A Step Forward for Smart Clothes: Printed Fabric-Based Hybrid Electronics for Wearable Health Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
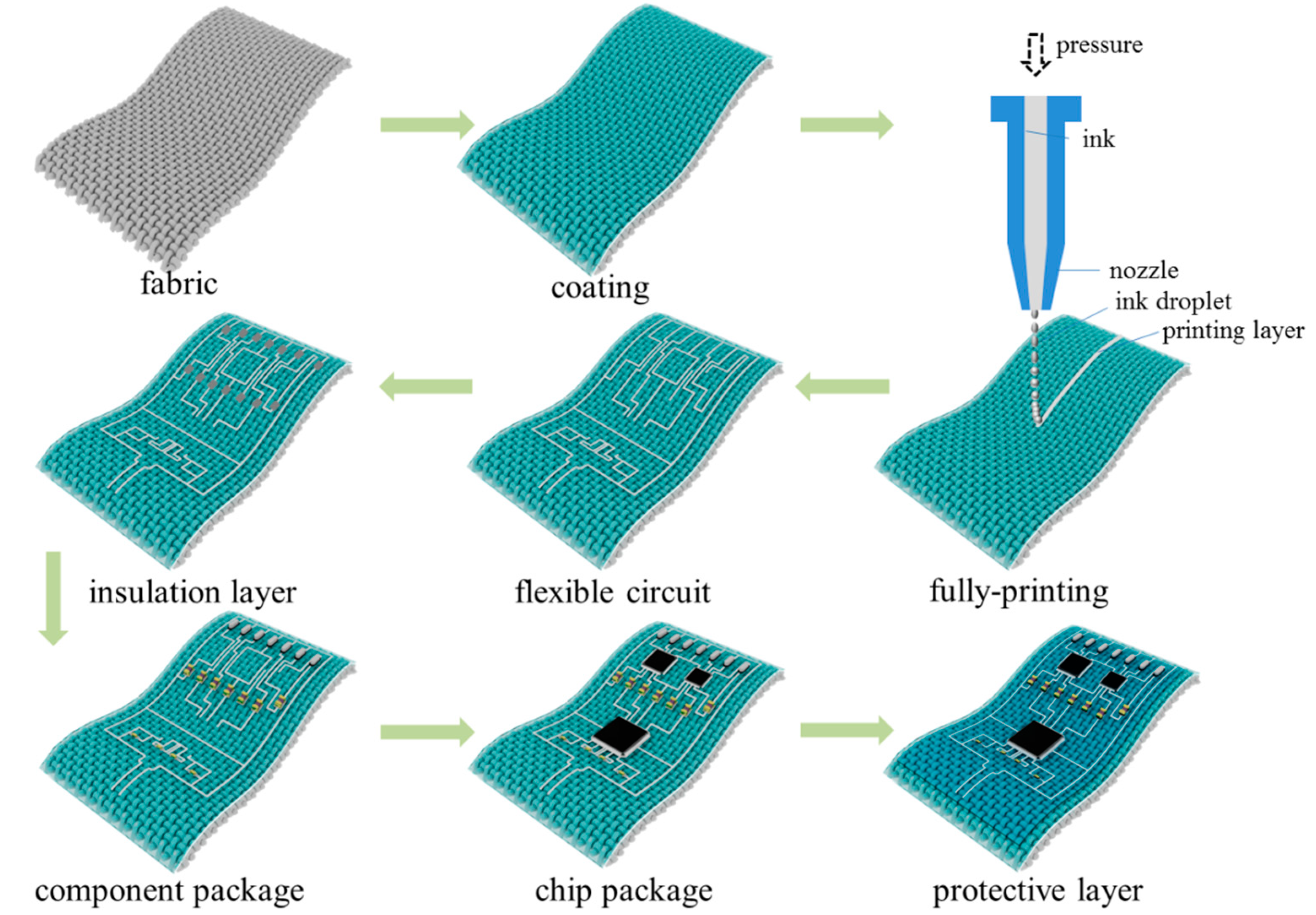
2.2. Design and Fabrication
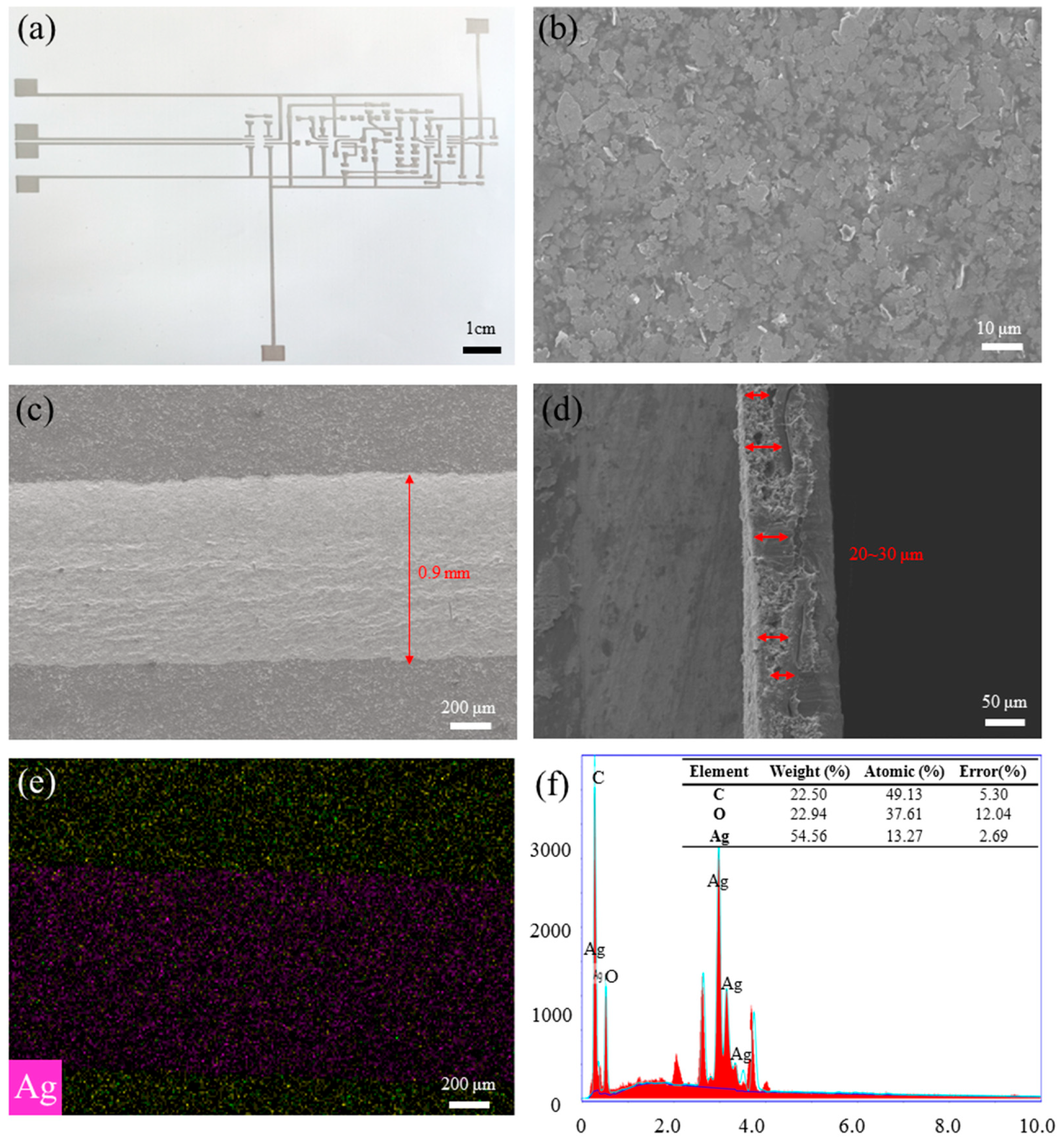
2.3. Microscopic/Epidermal Morphological Characterization
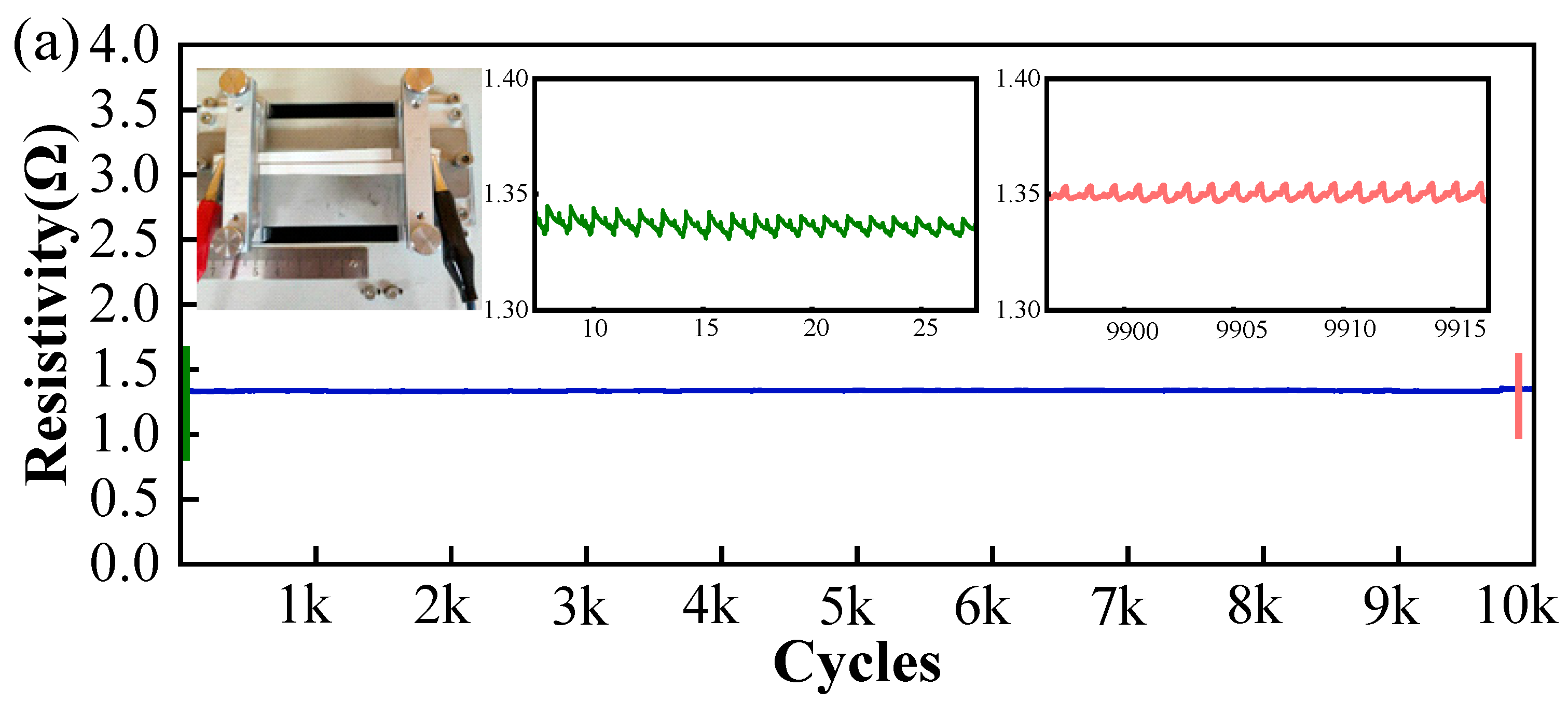
2.4. Electrical/Electrochemical Characterization
3. Results and Discussion
3.1. ECG Signal Acquisition
3.2. Real-Time Electrophysiological Signal Monitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannigan, B.C.; Cuthbert, T.J.; Ahmadizadeh, C.; Menon, C. Distributed sensing along fibers for smart clothing. Sci. Adv. 2024, 10, eadj9708. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.; Teay, S.H.; Sayem, A.S.M.; Albarbar, A. Smart clothing framework for health monitoring applications. Signals 2022, 3, 113–145. [Google Scholar] [CrossRef]
