The Effects of a Single Vagus Nerve’s Neurodynamics on Heart Rate Variability in Chronic Stress: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
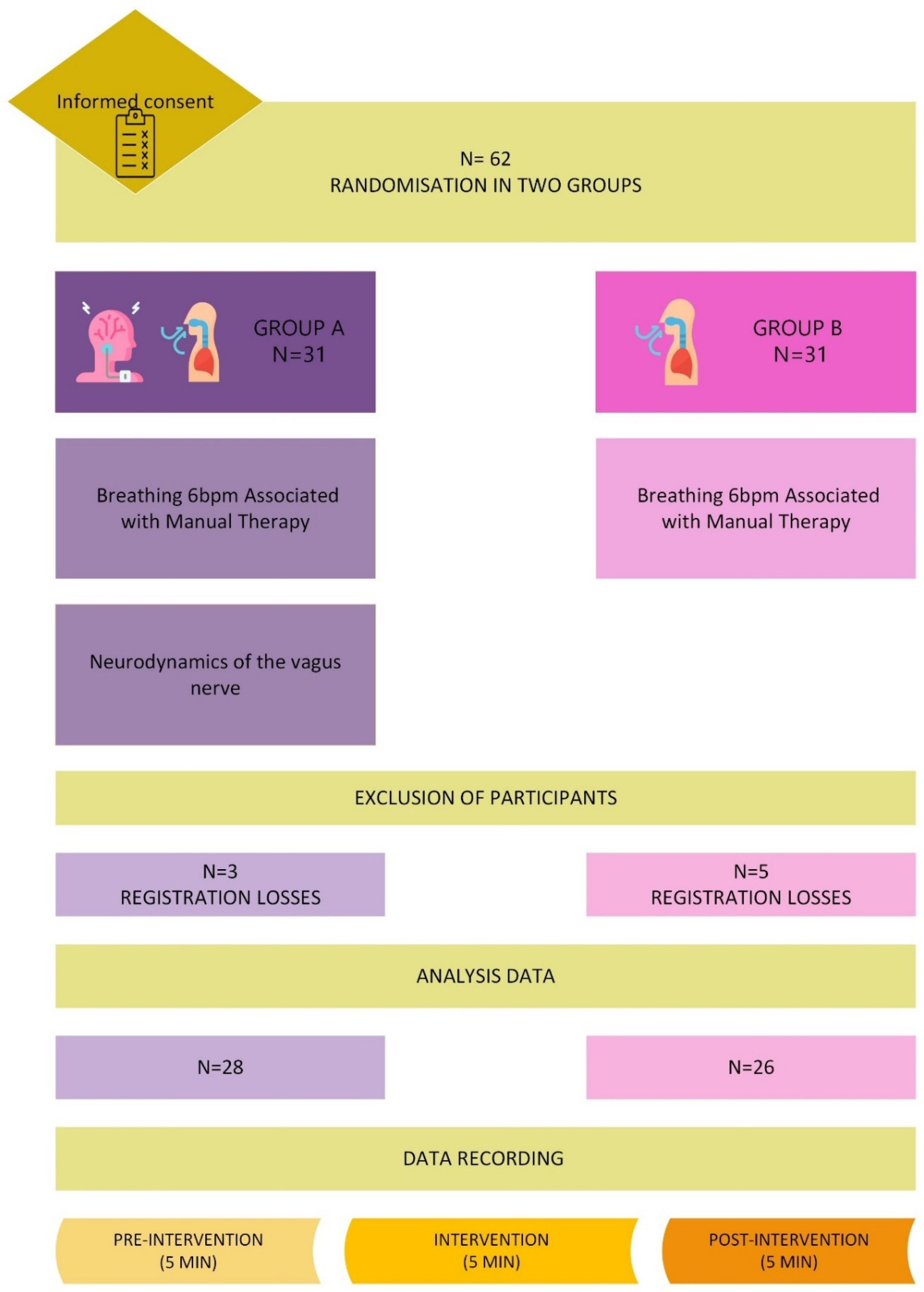
2.1. The Study Design
2.2. Study Subjects
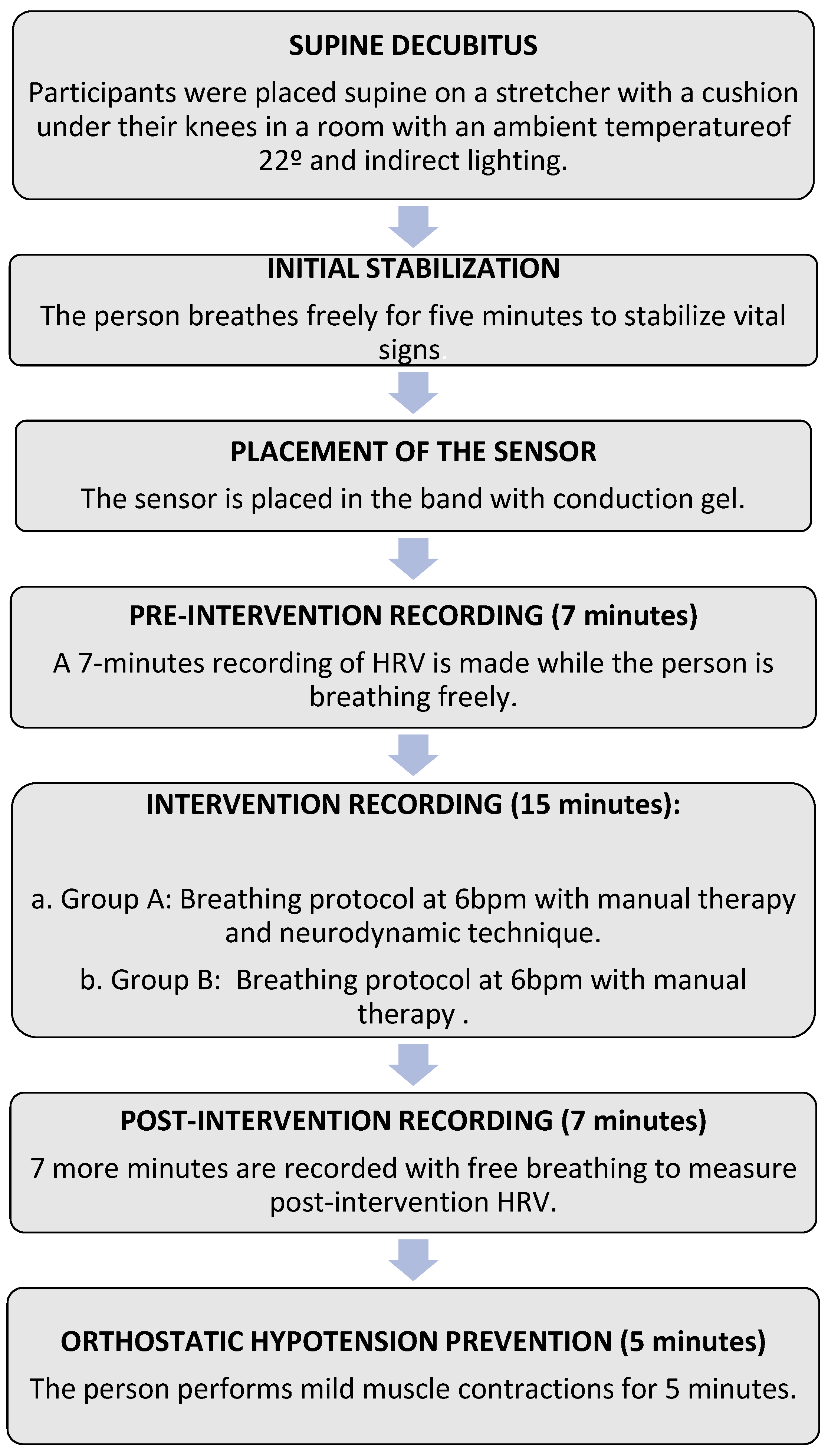
2.3. Intervention Protocol
2.3.1. Breathing Protocol Associated with Manual Therapy
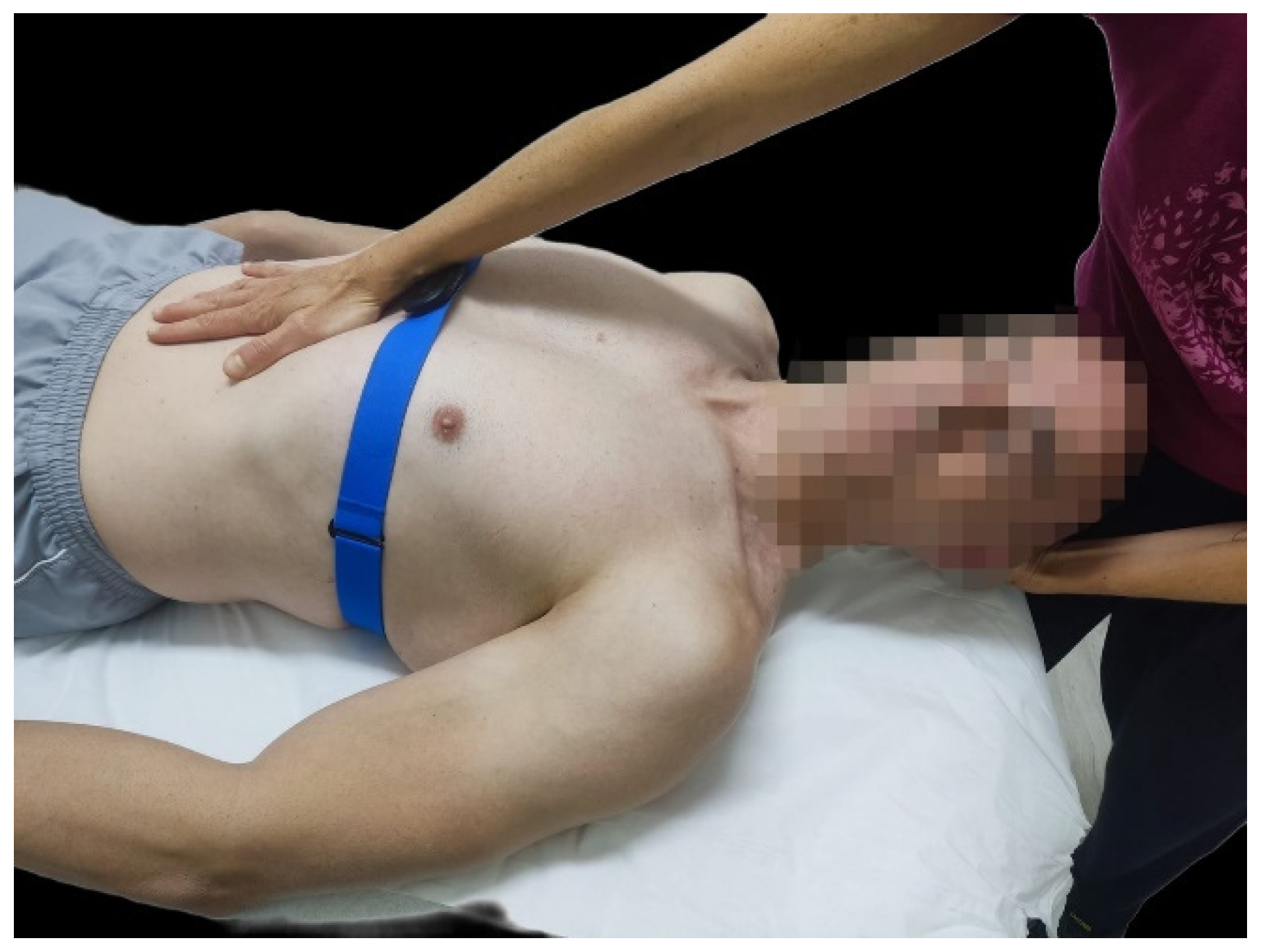
2.3.2. Neurodynamic Technique
2.4. Data Analysis Procedure
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Study Limitations
4.2. Future Research
4.3. Clinical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6 bpm | 6 breaths per minute |
| AAQ-II | Acceptance and Action Questionnaire-II |
| ANS | Autonomic Nervous System |
| BMI | Body Mass Index |
| HR Diff | Difference between Maximum and Minimum Heart Rate |
| HF | High-Frequency Component |
| HRV | Heart Rate Variability |
| LF/HF | Ratio between the low- and high-frequency bands |
| LF | Low-Frequency Component |
| max HR | Maximum Heart Rate |
| Mean HR | Mean Heart Rate |
| min HR | Minimum Heart Rate |
| NN | Intervals between consecutive heartbeats when interference is removed |
