Motion Tape Strain During Trunk Muscle Engagement in Young, Healthy Participants
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Motion Tape
2.3. Motion Tape Placement
2.4. Three-Dimensional Optical Motion Capture
2.5. MVIC Testing Protocol
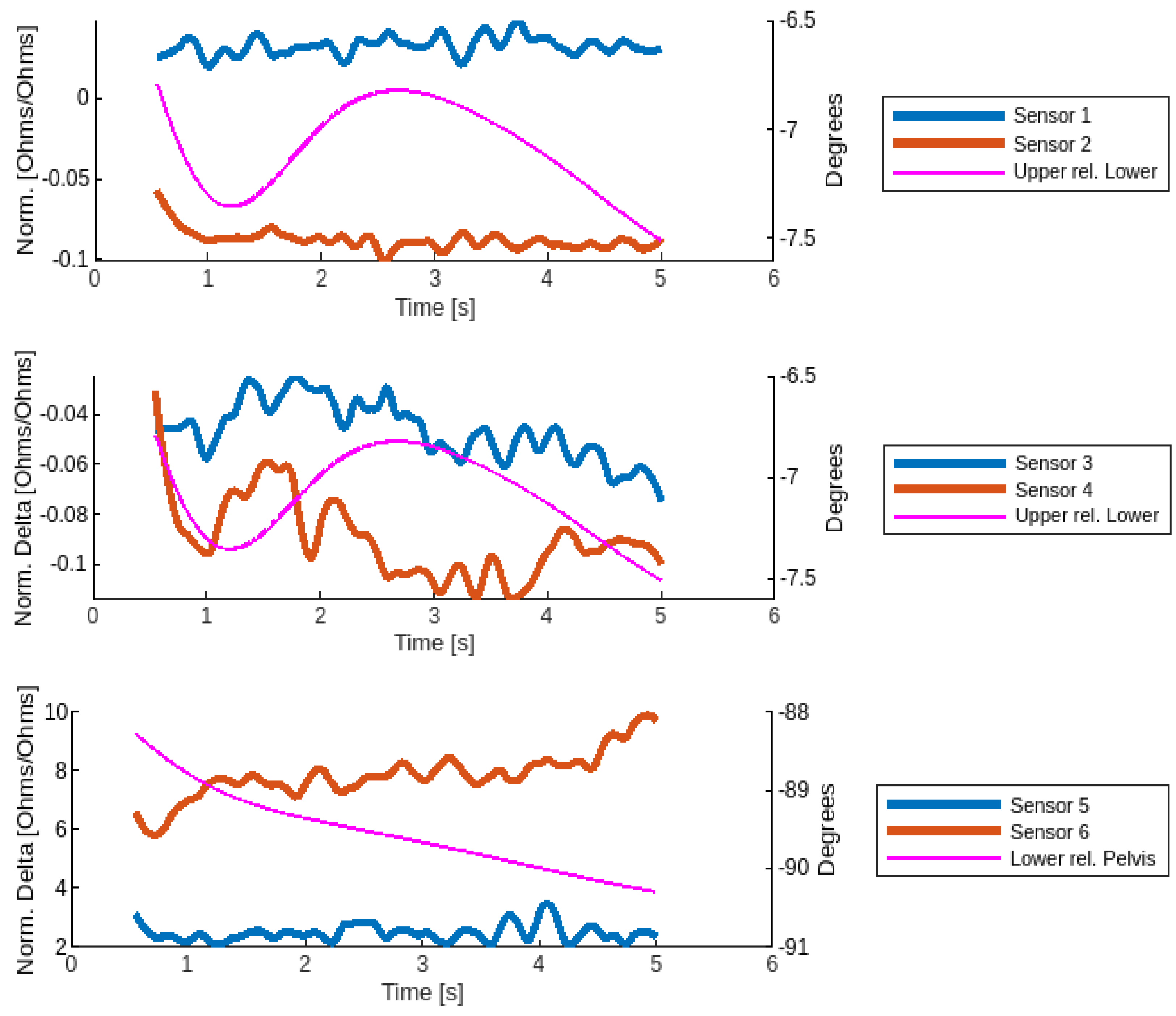
2.6. Data Processing
2.7. Data Analysis
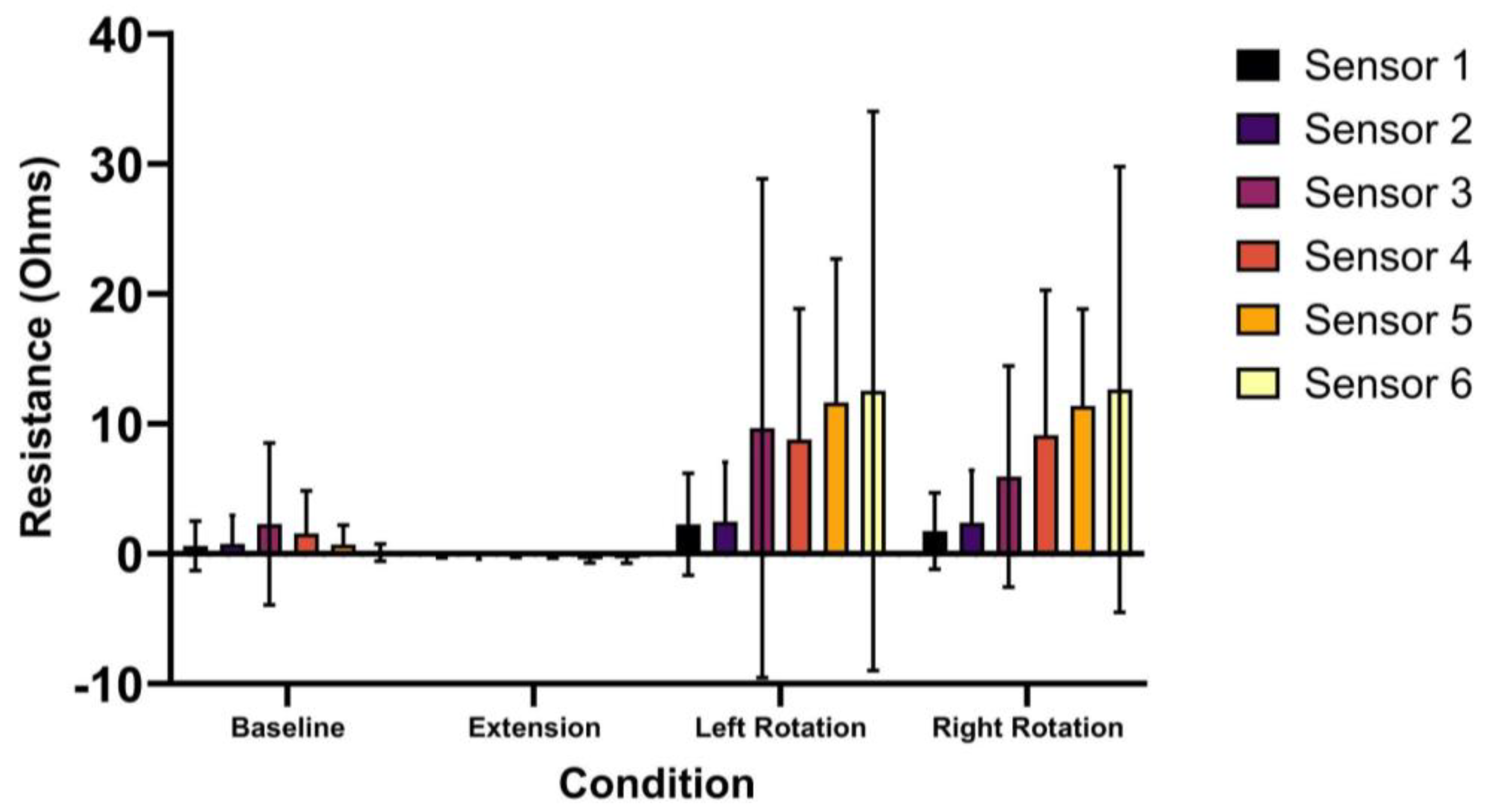
3. Results
4. Discussion
4.1. Comparison to Existing Technology
4.2. Clinical Implications
4.3. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, S.; Caro, J.; Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef] [PubMed]
- van Dieen, J.H.; Selen, L.P.; Cholewicki, J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J. Electromyogr. Kinesiol. 2003, 13, 333–351. [Google Scholar] [CrossRef]
- Geisser, M.E.; Ranavaya, M.; Haig, A.J.; Roth, R.S.; Zucker, R.; Ambroz, C.; Caruso, M. A meta-analytic review of surface electromyography among persons with low back pain and normal, healthy controls. J. Pain 2005, 6, 711–726. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Wright, A. The effect of musculoskeletal pain on motor activity and control. J. Pain 2001, 2, 135–145. [Google Scholar] [CrossRef]
- Oddsson, L.I.; De Luca, C.J. Activation imbalances in lumbar spine muscles in the presence of chronic low back pain. J. Appl. Physiol. (1985) 2003, 94, 1410–1420. [Google Scholar] [CrossRef]
- van Dieen, J.H.; Reeves, N.P.; Kawchuk, G.; van Dillen, L.R.; Hodges, P.W. Motor Control Changes in Low Back Pain: Divergence in Presentations and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 370–379. [Google Scholar] [CrossRef]
