Changes in Motor Strategy and Neuromuscular Control During Balance Tasks in People with a Bimalleolar Ankle Fracture: A Preliminary and Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedure
2.1.1. Descriptive and Anthropometric Measurements
2.1.2. Ankle Functionality Questionnaire Post-Surgery
2.1.3. Hip Strength and Ankle Range of Movement
2.1.4. Instrumentation and Electrode Placement for Muscle Activity
2.1.5. Balance Assessment
Static Balance
Dynamic Balance
2.2. Data Reduction
2.3. Statistical Analysis
3. Results
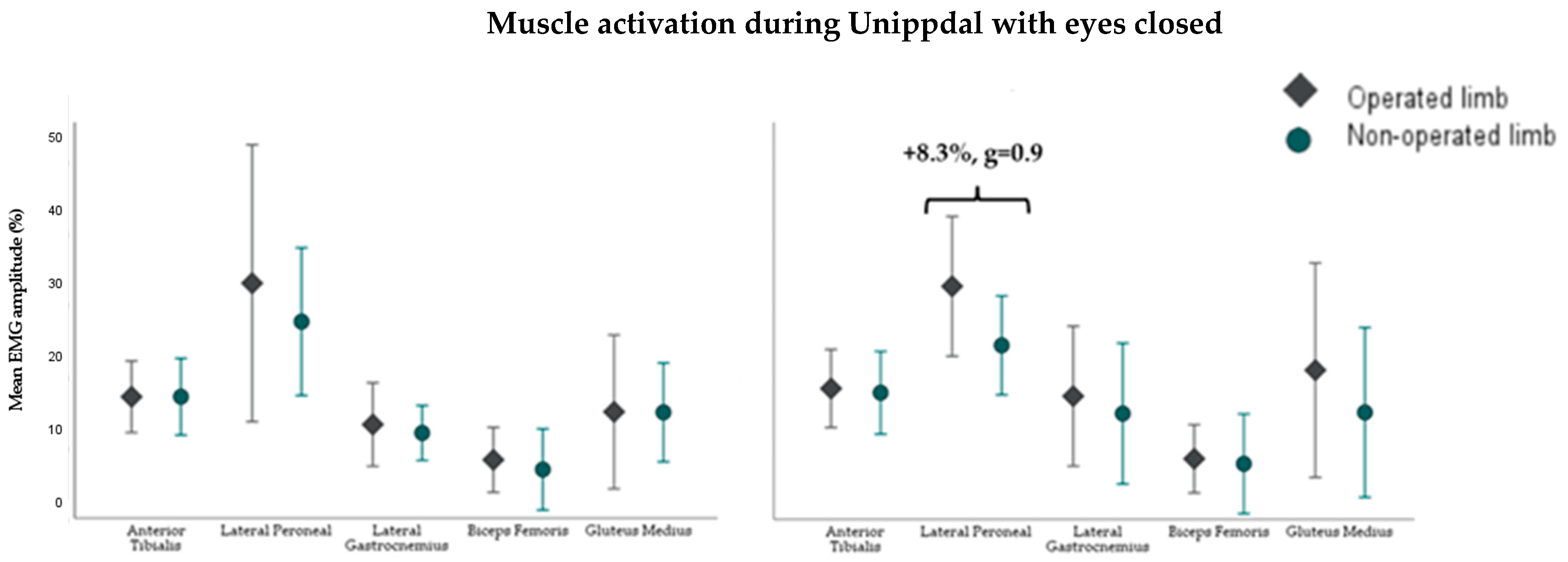
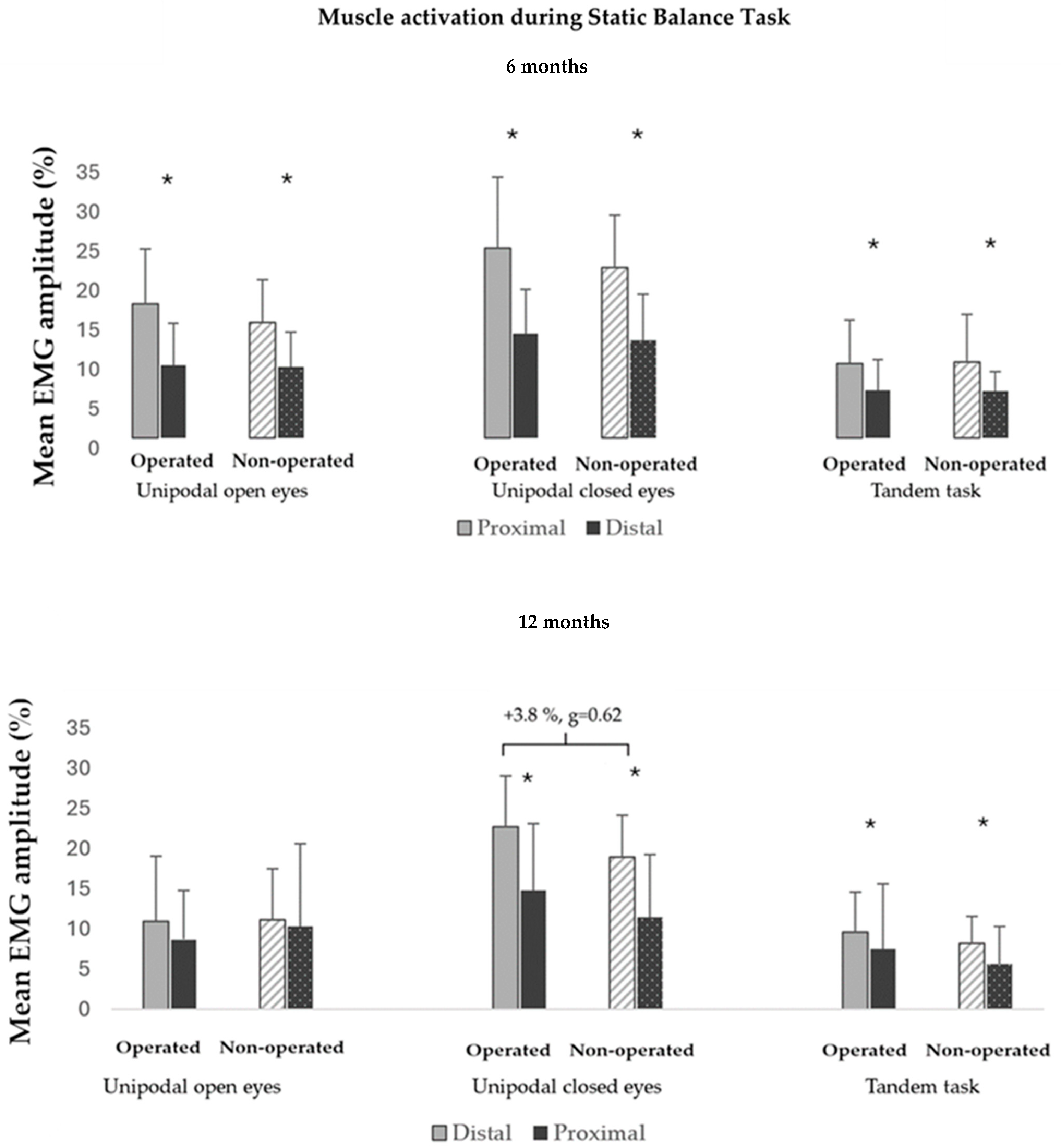
3.1. Muscle Activity During Static Balance Tests
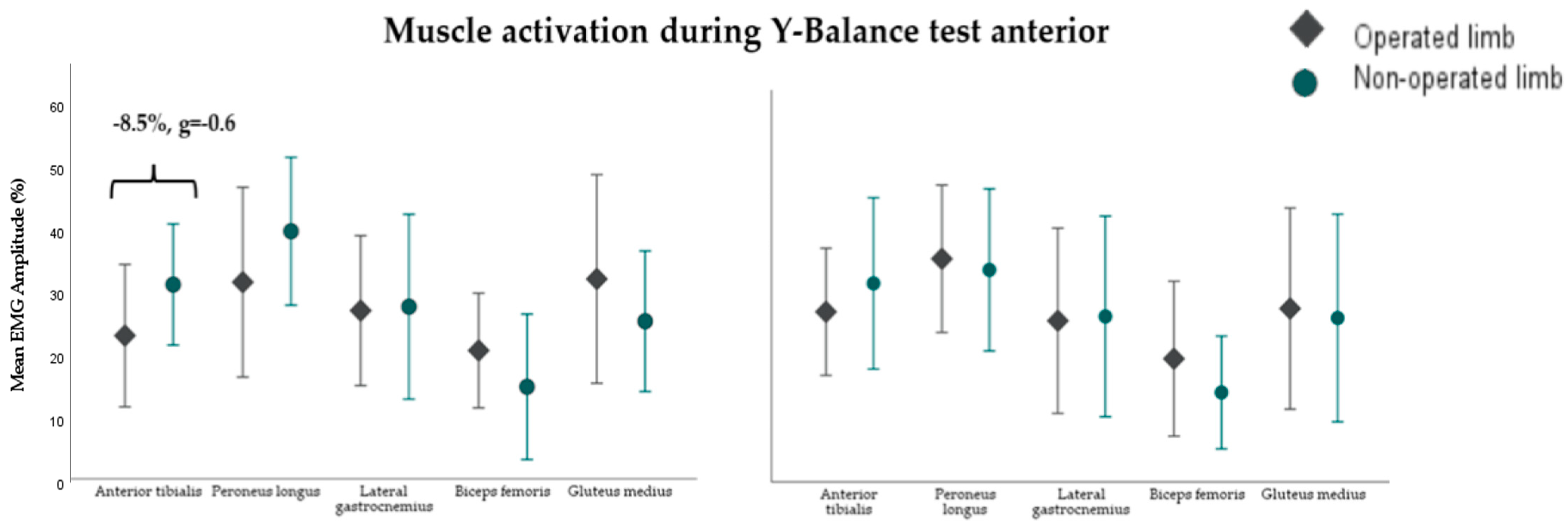
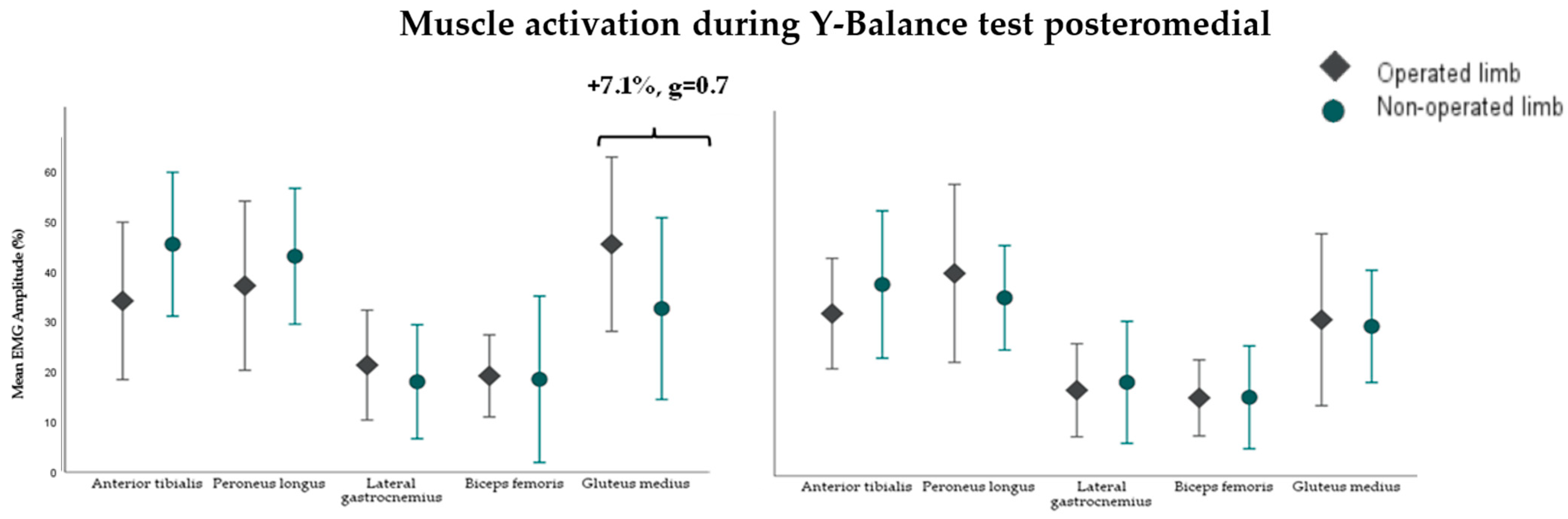
3.2. Muscle Activity During the Y-Balance Test (YBT)
3.3. Relationship Between Balance, Dorsiflexion, Hip Strength, AOFAS, and Activation of Distal and Proximal Musculature
4. Discussion
4.1. Main Results
4.2. Neuromuscular Control in Static Balance
4.3. Neuromuscular Control in Dynamic Balance
4.4. Neuromuscular Behavior and Its Relationship to Balance and Musculoskeletal Parameters
4.5. Limitations, Practical Implications, and Future Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noback, P.C.; Freibott, C.E.; Dougherty, T.; Swart, E.F.; Rosenwasser, M.P.; Vosseller, J.T. Estimates of Direct and Indirect Costs of Ankle Fractures: A Prospective Analysis. J. Bone Jt. Surg. Am. 2020, 102, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Albin, S.R.; Koppenhaver, S.L.; Marcus, R.; Dibble, L.; Cornwall, M.; Fritz, J.M. Short-term Effects of Manual Therapy in Patients After Surgical Fixation of Ankle and/or Hindfoot Fracture: A Randomized Clinical Trial. J. Orthop. Sports Phys. Ther. 2019, 49, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Black, J.D.J.; Bhavikatti, M.; Al-Hadithy, N.; Hakmi, A.; Kitson, J. Early Weight-Bearing in Operatively Fixed Ankle Fractures: A Systematic Review. Foot 2013, 23, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Drużbicki, M.; Przysada, G.; Śpiewak, D. Assessment of standing balance in patients after ankle fractures. Acta Bioeng. Biomech. 2014, 16, 59–65. [Google Scholar] [PubMed]