| NN50 | Number of NN Intervals differing by more than 50 ms |
| pNN50 | Percentage of NN Intervals differing by more than 50 ms |
| PNS | Parasympathetic Nervous System |
| PSS | Perceived Stress Scale |
| RMSSD | Root Mean Square Difference of the Successive Differences |
| RR | Intervals between consecutive heartbeats |
| SDNN | Standard Deviation of NN Intervals |
| SNS | Sympathetic Nervous System |
| UCLA | University of California, Los Angeles (Loneliness Scale) |
| WHO | World Health Organization |
References
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Porges, S. Brain-Body Connection May Ease Autistic People’s Social Problems [Internet]. 2019. Available online: https://www.spectrumnews.org (accessed on 3 August 2024).
- Lee, R.S. Chapter 1—The physiology of stress and the human body’s response to stress. In Epigenetics of Stress and Stress Disorders; Youssef, N.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–18. Available online: https://www.sciencedirect.com/science/article/pii/B9780128230398000174 (accessed on 17 August 2024).
- Santos, G.S.P.; Costa, A.C.; Picoli, C.C.; Rocha, B.G.S.; Sulaiman, S.O.; Radicchi, D.C.; Pinto, M.C.X.; Batista, M.L.; Amorim, J.H.; Azevedo, V.A.C.; et al. Sympathetic nerve-adipocyte interactions in response to acute stress. J. Mol. Med. 2021, 100, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Elamin, A.B.A.; Forsat, K.; Senok, S.S.; Goswami, N. Vagus Nerve Stimulation and Its Cardioprotective Abilities: A Systematic Review. J. Clin. Med. 2023, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Duque-Parra, J.E.; Barco-Ríos, J.; Barco-Cano, J.A. The true apparent origin of glossopharyngeal, vagus and accessory nerves. Rev. Fac. De Med. 2019, 67, 217–220. [Google Scholar] [CrossRef]
- Verlinden, T.J.M.; Rijkers, K.; Hoogland, G.; Herrler, A. Morphology of the human cervical vagus nerve: Implications for vagus nerve stimulation treatment. Acta Neurol. Scand. 2015, 133, 173–182. [Google Scholar] [CrossRef]
- OMS. World Mental Health Report: Transforming Mental Health for All. Executive Summary [Internet]. Ginebra: OMS. 2022. Available online: https://www.who.int/publications/i/item/9789240050860 (accessed on 10 July 2024).
- Gallup Inc. Gallup Global Emotions [Internet]. 2022. Available online: https://www.gallup.com/analytics/349280/gallup-global-emotions-report.aspx (accessed on 20 August 2024).
