temporal Distributions
4.4. Visualization and Identification of Multiple Gases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic Noses for Environmental Monitoring Applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic–Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.E. Semiconductor-Type MEMS Gas Sensor for Real-Time Environmental Monitoring Applications. ETRI J. 2013, 35, 617–624. [Google Scholar] [CrossRef]
- Ko, G.-J.; Han, S.D.; Kim, J.-K.; Zhu, J.; Han, W.B.; Chung, J.; Yang, S.M.; Cheng, H.; Kim, D.-H.; Kang, C.-Y.; et al. Biodegradable, flexible silicon nanomembrane-based NOx gas sensor system with record-high performance for transient environmental monitors and medical implants. NPG Asia Mater. 2020, 12, 71. [Google Scholar] [CrossRef]
- Ye, M.; Chien, P.-J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef]
- Yuan, Z.; Bariya, M.; Fahad, H.M.; Wu, J.; Han, R.; Gupta, N.; Javey, A. Trace-Level, Multi-Gas Detection for Food Quality Assessment Based on Decorated Silicon Transistor Arrays. Adv. Mater. 2020, 32, 1908385. [Google Scholar] [CrossRef]
- Viciano-Tudela, S.; Sendra, S.; Parra, L.; Jimenez, J.M.; Lloret, J. Proposal of a Gas Sensor-Based Device for Detecting Adulteration in Essential Oil of Cistus ladanifer. Sustainability 2023, 15, 3357. [Google Scholar] [CrossRef]
- Rasooli Sharabiani, V.; Khorramifar, A.; Karami, H.; Lozano, J.; Tabor, S.; Darvishi, Y.; Gancarz, M. Non-destructive test to detect adulteration of rice using gas sensors coupled with chemometrics methods. Int. Agrophysics 2023, 37, 235–244. [Google Scholar] [CrossRef]
- Jalal, A.H.; Alam, F.; Roychoudhury, S.; Umasankar, Y.; Pala, N.; Bhansali, S. Prospects and Challenges of Volatile Organic Compound Sensors in Human Healthcare. ACS Sens. 2018, 3, 1246–1263. [Google Scholar] [CrossRef]
- Yi, J.; Xianyu, Y. Gold Nanomaterials-Implemented Wearable Sensors for Healthcare Applications. Adv. Funct. Mater. 2022, 32, 2113012. [Google Scholar] [CrossRef]
- Leal-Junior, A.; Avellar, L.; Biazi, V.; Soares, M.S.; Frizera, A.; Marques, C. Multifunctional flexible optical waveguide sensor: On the bioinspiration for ultrasensitive sensors development. Opto-Electron. Adv. 2022, 5, 210098. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, A.; Baghaei Nejad, M.; Shahrokh Abadi, M.H.; Zou, Z.; Zheng, L.-R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, G.; Nejad, M.B.; Shahrokhabadi, M.H. Polymer-based gas sensors to detect meat spoilage: A review. React. Funct. Polym. 2021, 165, 104962. [Google Scholar] [CrossRef]
- Yang, Z.; Sassa, F.; Hayashi, K. A Robot Equipped with a High-Speed LSPR Gas Sensor Module for Collecting Spatial Odor Information from On-Ground Invisible Odor Sources. ACS Sens. 2018, 3, 1174–1181. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J. Odor source localization algorithms on mobile robots: A review and future outlook. Robot. Auton. Syst. 2019, 112, 123–136. [Google Scholar] [CrossRef]
- Terutsuki, D.; Uchida, T.; Fukui, C.; Sukekawa, Y.; Okamoto, Y.; Kanzaki, R. Real-time odor concentration and direction recognition for efficient odor source localization using a small bio-hybrid drone. Sens. Actuators B Chem. 2021, 339, 129770. [Google Scholar] [CrossRef]
- Chen, L.; Guo, H.; Wang, C.; Chen, B.; Sassa, F.; Hayashi, K. Two-Dimensional SERS Sensor Array for Identifying and Visualizing the Gas Spatial Distributions of Two Distinct Odor Sources. Sensors 2024, 24, 790. [Google Scholar] [CrossRef]
- Yoshioka, H.-T.; Liu, C.; Hayashi, K. Multispectral fluorescence imaging for odorant discrimination and visualization. Sens. Actuators B Chem. 2015, 220, 1297–1304. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Ma, C.; Luo, S.; Xu, M.; Wu, Z.; Li, W.; Liu, S. Luminescent Transparent Wood Based on Lignin-Derived Carbon Dots as a Building Material for Dual-Channel, Real-Time, and Visual Detection of Formaldehyde Gas. ACS Appl. Mater. Interfaces 2020, 12, 36628–36638. [Google Scholar] [CrossRef] [PubMed]
- Iitani, K.; Toma, K.; Arakawa, T.; Mitsubayashi, K. Transcutaneous Blood VOC Imaging System (Skin-Gas Cam) with Real-Time Bio-Fluorometric Device on Rounded Skin Surface. ACS Sens. 2020, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lv, H.; Liu, Z.; Chen, J.; Wang, J.; Sun, B.; Zhang, Y.; Wang, R.; Shi, K. Thin-layered MoS2 nanoflakes vertically grown on SnO2 nanotubes as highly effective room-temperature NO2 gas sensor. J. Hazard. Mater. 2021, 416, 125830. [Google Scholar] [CrossRef]