- Wang, R.; Thur, C.K.; Gutierrez-Farewik, E.M.; Wretenberg, P.; Broström, E. One year follow-up after operative ankle fractures: A prospective gait analysis study with a multi-segment foot model. Gait Posture 2010, 31, 234–240. [Google Scholar] [CrossRef]
- Nilsson, G.; Nyberg, P.; Ekdahl, C.; Eneroth, M. Performance after surgical treatment of patients with ankle fractures—14-month follow-up. Physiother. Res. Int. 2003, 8, 69–82. [Google Scholar] [CrossRef]
- Salas-Gómez, D.; Fernández-Gorgojo, M.; Sanchez-Juan, P.; Bercero, E.L.; Isabel Perez-Núñez, M.; Barbado, D. Quantifying balance deficit in people with ankle fracture six months after surgical intervention through the Y-Balance test. Gait Posture 2020, 95, 249–255. [Google Scholar] [CrossRef]
- Loram, I.D.; Maganaris, C.N.; Lakie, M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J. Physiol. 2005, 564, 295–311. [Google Scholar] [CrossRef]
- Barbado Murillo, D.; Sabido Solana, R.; Vera-Garcia, F.J.; Gusi Fuertes, N.; Moreno, F.J. Effect of increasing difficulty in standing balance tasks with visual feedback on postural sway and EMG: Complexity and performance. Hum. Mov. Sci. 2012, 31, 1224–1237. [Google Scholar] [CrossRef]
- Loram, I.D.; Lakie, M. Human balancing of an inverted pendulum: Position control by small, ballistic-like, throw and catch movements. J. Physiol. 2002, 540, 1111–1124. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Song, S.; Hou, Y.; Hong, Y.; Yue, S.; Li, K. Dynamical Analysis of Standing Balance Control on Sloped Surfaces in Individuals with Lumbar Disc Herniation. Sci. Rep. 2020, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Jaber, H.; Lohman, E.; Daher, N.; Bains, G.; Nagaraj, A.; Mayekar, P.; Shanbhag, M.; Alameri, M. Neuromuscular control of ankle and hip during performance of the star excursion balance test in subjects with and without chronic ankle instability. PLoS ONE 2018, 13, e0201479. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.A.; Pietrosimone, B.G.; Gribble, P.A. Muscle Activation During Landing Before and After Fatigue in Individuals with or without Chronic Ankle Instability. J. Athl. Train. 