- Hodges, P.W.; van den Hoorn, W. A vision for the future of wearable sensors in spine care and its challenges: Narrative review. J. Spine Surg. 2022, 8, 103–116. [Google Scholar] [CrossRef]
- Papi, E.; Koh, W.; Mcgregor, A. Wearable technology for spine movement assessment: A systematic review. J. Biomech. 2017, 64, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Claus, A.P.; Hides, J.A.; Moseley, G.L.; Hodges, P.W. Thoracic and lumbar posture behaviour in sitting tasks and standing: Progressing the biomechanics from observations to measurements. Appl. Ergon. 2016, 53 Pt A, 161–168. [Google Scholar] [CrossRef]
- Feng, T.; Ling, D.; Li, C.; Zheng, W.; Zhang, S.; Li, C.; Emel’yanov, A.; Pozdnyakov, A.S.; Lu, L.; Mao, Y. Stretchable on-skin touchless screen sensor enabled by ionic hydrogel. Nano Res. 2024, 17, 4462–4470. [Google Scholar] [CrossRef]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire-elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef]
- Lin, Y.A.; Mhaskar, Y.; Silder, A.; Sessoms, P.H.; Fraser, J.J.; Loh, K.J. Muscle Engagement Monitoring Using Self-Adhesive Elastic Nanocomposite Fabrics. Sensors 2022, 22, 6768. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Schraefel, M.; Chiang, W.; Loh, K. Wearable nanocomposite kinesiology tape for distributed muscle engagement monitoring. MRS Adv. 2020, 6, 6–13. [Google Scholar] [CrossRef]
- Appelle, A.; Lin, Y.-A.; Noble, E.; Salvino, L.; Loh, K.J.; Lynch, J.P. Wearable Sensor Platform to Monitor Physical Exertion Using Graphene Motion Tape. In European Workshop on Structural Health Monitoring; Springer: Cham, Switzerland, 2023; pp. 894–904. [Google Scholar]