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Zhang, C.; Dulinskas, R.; Ineichen, C.; Greter, A.; Sigrist, H.; Li, Y.; Alanis-Lobato, G.; Hengerer, B.; Pryce, C.R. Chronic stress deficits in reward behaviour co-occur with low nucleus accumbens dopamine activity during reward anticipation specifically. Commun. Biol. 2024, 7, 966. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; Wen, P.; Chen, Y.; Cao, J.; Sun, X.; Dong, Y. Chronic Stress-induced Serotonin Impairs Intestinal Epithelial Cell Mitochondrial Biogenesis via the AMPK-PGC-1α Axis. Int. J. Biol. Sci. 2024, 20, 4476–4495. [Google Scholar] [CrossRef]
- Morera, L.P.; Tempesti, T.C.; Pérez, E.; Medrano, L.A. Biomarkers in Stress Measurement: A Systematic Review; Elsevier Espana S.L.U: Amsterdam, The Netherlands, 2019; Volume 25, pp. 49–58. [Google Scholar] [CrossRef]
- Ramos-Martínez, I.E.; Rodríguez, M.C.; Cerbón, M.; Ramos-Martínez, J.C.; Ramos-Martínez, E.G. Role of the cholinergic anti-inflammatory reflex in central nervous system diseases. Int. J. Mol. Sci. 2021, 22, 13427. [Google Scholar] [CrossRef]
- Zárate, S.; Acevedo-Triana, C.A.; Sarmiento-Bolaños, M.; Cardenas, P.L.F.; León, L.A. Efectos del estrés sobre los procesos de plasticidad y neurogénesis: Una revisión. Univ. Psychol. 2014, 13, 1181–1214. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Wang, C.-Y.; Wang, Y.; Cai, M.-T.; Zhong, W.-X.; Liu, T.; Wang, Z.-H.; Pan, H.-Q.; Zhang, W.-H.; Pan, B.-X. Circuit- and laminar-specific regulation of medial prefrontal neurons by chronic stress. Cell Biosci. 2023, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Orock, A.; Johnson, A.; Mohammadi, E.; Meerveld, B.G.-V. Environmental enrichment reverses stress-induced changes in the brain-gut axis to ameliorate chronic visceral and somatic hypersensitivity. Neurobiol. Stress 2023, 28, 100590. [Google Scholar] [CrossRef] [PubMed]
- Arzate-Mejia, R.G.; Carullo, N.V.N.; Mansuy, I.M. The Epigenome Under Pressure: On Regulatory Adaptation to Chronic Stress in the Brain [Internet]. 2024. Available online: https://www.zora.uzh.ch/id/eprint/253254/ (accessed on 18 July 2024).
- Xu, J.; Li, C.; Kang, X. The epigenetic regulatory effect of histone acetylation and deacetylation on skeletal muscle metabolism-a review. Front. Physiol. 2023, 14, 1267456. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Xia, T.-J.; Wang, Z.; Jin, S.-W.; Liu, X.-M.; Liu, Y.-G.; Zhang, S.-S.; Pan, R.-L.; Jiang, N.; Liao, Y.-H.; Yan, M.-Z.; et al. Melatonin-related dysfunction in chronic restraint stress triggers sleep disorders in mice. Front. Pharmacol. 2023, 14, 1210393. [Google Scholar] [CrossRef]
- Fonte, C.; Sá, D.; Pimentão, C. Relação entre sentido de vida, ansiedade, depressão e stress em adultos. Inf. Psicológicos 2024, 24, 155–157. [Google Scholar]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic regulation in major depression and other stress-related disorders: Molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, R.; Zhang, Y.; Zhang, R.; Liang, L.; Wang, Y.; Wei, Y.; Zhu, R.; Wang, F. Association between perceived stress and depression among medical students during the outbreak of COVID-19: The mediating role of insomnia. J. Affect. Disord. 2021, 292, 89–94. [Google Scholar] [CrossRef]
- Feng, G.; Xu, X.; Lei, J. Tracking perceived stress, anxiety, and depression in daily life: A double-downward spiral process. Front. Psychol. 2023, 14, 1114332. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J., III; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Schwerdtfeger, A. Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: A meta-analysis. Psychol. Med. 2020, 50, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Massaro, S.; Pecchia, L. Heart Rate Variability (HRV) Analysis: A Methodology for Organizational Neuroscience. Organ. Res. Methods 2016, 22, 354–393. [Google Scholar] [CrossRef]
- Teodorescu, V.; Nanea, T. Măsurătorile standard, interpretarea fi ziologică şi clinică a variabilităţii ritmului cardiac. Perspective clinice postinfarct. Standards of measurement, physiological and clinical interpretation of heart rate variability. Postinfarction clinical perspectives. Rom. J. Med. Pract. 2014, 9, 148–153. [Google Scholar]
- Shaffer, F.; Meehan, Z.M. A Practical Guide to Resonance Frequency Assessment for Heart Rate Variability Biofeedback. Front. Neurosci. 2020, 14, 570400. [Google Scholar] [CrossRef]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal alance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Lehrer, P. How Does Heart Rate Variability Biofeedback Work? Resonance, the Baroreflex, and Other Mechanisms. Biofeedback 2013, 41, 26–31. [Google Scholar] [CrossRef]