2016, 51, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, S.; Staes, F.F.; Stappaerts, K.H.; Janssens, L.; Levin, O.; Peers, K.K.H. Relationship of chronic ankle instability to muscle activation patterns during the transition from double-leg to single-leg stance. Am. J. Sports Med. 2007, 35, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Molina-Rueda, F.; Cuesta-Gómez, A.; Carratalá-Tejada, M.; Koutsou, A.; Fernández-González, P.; Alguacil-Diego, I.M. Ankle muscle activation during the limits of stability test in subjects with chronic ankle instability. Phys. Ther. Sport 2021, 47, 134–139. [Google Scholar] [CrossRef]
- Webster, K.A.; Gribble, P.A. A comparison of electromyography of gluteus medius and maximus in subjects with and without chronic ankle instability during two functional exercises. Phys. Ther. Sport 2013, 14, 17–22. [Google Scholar] [CrossRef]
- Feger, M.A.; Donovan, L.; Hart, J.M.; Hertel, J. Lower extremity muscle activation during functional exercises in patients with and without chronic ankle instability. PM&R 2014, 6, 602–611; quiz 611. [Google Scholar] [CrossRef]
- Feger, M.A.; Donovan, L.; Hart, J.M.; Hertel, J. Lower extremity muscle activation in patients with or without chronic ankle instability during walking. J. Athl. Train. 2015, 50, 350–357. [Google Scholar] [CrossRef]
- Jeon, H.G.; Lee, S.Y.; Park, S.E.; Ha, S. Ankle Instability Patients Exhibit Altered Muscle Activation of Lower Extremity and Ground Reaction Force during Landing: A Systematic Review and Meta-Analysis. J. Sports Sci. Med. 2021, 20, 373. [Google Scholar] [CrossRef]
- Salas-Gómez, D.; Fernández-Gorgojo, M.; Sánchez-Juan, P.; Pérez-Núñez, M.I.; Laguna-Bercero, E.; Prat-Luri, A.; Barbado, D. Measuring Recovery and Understanding Long-Term Deficits in Balance, Ankle Mobility and Hip Strength in People after an Open Reduction and Internal Fixation of Bimalleolar Fracture and Their Impact on Functionality: A 12-Month Longitudinal Study. J. Clin. Med. 2022, 11, 2539. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Calderazzi, F.; Pedrazzi, G. Is There a Relation between AOFAS Ankle-Hindfoot Score and SF-36 in Evaluation of Achilles Ruptures Treated by Percutaneous Technique? J. Foot Ankle Surg. 2013, 53, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ishøi, L.; Thorborg, K.; Krohn, L.; Louis Andersen, L.; Møller Nielsen, A.; Bek Clausen, M. Maximal and Explosive Muscle Strength During Hip Adduction Squeeze and Hip Abduction Press Test Using A Handheld Dynamometer: An Intra- and Inter-tester Reliability Study. Int. J. Sports Phys. Ther. 2023, 18, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Dill, K.E.; Begalle, R.L.; Frank, B.S.; Zinder, S.M.; Padua, D.A. Altered knee and ankle kinematics during squatting in those with limited weight-bearing-lunge ankle-dorsiflexion range of motion. J. Athl. Train. 2014, 49, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.; Nielsen, H.B.; Lund, C.; Sørensen, D.S.; Larsen, B.T.; Matthews, M.; Vicenzino, B.; Elsoe, R. A novel tool for measuring ankle dorsiflexion: A study of its reliability in patients following ankle fractures. Foot Ankle Surg. 2016, 22, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Gosse, G.; Ward, E.; McIntyre, A.; Banwell, H.A. The reliability and validity of the weight-bearing lunge test in a Congenital Talipes Equinovarus population (CTEV). PeerJ 2021, 9, e10253. [Google Scholar] [CrossRef]
- Hirata, K.; Yamadera, R.; Akagi, R. Associations between Range of Motion and Tissue Stiffness in Young and Older People. Med. Sci. Sports Exerc. 2020, 52, 2179–2188. [Google Scholar] [CrossRef]
- Baumgarten, A.; Kim, J.K.; Robison, J.; Mayer, J.; Hardwick, D.; Patel, T. Analysis of surgeon biometrics during open and robotic radical cystectomy with electromyography and motion capture analysis. Int. Braz. J. Urol. 2020, 46, 138. [Google Scholar] [CrossRef]
- Cho, C.; Jeong, J.-H.; Kim, Y.; Jang, J.H.; Lee, S.-S.; Lee, K. Comparison of Endurance Time Prediction of Biceps Brachii Using Logarithmic Parameters of a Surface Electromyogram during Low-Moderate Level Isotonic Contractions. Appl. Sci. 2021, 11, 2861. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Besomi, M.; Hodges, P.W.; Clancy, E.A.; Van Dieën, J.; Hug, F.; Lowery, M.; Merletti, R.; Søgaard, K.; Wrigley, T.; Besier, T.; et al. Consensus for experimental design in electromyography (CEDE) project: Amplitude normalization matrix. J. Electromyogr. Kinesiol. 2020, 53, 102438. [Google Scholar] [CrossRef]