- Lee, A.; Wyckoff, E.; Farcas, E.; Godino, J.; Patrick, K.; Spiegel, S.; Yu, R.; Kumar, A.; Loh, K.J.; Gombatto, S. Preliminary Validity and Acceptability of Motion Tape for Measuring Low Back Movement: Mixed Methods Study. JMIR Rehabil. Assist. Technol. 2024, 11, e57953. [Google Scholar] [CrossRef]
- Lee, A.; Dionicio, P.; Farcas, E.; Godino, J.; Patrick, K.; Wyckoff, E.; Loh, K.J.; Gombatto, S.P. Physical Therapists’ Acceptance of a Wearable, Fabric-Based Sensor System (Motion Tape) for Use in Clinical Practice: Qualitative Focus Group Study. JMIR Hum. Factors 2024, 11, e55246. [Google Scholar] [CrossRef]
- Lin, Y.-A.; Zhao, Y.; Wang, L.; Park, Y.; Yeh, Y.-J.; Chiang, W.-H.; Loh, K.J. Graphene K-Tape Meshes for Densely Distributed Human Motion Monitoring. Adv. Mater. Technol. 2021, 6, 2000861. [Google Scholar] [CrossRef]
- Pierce, T.; Lin, Y.-A.; Loh, K.J. Wireless Gait and Respiration Monitoring using Nanocomposite Sensors. In Proceedings of the Structural Health Monitoring 2023, Stanford, CA, USA, 12–14 September 2023; pp. 1997–2004. [Google Scholar]
- Gombatto, S.E.M.; Joyce, C.; Linske, G.; Muehlenkamp, P. Validity and reliability of a multi-segmental lumbar spine model using a 3D motion analysis system. In Proceedings of the American Physical Therapy Association Combined Sections Meeting, San Diego, CA, USA, 21–24 January 2013. [Google Scholar]