- Sasaki, K.; Maruyama, R. Consciously controlled breathing decreases the high-frequency component of heart rate variability by inhibiting cardiac parasympathetic nerve activity. Tohoku J. Exp. Med. 2014, 233, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, K.E.; Bishop, M.D. Chronic Stress, Cortisol Dysfunction, and Pain: A Psychoneuroendocrine Rationale for Stress Management in Pain Rehabilitation. Phys Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Hilton, L.; Hempel, S.; Ewing, B.A.; Apaydin, E.; Xenakis, L.; Newberry, S.; Colaiaco, B.; Maher, A.R.; Shanman, R.M.; Sorbero, M.E.; et al. Mindfulness Meditation for Chronic Pain: Systematic Review and Meta-analysis. Ann. Behav. Med. 2016, 51, 199–213. [Google Scholar] [CrossRef]
- Tang, X.; Lin, S.; Fang, D.; Lin, B.; Yao, L.; Wang, L.; Xu, Q.; Lu, L.; Xu, N. Efficacy and underlying mechanisms of acupuncture therapy for PTSD: Evidence from animal and clinical studies. Front. Behav. Neurosci. 2023, 17, 1163718. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by α-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Zaccaro, A.; Piarulli, A.; Laurino, M.; Garbella, E.; Menicucci, D.; Neri, B.; Gemignani, A. How Breath-Control Can Change Your Life: A Systematic Review on Psycho-Physiological Correlates of Slow Breathing. Front. Hum. Neurosci. 2018, 12, 353. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Park, B.J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Psychological benefits of walking through forest areas. Int. J. Environ. Res. Public Health 2020, 15, 2804. [Google Scholar] [CrossRef]
- Park, B.-J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)—Using salivary cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef]
- Vickhoff, B.; Malmgren, H.; Åström, R.; Nyberg, G.; Ekström, S.R.; Engwall, M.; Jörnsten, R. Music structure determines heart rate variability of singersmusic structure determines heart rate variability of singers. Front. Psychol. 2013, 4, 334. [Google Scholar]
- Arvidson, E.; Dahlman, A.S.; Börjesson, M.; Gullstrand, L.; Jonsdottir, I.H. The effects of exercise training on hypothalamic-pituitary-adrenal axis reactivity and autonomic response to acute stress—A randomized controlled study. Trials 2020, 21, 888. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Rusia, A.; Haskin-Popp, C.; Tawney, A. Chronic stress, exercise and cardiovascular disease: Placing the benefits and risks of physical activity into perspective. Int. J. Environ. Res. Public Health 2021, 18, 9922. [Google Scholar] [CrossRef] [PubMed]
- Gurel, N.Z.; Huang, M.; Wittbrodt, M.T.; Jung, H.; Ladd, S.L.; Shandhi, M.H.; Ko, Y.-A.; Shallenberger, L.; Nye, J.A.; Pearce, B.; et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2019, 13, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Parker, S.H. Biofeedback as a stress management tool: A systematic review. Cogn. Technol. Work 2018, 21, 161–190. [Google Scholar] [CrossRef]
- Tanzmeister, S.; Rominger, C.; Weber, B.; Tatschl, J.M.; Schwerdtfeger, A.R. Singing at 0.1 Hz as a Resonance Frequency Intervention to Reduce Cardiovascular Stress Reactivity? Front. Psychiatry 2022, 13, 876344. [Google Scholar] [CrossRef]
- Basson, A.; Olivier, B.; Ellis, R.; Coppieters, M.; Stewart, A.; Mudzi, W. The effectiveness of neural mobilization for neuromusculoskeletal conditions: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 593–615. [Google Scholar] [CrossRef]
- Shamsi, H.; Khademi-Kalantari, K.; Okhovatian, F. Effects of Neural Mobilization Techniques on Pain and Disability in Patients With Neurodynamic Dysfunction: A Systematic Review of Randomized Controlled Trials. J. Mod. Rehabil. 2021, 15, 209–218. [Google Scholar]
- de Faria, S.C.S.; Vilella, R.C.; Guimaraes, L.H.d.C.T.; Lunkes, L.C.; Motta, U.C.U.A. Effects of neural mobilization in the treatment of chronic low back pain: A systematic review. Iberoam. J. Med. 2022, 4, 157–163. [Google Scholar] [CrossRef]
- Carta, G.; Seregni, A.; Casamassima, A.; Manuela, G.; Geuna, S.; Zago, M. Discovering the Vagus: Validation and Inter-Rater Reliability of the Vagus Nerve Neuro-dynamic Test Among Healthy Subjects. 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3529450 (accessed on 23 March 2023).
- Felten, D.L.; Netter, F.H.; Machado, C.A.G.; Perkins, J.A.; Maida, M.S.; Craig, J.A. Netter Atlas de Neurociencia [Internet]. 2016. Available online: https://search.ebscohost.com/login.aspx?direct=true&db=cat06387a&AN=crai.88772&site=eds-live (accessed on 20 July 2024).