- Plisky, P.J.; Gorman, P.P.; Butler, R.J.; Kiesel, K.B.; Underwood, F.B.; Elkins, B. The reliability of an instrumented device for measuring components of the star excursion balance test. N. Am. J. Sports Phys. Ther. 2009, 4, 92–99. [Google Scholar] [PubMed]
- Morasso, P.G.; Sanguineti, V. Ankle muscle stiffness alone cannot stabilize balance during quiet standing. J. Neurophysiol. 2002, 88, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- van Dieën, J.H.; Koppes, L.L.J.; Twisk, J.W.R. Postural sway parameters in seated balancing; their reliability and relationship with balancing performance. Gait Posture 2010, 31, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Khaliliyan, H.; Sharafatvaziri, A.; Safaeepour, Z.; Bahramizadeh, M. Gait and muscle activity measures after biomechanical device therapy in subjects with ankle instability: A systematic review. Foot 2024, 59, 102083. [Google Scholar] [CrossRef]
- Bertrand-Charette, M.; Dambreville, C.; Bouyer, L.J.; Roy, J.-S. Systematic review of motor control and somatosensation assessment tests for the ankle. BMJ Open Sport Exerc. Med. 2020, 6, e000685. [Google Scholar] [CrossRef]
- Delahunt, E.; Monaghan, K.; Caulfield, B. Changes in lower limb kinematics, kinetics, and muscle activity in subjects with functional instability of the ankle joint during a single leg drop jump. J. Orthop. Res. 2006, 24, 1991–2000. [Google Scholar] [CrossRef]
- Myers, J.B.; Riemann, B.L.; Hwang, J.-H.; Fu, F.H.; Lephart, S.M. Effect of Peripheral Afferent Alteration of the Lateral Ankle Ligaments on Dynamic Stability. Am. J. Sports Med. 2003, 31, 498–506. Available online: https://journals.sagepub.com/doi/abs/10.1177/03635465030310040401 (accessed on 13 August 2021). [CrossRef]
- Pozzi, F.; Moffat, M.; Gutierrez, G. Neuromuscular control during performance of a dynamic balance task in subjects with and without ankle instability. Int. J. Sports Phys. Ther. 2015, 10, 520–529. [Google Scholar]
- Cimadoro, G.; Paizis, C.; Alberti, G.; Babault, N. Effects of different unstable supports on EMG activity and balance. Neurosci. Lett. 2013, 548, 228–232. [Google Scholar] [CrossRef]