- Mitchell, K.; Porter, M.; Anderson, L.; Phillips, C.; Arceo, G.; Montz, B.; Levy, S.; Gombatto, S.P. Differences in lumbar spine and lower extremity kinematics in people with and without low back pain during a step-up task: A cross-sectional study. BMC Musculoskelet. Disord. 2017, 18, 369. [Google Scholar] [CrossRef]
- Gombatto, S.P.; Brock, T.; DeLork, A.; Jones, G.; Madden, E.; Rinere, C. Lumbar spine kinematics during walking in people with and people without low back pain. Gait Posture 2015, 42, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lehman, G.J.; McGill, S.M. The importance of normalization in the interpretation of surface electromyography: A proof of principle. J. Manip. Physiol. Ther. 1999, 22, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-C.; Lin, Y.-A.; Pierce, T.; Wyckoff, E.; Loh, K.J. Measuring the Golf Swing Pattern Using Motion Tape for Feedback and Fault Detection. In Proceedings of the Structural Health Monitoring 2023, Stanford, CA, USA, 12–14 September 2023. [Google Scholar]
- Ekstrom, R.A.; Osborn, R.W.; Hauer, P.L. Surface electromyographic analysis of the low back muscles during rehabilitation exercises. J. Orthop. Sports Phys. Ther. 2008, 38, 736–745. [Google Scholar] [CrossRef] [PubMed]
- van Dieen, J.H.; Reeves, N.P.; Kawchuk, G.; van Dillen, L.R.; Hodges, P.W. Analysis of Motor Control in Patients with Low Back Pain: A Key to Personalized Care? J. Orthop. Sports Phys. Ther. 2019, 49, 380–388. [Google Scholar] [CrossRef]
- Kent, P.; Laird, R.; Haines, T. The effect of changing movement and posture using motion-sensor biofeedback, versus guidelines-based care, on the clinical outcomes of people with sub-acute or chronic low back pain-a multicentre, cluster-randomised, placebo-controlled, pilot trial. BMC Musculoskelet. Disord. 2015, 16, 131. [Google Scholar] [CrossRef]