- Mccraty, R.; Atkinson, M.; Tomasino, D.; Bradley, R.T. The Coherent Heart Heart-Brain Interactions, Psychophysiological Coherence, and the Emergence of System-Wide Order. Integral Rev. 2009, 5, 25. [Google Scholar]
- Schwerdtfeger, A.R.; Schwarz, G.; Pfurtscheller, K.; Thayer, J.F.; Jarczok, M.N.; Pfurtscheller, G. Heart rate variability (HRV): From brain death to resonance breathing at 6 breaths per minute. Clin. Neurophysiol. 2019, 131, 676–693. [Google Scholar] [CrossRef]
- Pagaduan, J.; Wu, S.S.; Kameneva, T.; Lambert, E. Acute effects of resonance frequency breathing on cardiovascular regulation. Physiol. Rep. 2019, 7, e14295. [Google Scholar] [CrossRef] [PubMed]
- Bouny, P.; Arsac, L.M.; Guérin, A.; Nerincx, G.; Deschodt-Arsac, V. Guiding Breathing at the Resonance Frequency with Haptic Sensors Potentiates Cardiac Coherence. Sensors 2023, 23, 4494. [Google Scholar] [CrossRef] [PubMed]
- Giles, P.D.; Hensel, K.L.; Pacchia, C.F.; Smith, M.L. Suboccipital decompression enhances heart rate variability indices of cardiac control in healthy subjects. J. Altern. Complement. Med. 2013, 19, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Kulakowski, P.; Brignole, M.; de Lange, F.J.; Kenny, R.A.; Moya, A.; Rivasi, G.; Sheldon, R.; Van Dijk, G.; Sutton, R.; et al. Twenty-five years of research on syncope. Europace 2023, 25, euad163. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, T.; Raj, S.; Sheldon, R.S. Current approach to the treatment of vasovagal syncope in adults. Intern. Emerg. Med. 2022, 18, 23–30. [Google Scholar] [CrossRef]
- Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing. Heart Rate Variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Hernando, A.; Lazaro, J.; Gil, E.; Arza, A.; Garzon, J.M.; Lopez-Anton, R.; de la Camara, C.; Laguna, P.; Aguilo, J.; Bailon, R. Inclusion of Respiratory Frequency Information in Heart Rate Variability Analysis for Stress Assessment. IEEE J. Biomed. Health Informatics 2016, 20, 1016–1025. [Google Scholar] [CrossRef]
- Tabor, A.; Bateman, S.; Scheme, E.J.; Schraefel, M. Comparing heart rate variability biofeedback and simple paced breathing to inform the design of guided breathing technologies. Front. Comput. Sci. 2022, 4, 926649. [Google Scholar] [CrossRef]
- Magnon, V.; Dutheil, F.; Vallet, G.T. Benefits from one session of deep and slow breathing on vagal tone and anxiety in young and older adults. Sci. Rep. 2021, 11, 19267. [Google Scholar] [CrossRef]
- Laborde, S.; Allen, M.S.; Borges, U.; Iskra, M.; Zammit, N.; You, M.; Hosang, T.; Mosley, E.; Dosseville, F. Psychophysiological effects of slow-paced breathing at six cycles per minute with or without heart rate variability biofeedback. Psychophysiology 2021, 59, e13952. [Google Scholar] [CrossRef]
- Steffen, P.R.; Austin, T.; DeBarros, A.; Brown, T. The Impact of Resonance Frequency Breathing on Measures of Heart Rate Variability, Blood Pressure, and Mood. Front. Public Health 2017, 5, 222. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.; Tai, L.; Fan, S. Breathing at a rate of 5.5breaths per minute with equal inhalation-to-exhalation ratio increases heart rate variability. Int. J. Psychophysiol. 2014, 91, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Jiang, X.; Hu, Y.-Y.; Liang, Q.-S.; He, Z.-S.; Xue, Y.; Zhu, W.; Tang, Z.-X.; Hou, Y.-Y.; et al. Effect of voluntary breathing exercises on stable coronary artery disease in heart rate variability and rate-pressure product: A study protocol for a single-blind, prospective, randomized controlled trial. Trials 2020, 21, 602. [Google Scholar] [CrossRef] [PubMed]