- Donath, L.; Kurz, E.; Roth, R.; Zahner, L.; Faude, O. Leg and trunk muscle coordination and postural sway during increasingly difficult standing balance tasks in young and older adults. Maturitas 2016, 91, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gebel, A.; Lüder, B.; Granacher, U. Effects of Increasing Balance Task Difficulty on Postural Sway and Muscle Activity in Healthy Adolescents. Front. Physiol. 2019, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-M. Higher visual reliance during single-leg balance bilaterally occurring following acute lateral ankle sprain: A potential central mechanism of bilateral sensorimotor deficits. Gait Posture 2020, 78, 26–29. [Google Scholar] [CrossRef] [PubMed]
- DeJong, A.F.; Mangum, L.C.; Hertel, J. Ultrasound Imaging of the Gluteal Muscles During the Y-Balance Test in Individuals with or without Chronic Ankle Instability. J. Athl. Train. 2019, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Aminaka, N.; Gribble, P.A. Lower-Extremity Muscle Activity, Kinematics, and Dynamic Postural Control in Individuals with Patellofemoral Pain. J. Sport Rehabil. 2018, 27, 505–512. [Google Scholar] [CrossRef]
- Nilsson, G.; Ageberg, E.; Ekdahl, C.; Eneroth, M. Balance in Single-Limb Stance After Surgically Treated Ankle Fractures: A 14-Month Follow-Up. BMC Musculoskelet. Disord. 2006, 7, 35. [Google Scholar] [CrossRef]
- Dejong, A.F.; Koldenhoven, R.M.; Hertel, J. Proximal Adaptations in Chronic Ankle Instability: Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2020, 52, 1563–1575. [Google Scholar] [CrossRef]
- Bisson, E.J.; McEwen, D.; Lajoie, Y.; Bilodeau, M. Effects of ankle and hip muscle fatigue on postural sway and attentional demands during unipedal stance. Gait Posture 2011, 33, 83–87. [Google Scholar] [CrossRef]
- Gribble, P.A.; Hertel, J.; Denegar, C.R.; Buckley, W.E. The Effects of Fatigue and Chronic Ankle Instability on Dynamic Postural Control. J. Athl. Train. 2004, 39, 321–329. [Google Scholar]
- Bastien, M.; Moffet, H.; Bouyer, L.J.; Perron, M.; Hébert, L.J.; Leblond, J. Alteration in global motor strategy following lateral ankle sprain. BMC Musculoskelet. Disord. 2014, 15, 436. [Google Scholar] [CrossRef]