- Loh, K.; Huang, S.-C.; Lin, Y.-A. Enhancing Warfighter Marksmanship Performance using Motion Tape Elastic Fabric Sensors. In Proceedings of the Interservice/Industry Training, Simulation, and Education Conference, Orlando, FL, USA, 28 November–2 December 2022. [Google Scholar]
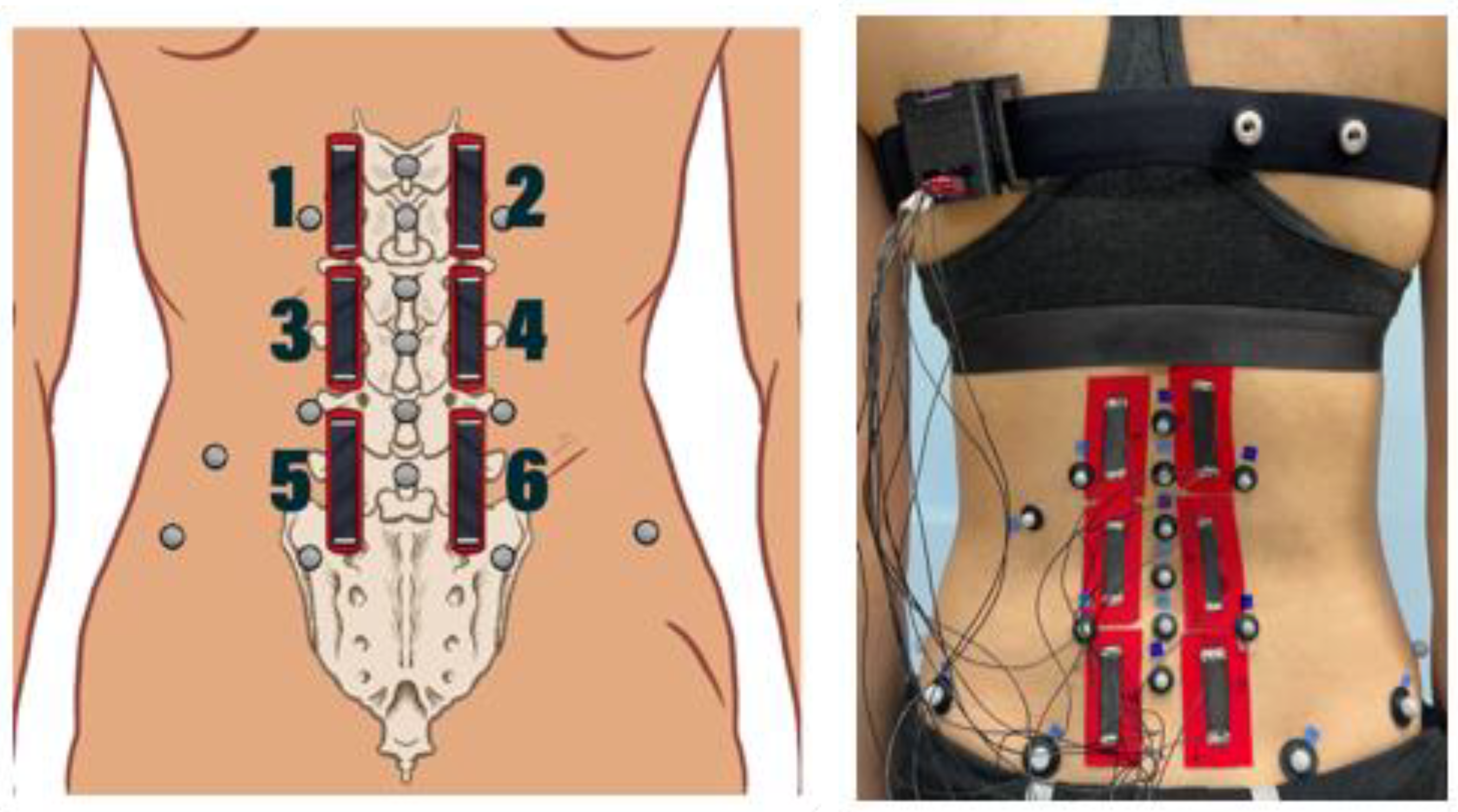
| Condition | Description | Visual |
|---|---|---|
| Baseline (BASE) | Participant is prone on the treatment table with low back muscles relaxed. | 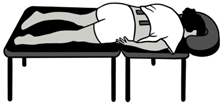 |
| MVIC Extension (EXT) | Participant is prone with the pelvis supported on the treatment table up to the level of the anterior superior iliac spine. The participant holds the trunk level with the table in a neutral position against maximal examiner resistance on the upper back. | 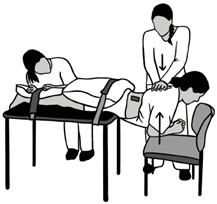 |
| MVIC Left/Right Rotation (LROT/RROT) | Participant is seated upright at the edge of the treatment table, with feet supported on the ground and arms crossed. Participant pushes against maximal examiner resistance into the left rotation direction. This was repeated for the right rotation direction. |  |
| Baseline (BASE) | MVIC Extension (EXT) | MVIC Left Rotation (LROT) | MVIC Right Rotation (RROT) | |
|---|---|---|---|---|
| Upper relative to Lower (degrees) | 1.0° (1.2°) | 3.3° (2.8°) | 3.3° (4.7°) | 2.3° (2.1°) |
| Lower relative to Pelvis (degrees) | 1.1° (1.9°) | 3.3° (1.9°) | 2.1° (3.0°) | 1.7° (1.1°) |