- Soer, R.; Dijkstra, M.W.S.; Bieleman, H.J.; Oosterveld, F.G.; Rijken, N.H.; Bieleman, A. Influence of respiration frequency on heart rate variability parameters: A randomized cross-sectional study. J. Back Musculoskelet. Rehabil. 2021, 34, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.; Vanacker, L.; De Couck, M.; De Leeuw, I.; Gidron, Y. Neuromodulation Applied to Diseases: The Case of HRV Biofeedback. J. Clin. Med. 2022, 11, 5927. [Google Scholar] [CrossRef]
- Hayano, J.; Yuda, E. Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthr. 2019, 38, 3. [Google Scholar] [CrossRef]
- You, M.; Laborde, S.; Zammit, N.; Iskra, M.; Borges, U.; Dosseville, F.; Vaughan, R.S. Emotional intelligence training: Influence of a brief slow-paced breathing exercise on psychophysiological variables linked to emotion regulation. Int. J. Environ. Res. Public Health 2021, 18, 6630. [Google Scholar] [CrossRef]
- Kromenacker, B.W.M.; Sanova, A.A.B.; Marcus, F.I.; Allen, J.J.; Lane, R.D. Vagal Mediation of Low-Frequency Heart Rate Variability During Slow Yogic Breathing. Psychosom. Med. 2018, 80, 581–587. [Google Scholar] [CrossRef]
- Young, F.L.; Leicht, A.S. Short-term stability of resting heart rate variability: Influence of position and gender. Appl. Physiol. Nutr. Metab. 2011, 36, 210–218. [Google Scholar] [CrossRef]
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, I.; Adam, D.; Gordon, D.A.V.I.D.; Cohen, R.J. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol.-Heart Circ. Physiol. 1985, 248, H151–H153. [Google Scholar] [CrossRef]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (n = 54) | Group with Neurodynamics (n = 28) | Group Without Neurodynamics (n = 26) | p |
|---|---|---|---|---|
| Age (years) | 45.5 (40.0, 52.0) | 44.5 (35.5, 52.3) | 46.5 (42.3, 52.0) | 0.204 |
| Sex (Male, n (%)) | 13 (24.1) | 9 (32.1) | 4 (15.4) | 0.150 |
| Height (cm) | 167.0 (160.0, 170.0) | 165.0 (160.8, 169.3) | 167.0 (160.0, 170.0) | 0.975 |
| Weight (kg) | 67.0 (60.5, 75.0) | 70.0 (61.5, 75.5) | 65.0 (60.5, 73.3) | 0.415 |
| BMI (kg/m2) | 24.1 (22.9, 26.0) | 24.1 (22.8, 26.1) | 24.1 (22.9, 25.9) | 0.556 |
| Variable | Total (n = 54) | Group with Neurodynamics (n = 28) | Group Without Neurodynamics (n = 26) | p |
|---|---|---|---|---|
| Pre-intervention | ||||
| Mean HR (bpm) | 66.3 [60.5, 71.5] | 64.7 [60.3, 70.2] | 67.0 [62.5, 76.7] | 0.497 |
| SDNN (ms) | 34.6 [25.1, 48.1] | 33.3 [25.4, 46.2] | 36.2 [25.1, 52.7] | 0.455 |
| Diff HR (bpm) | 15.1 [11.4, 18.1] | 14.6 [10.9, 17.6] | 15.8 [12.7, 18.1] | 0.508 |
| RMSSD (ms) | 31.9 [21.1, 45.6] | 30.7 [20.3, 44.4] | 33.5 [22.9, 47.6] | 0.790 |
| NN50 (number of NN intervals) | 23.0 [7.0, 73.3] | 22.0 [7.0, 75.3] | 33.5 [10.3, 69.8] | 0.723 |
| pNN50 (%) | 0.07 [0.02, 0.24] | 0.07 [0.02, 0.23] | 0.09 [0.03, 0.24] | 0.684 |
| HF (n.u.) | 35.0 [18.6, 53.7] | 35.4 [17.1, 54.1] | 34.8 [24.7, 52.7] | 0.911 |
| During intervention | ||||
| Mean HR (bpm) | 64.0 [58.6, 71.5] | 61.4 [59.5, 66.1] | 66.5 [56.8, 73.1] | 0.444 |
| SDNN (ms) | 50.5 [39.5, 71.6] | 50.5 [40.6, 67.5] | 51.2 [36.2, 76.1] | 0.843 |
| Diff HR (bpm) | 16.5 [12.5, 21.1] | 14.8 [12.1, 21.7] | 17.0 [13.7, 19.9] | 0.665 |
| RMSSD (ms) | 36.6 [25.1, 50.9] | 38.6 [29.0, 50.7] | 36.2 [21.4, 61.1] | 0.519 |
| NN50 (number of NN intervals) | 48.0 [18.3, 90.0] | 49.0 [24.3, 81.8] | 47.0 [12.0, 94.3] | 0.562 |
| pNN50 (%) | 0.15 [0.06, 0.32] | 0.15 [0.07, 0.29] | 0.16 [0.04, 0.33] | 0.917 |
| HF (n.u.) | 12.1 [8.1, 19.4] | 14.3 [10.1, 20.4] | 9.2 [6.8, 13.2] | 0.012 |
| Post intervention | ||||
| Mean HR (bpm) | 63.1 [57.7, 71.9] | 62.7 [57.3, 71.7] | 67.3 [59.1, 72.3] | 0.519 |
| SDNN (ms) | 32.3 [24.7, 41.9] | 32.3 [23.6, 44.1] | 32.4 [26.7, 41.7] | 0.712 |
| Diff HR (bpm) | 13.3 [10.2, 18.7] | 12.6 [9.8, 18.8] | 13.6 [12.3, 18.5] | 0.260 |