- Macrum, E.; Bell, D.R.; Boling, M.; Lewek, M.; Padua, D. Effect of limiting ankle-dorsiflexion range of motion on lower extremity kinematics and muscle-activation patterns during a squat. J. Sport Rehabil. 2012, 21, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Khou, S.B.; Saki, F.; Tahayori, B. Muscle activation in the lower limb muscles in individuals with dynamic knee valgus during single-leg and overhead squats: A meta-analysis study. BMC Musculoskelet. Disord. 2024, 25, 652. [Google Scholar] [CrossRef] [PubMed]


| Operated Limb | Non-Operated Limb | p-Value | Operated Limb | Non-Operated Limb | p-Value | |
|---|---|---|---|---|---|---|
| (N = 14) | (N = 14) | (N = 21) | (N = 21) | |||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Age | 45.6 ± 9.4 | 44.1 ± 11.1 | ||||
| Sex (% of women) | 50% | 47.60% | ||||
| Height (cm) | 166.9 ± 8.7 | 168.8 ± 9.2 | ||||
| Weight (kg) | 76.1 ± 9.5 | 77.9 ± 17.0 | ||||
| Immobilization time (weeks) | 3.4 ± 1.2 | |||||
| Unloading period (weeks) | 6.1 ± 1.3 | |||||
| Rehabilitation time (months) | 3.2 ± 2.5 | |||||
| AOFAS | 76.1 ± 9.5 | 84.4 ± 12.7 | ||||
| FDTROM (°) | 24.4 ± 8.7 | 29.0 ± 8.3 | <0.001 | 30.4 ± 9.4 | 37.4 ± 6.1 | <0.001 |
| ABD (%) | 28.2 ± 5.2 | 32.4 ± 8.2 | 0.025 | 30.4 ± 7.7 | 27.0 ± 7.2 | <0.001 |
| Muscle Activity of the Ankle Muscles | Muscle Activity of the Hip Muscles | |||||||
|---|---|---|---|---|---|---|---|---|
| Operated Limb | Non-Operated Limb | Operated Limb | Non-Operated Limb | Operated Limb | Non-Operated Limb | Operated Limb | Non-Operated Limb | |
| 6 Months | 12 Months | 6 Months | 12 Months | |||||
| Anterior direction of the Y-Balance Test | ||||||||
| YBTA-Distance | −0.185 | −0.082 | −0.281 | −0.218 | −0.365 | 0.058 | −0.421 | −0.013 |
| FDTROM (°) | −0.603 GL *** | −0.371 | −0.553 | −0.253 | −0.392 | −0.279 | −0.308 | −0.136 |
| ABD (%) | −0.340 | −0.234 | −0.427 | −0.172 | −0.153 | −0.143 | −0.355 | 0.023 |
| AOFAS | −0.133 | 0.351 | −0.410 | −0.072 | −0.047 | 0.062 | −0.365 | 0.143 |
| Posterior medial direction of the Y-Balance Test | ||||||||