- Soo, M.T.; Cheong, K.Y.; Noor, A.F.M. Advances of SiC-based MOS capacitor hydrogen sensors for harsh environment applications. Sens. Actuators B Chem. 2010, 151, 39–55. [Google Scholar] [CrossRef]
- Cui, S.; Lu, Y.; Kong, D.; Luo, H.; Peng, L.; Yang, G.; Yang, H.; Xu, K. Laser direct writing of Ga2O3/liquid metal-based flexible humidity sensors. Opto-Electron. Adv. 2023, 6, 220172. [Google Scholar] [CrossRef]
- Rai, P.; Raj, S.; Ko, K.-J.; Park, K.-K.; Yu, Y.-T. Synthesis of flower-like ZnO microstructures for gas sensor applications. Sens. Actuators B Chem. 2013, 178, 107–112. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Liu, C.; Wyszynski, B.; Yatabe, R.; Hayashi, K.; Toko, K. Molecularly Imprinted Sol-Gel-Based QCM Sensor Arrays for the Detection and Recognition of Volatile Aldehydes. Sensors 2017, 17, 382. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Zhou, T.; Zhang, T. Nanoscale MOF-74-based QCM gas sensor for CO2 detection at room temperature. Sens. Actuators B Chem. 2024, 413, 135874. [Google Scholar] [CrossRef]
- Qi, P.; Zhao, C.; Wang, R.; Fei, T.; Zhang, T. High-Performance QCM Humidity Sensors Using Acidized-Multiwalled Carbon Nanotubes as Sensing Film. IEEE Sens. J. 2018, 18, 5278–5283. [Google Scholar] [CrossRef]
- Kaaliveetil, S.; Lee, Y.-Y.; Li, Z.; Cheng, Y.-H.; Menon, N.H.; Dongare, S.; Gurkan, B.; Basuray, S. Ionic Liquid-Packed Microfluidic Device with Non-Planar Microelectrode as a Miniaturized Electrochemical Gas Sensor. J. Electrochem. Soc. 2023, 170, 087508. [Google Scholar] [CrossRef]
- Jiang, T.; Ye, X.; Guo, H.; Wang, C.; Ge, L.; Sassa, F.; Hayashi, K. Integrated Subpixel-Patterned LSPR Gas Sensor via Inkjet Printing of Au/Ag Nanoparticles and Pigments for Multigas Detection. IEEE Sens. Lett. 2024, 8, 4501804. [Google Scholar] [CrossRef]
- Jiang, T.; Ye, X.; Ge, L.; Guo, H.; Sassa, F.; Hayashi, K. Subpixel Patterned LSPR Gas Sensor Array with Using Inkjet Printing Au/Ag Nanoparticle to Enhance the Selectivity. IEEE Sens. Lett. 2023, 7, 4502304. [Google Scholar] [CrossRef]
- Ishida, H.; Tokuhiro, T.; Nakamoto, T.; Moriizumi, T. Improvement of olfactory video camera: Gas/odor flow visualization system. Sens. Actuators B Chem. 2022, 83, 256–261. [Google Scholar] [CrossRef]
- Tao, Y.; Tian, H.; Zhao, K.; Yu, Y.; Guo, L.; Liu, G.; Bai, X. High-precision discrimination of maize silage based on olfactory visualization technology integrated with chemometrics analysis. BioResources 2024, 19, 3597–3613. [Google Scholar] [CrossRef]
- Matsuoka, M.; Lingpu, G.; Sassa, F.; Hayashi, K. Spatiotemporal Visualization of Gases Using 2-D LSPR Gas Sensor. IEEE Sens. Lett. 2023, 7, 5000704. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Chen, B.; Hayashi, K. Development of molecular imprinted sol-gel based LSPR sensor for detection of volatile cis-jasmone in plant. Sens. Actuators B Chem. 2018, 260, 617–626. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Watanabe, M.; Chen, B.; Hayashi, K. LSPR sensor array based on molecularly imprinted sol-gels for pattern recognition of volatile organic acids. Sens. Actuators B Chem. 2017, 249, 14–21. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Biswas, R. LSPR enhanced gasoline sensing with a U-bent optical fiber. J. Phys. Appl. Phys. 2016, 49, 305104. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Guo, H.; Ge, L.; Sassa, F.; Hayashi, K. Inkjet-Printed Localized Surface Plasmon Resonance Subpixel Gas Sensor Array for Enhanced Identification and Visualization of Gas Spatial Distributions from Multiple Odor Sources. Sensors 2024, 24, 6731. https://doi.org/10.3390/s24206731
Jiang T, Guo H, Ge L, Sassa F, Hayashi K. Inkjet-Printed Localized Surface Plasmon Resonance Subpixel Gas Sensor Array for Enhanced Identification and Visualization of Gas Spatial Distributions from Multiple Odor Sources. Sensors. 2024; 24(20):6731. https://doi.org/10.3390/s24206731
Chicago/Turabian StyleJiang, Tianshu, Hao Guo, Lingpu Ge, Fumihiro Sassa, and Kenshi Hayashi. 2024. "Inkjet-Printed Localized Surface Plasmon Resonance Subpixel Gas Sensor Array for Enhanced Identification and Visualization of Gas Spatial Distributions from Multiple Odor Sources" Sensors 24, no. 20: 6731. https://doi.org/10.3390/s24206731
APA StyleJiang, T., Guo, H., Ge, L., Sassa, F., & Hayashi, K. (2024). Inkjet-Printed Localized Surface Plasmon Resonance Subpixel Gas Sensor Array for Enhanced Identification and Visualization of Gas Spatial Distributions from Multiple Odor Sources. Sensors, 24(20), 6731. https://doi.org/10.3390/s24206731