| RMSSD (ms) | 29.3 [20.0, 39.2] | 28.6 [19.9, 39.4] | 29.3 [21.0, 37.9] | 0.938 |
| NN50 (number of NN intervals) | 29.5 [9.0, 48.0] | 22.5 [6.0, 52.3] | 32.0 [9.0, 43.3] | 0.703 |
| pNN50 (%) | 0.08 [0.02, 0.16] | 0.06 [0.02, 0.20] | 0.08 [0.03, 0.13] | 0.710 |
| HF (n.u.) | 35.3 [25.4, 49.0] | 34.2 [26.2, 55.4] | 36.2 [24.1, 46.3] | 0.764 |
| Pre-Intervention vs. Intervention | ||
| Variable | Group with neurodynamics (n = 28) | Group without neurodynamics (n = 26) |
| Mean HR (bpm) | 0.002 | <0.001 |
| SDNN (ms) | <0.001 | <0.001 |
| Diff HR (bpm) | 0.032 | 0.380 |
| RMSSD (ms) | <0.001 | 0.049 |
| NN50 (number of NN intervals) | 0.004 | 0.100 |
| pNN50 (%) | 0.572 | 0.080 |
| HF (n.u.) | <0.001 | <0.001 |
| Pre-Intervention vs. Post Intervention | ||
| Variable | Group with neurodynamics (n = 28) | Group without neurodynamics (n = 26) |
| Mean HR (bpm) | 0.010 | 0.024 |
| SDNN (ms) | 0.537 | 0.105 |
| Diff HR (bpm) | 0.662 | 0.437 |
| RMSSD (ms) | 0.274 | 0.053 |
| NN50 (number of NN intervals) | 0.263 | 0.018 |
| pNN50 (%) | 0.572 | 0.041 |
| HF (n.u.) | 0.762 | 0.499 |
| Variable | Group with Neurodynamics (n = 28) | Group Without Neurodynamics (n = 26) | p |
|---|---|---|---|
| Pre-intervention—during intervention | |||
| Mean HR (bpm) | 2.48 [0.92, 4.04] | 3.05 [1.74, 4.35] | 0.661 |
| SDNN (ms) | −23.30 [−31.70, −15.00] | −16.70 [−22.50, −10.90] | 0.322 |
| Diff HR (bpm) | −1.56 [−2.99, −0.13] | −1.12 [−3.22, 0.97] | 0.600 |
| RMSSD (ms) | −11.50 [−18.30, −4.62] | −5.40 [−10.40, −0.43] | 0.267 |
| NN50 (number of NN intervals) | −20.10 [−32.20, −7.96] | −6.81 [−18.8, 5.14] | 0.215 |
| pNN50 (%) | −0.07 [−0.11, −0.03] | 1.99 [−2.39, 6.36] | 0.377 |
| HF (n.u.) | 22.10 [14.60, 29.60] | 26.10 [17.30, 34.90] | 0.465 |
| Pre-intervention—post intervention | |||
| Mean HR (bpm) | 2.02 [0.34, 3.70] | 1.79 [0.29, 3.29] | 0.803 |
| SDNN (ms) | 0.57 [−4.05, 5.20] | 3.64 [−0.77, 8.04] | 0.404 |
| Diff HR (bpm) | 0.39 [−1.53, 2.31] | 0.64 [−1.78, 3.05] | 0.870 |
| RMSSD (ms) | 2.40 [−1.44, 6.25] | 4.06 [0.37, 7.75] | 0.486 |
| NN50 (number of NN intervals) | 8.07 [−2.51, 18.60] | 13.90 [3.28, 24.60] | 0.359 |
| pNN50 (%) | 0.02 [−0.02, 0.05] | 2.16 [−2.21, 6.53] | 0.245 |
| HF (n.u.) | −0.52 [−8.72, 7.68] | 2.13 [−6.63, 10.9] | 0.476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Alcalde, A.I.; Galán-del-Río, F.; Fernández-Rodríguez, F.J.; de la Plaza San Frutos, M.; García-Arrabé, M.; Giménez, M.-J.; Ruiz-Ruiz, B. The Effects of a Single Vagus Nerve’s Neurodynamics on Heart Rate Variability in Chronic Stress: A Randomized Controlled Trial. Sensors 2024, 24, 6874. https://doi.org/10.3390/s24216874
Pérez-Alcalde AI, Galán-del-Río F, Fernández-Rodríguez FJ, de la Plaza San Frutos M, García-Arrabé M, Giménez M-J, Ruiz-Ruiz B. The Effects of a Single Vagus Nerve’s Neurodynamics on Heart Rate Variability in Chronic Stress: A Randomized Controlled Trial. Sensors. 2024; 24(21):6874. https://doi.org/10.3390/s24216874
Chicago/Turabian StylePérez-Alcalde, Ana Isabel, Fernando Galán-del-Río, Francisco J. Fernández-Rodríguez, Marta de la Plaza San Frutos, María García-Arrabé, María-José Giménez, and Beatriz Ruiz-Ruiz. 2024. "The Effects of a Single Vagus Nerve’s Neurodynamics on Heart Rate Variability in Chronic Stress: A Randomized Controlled Trial" Sensors 24, no. 21: 6874. https://doi.org/10.3390/s24216874
APA StylePérez-Alcalde, A. I., Galán-del-Río, F., Fernández-Rodríguez, F. J., de la Plaza San Frutos, M., García-Arrabé, M., Giménez, M.-J., & Ruiz-Ruiz, B. (2024). The Effects of a Single Vagus Nerve’s Neurodynamics on Heart Rate Variability in Chronic Stress: A Randomized Controlled Trial. Sensors, 24(21), 6874. https://doi.org/10.3390/s24216874