| YBTPM-Distance | −0.277 | −0.259 | −0.169 | −0.207 | −0.349 | −0.124 | −0.535 | 0.031 |
| FDTROM (°) | −0.616 GL *** | −0.424 | −0.699 | −0.361 | −0.586 | −0.167 | −0.371 | 0.054 |
| ABD (%) | −0.330 | −0.071 | −0.344 | −0.314 | −0.33 | −0.087 | −0.553 | 0.206 |
| AOFAS | −0.156 | −0.098 | −0.548 | −0.413 | −0.224 | 0.053 | −0.452 | 0.283 |
| Posterior lateral direction of the Y-Balance Test | ||||||||
| YBTPL-Distance | 0.104 | −0.421 | 0.273 | 0.133 | −0.388 | 0.269 | −0.630 | 0.175 |
| FDTROM (°) | −0.547 | −0.235 | −0.05 | −0.164 | −0.533 | −0.125 | −0.164 | −0.179 |
| ABD (%) | 0.072 | −0.279 | 0.235 | −0.008 | 0.306 | −0.072 | −0.093 | 0.046 |
| AOFAS | 0.090 | −0.374 | 0.029 | −0.205 | 0.087 | −0.160 | −0.281 | 0.141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Gómez, D.; Barbado, D.; Sánchez-Juan, P.; Pérez-Núñez, M.I.; Laguna-Bercero, E.; Lantarón-Juarez, S.; Fernandez-Gorgojo, M. Changes in Motor Strategy and Neuromuscular Control During Balance Tasks in People with a Bimalleolar Ankle Fracture: A Preliminary and Exploratory Study. Sensors 2024, 24, 6798. https://doi.org/10.3390/s24216798
Salas-Gómez D, Barbado D, Sánchez-Juan P, Pérez-Núñez MI, Laguna-Bercero E, Lantarón-Juarez S, Fernandez-Gorgojo M. Changes in Motor Strategy and Neuromuscular Control During Balance Tasks in People with a Bimalleolar Ankle Fracture: A Preliminary and Exploratory Study. Sensors. 2024; 24(21):6798. https://doi.org/10.3390/s24216798
Chicago/Turabian StyleSalas-Gómez, Diana, David Barbado, Pascual Sánchez-Juan, María Isabel Pérez-Núñez, Esther Laguna-Bercero, Saray Lantarón-Juarez, and Mario Fernandez-Gorgojo. 2024. "Changes in Motor Strategy and Neuromuscular Control During Balance Tasks in People with a Bimalleolar Ankle Fracture: A Preliminary and Exploratory Study" Sensors 24, no. 21: 6798. https://doi.org/10.3390/s24216798
APA StyleSalas-Gómez, D., Barbado, D., Sánchez-Juan, P., Pérez-Núñez, M. I., Laguna-Bercero, E., Lantarón-Juarez, S., & Fernandez-Gorgojo, M. (2024). Changes in Motor Strategy and Neuromuscular Control During Balance Tasks in People with a Bimalleolar Ankle Fracture: A Preliminary and Exploratory Study. Sensors, 24(21), 6798. https://doi.org/10.3390/s24216798