| BASE | EXT | LROT | RROT | |
|---|---|---|---|---|
| Sensor 1 | 0.6 (1.9) | −0.1 (0.1) | 2.3 (3.9) | 1.8 (2.9) |
| Sensor 2 | 0.8 (2.2) | −0.2 (0.2) | 2.5 (4.6) | 2.4 (4.0) |
| Sensor 3 | 2.3 (6.2) | −0.1 (0.1) | 9.7 (19.2) | 6.0 (8.5) |
| Sensor 4 | 1.6 (3.3) | −0.1 (0.3) | 8.8 * (10.1) | 9.1 * (11.2) |
| Sensor 5 | 0.7 (1.5) | −0.4 * (0.3) | 11.6 * (11.0) | 11.4 * (7.5) |
| Sensor 6 | 0.1 (0.7) | −0.4 * (0.4) | 12.5 (21.5) | 12.6 * (17.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiegel, S.; Wyckoff, E.; Barolo, J.; Lee, A.; Farcas, E.; Godino, J.; Patrick, K.; Loh, K.J.; Gombatto, S.P. Motion Tape Strain During Trunk Muscle Engagement in Young, Healthy Participants. Sensors 2024, 24, 6933. https://doi.org/10.3390/s24216933
Spiegel S, Wyckoff E, Barolo J, Lee A, Farcas E, Godino J, Patrick K, Loh KJ, Gombatto SP. Motion Tape Strain During Trunk Muscle Engagement in Young, Healthy Participants. Sensors. 2024; 24(21):6933. https://doi.org/10.3390/s24216933
Chicago/Turabian StyleSpiegel, Spencer, Elijah Wyckoff, Jay Barolo, Audrey Lee, Emilia Farcas, Job Godino, Kevin Patrick, Kenneth J. Loh, and Sara P. Gombatto. 2024. "Motion Tape Strain During Trunk Muscle Engagement in Young, Healthy Participants" Sensors 24, no. 21: 6933. https://doi.org/10.3390/s24216933
APA StyleSpiegel, S., Wyckoff, E., Barolo, J., Lee, A., Farcas, E., Godino, J., Patrick, K., Loh, K. J., & Gombatto, S. P. (2024). Motion Tape Strain During Trunk Muscle Engagement in Young, Healthy Participants. Sensors, 24(21), 6933. https://doi.org/10.3390/s24216933