- Wicaksono, I.; Tucker, C.I.; Sun, T.; Guerrero, C.A.; Liu, C.; Woo, W.M.; Pence, E.J.; Dagdeviren, C. A tailored, electronic textile conformable suit for large-scale spatiotemporal physiological sensing in vivo. NPJ Flex. Electron. 2020, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A Wearable Chemical-electrophysiological Hybrid Biosensing System for Real-time Health and Fitness Monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Khoshmanesh, F.; Thurgood, P.; Pirogova, E.; Nahavandi, S.; Baratchi, S. Wearable sensors: At the Frontier of Personalised Health Monitoring, Smart Prosthetics and Assistive Technologies. Biosens. Bioelectron. 2021, 176, 112946. [Google Scholar] [CrossRef]
- Qiu, Y.; He, X.; Li, Z.; Peng, Z.; Huang, Y.; Yu, X. Wearable Sensors for Motion and Electrophysiological Signal Tracking in XR. Korean J. Chem. Eng. 2024, 20, 1–26. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Silva, J.S.; Rodrigues, A.; Boavida, F. A Survey of Approaches to Unobtrusive Sensing of Humans. ACM Comput. Surv. 2022, 55, 1–28. [Google Scholar] [CrossRef]
- bin Ahmad, M.A.S.; Harun, F.K.C.; Wicaksono, D.H. Hybrid Flexible Circuit on Cotton Fabric for Wearable Electrocardiogram Monitoring. In Proceedings of the 2017 International Electronics Symposium on Engineering Technology and Applications (IES-ETA), Surabaya, Indonesia, 26–27 September 2017; pp. 217–222. [Google Scholar]
- Guo, H.; Saifi, S.; Fukuda, K.; Cheng, H.-M.; Lou, Z.; Xu, X. Flexible organic photodetectors and their use in wearable systems. Digit. Signal Process. 2022, 125, 103145. [Google Scholar] [CrossRef]
- Etana, B.B.; Malengier, B.; Krishnamoorthy, J.; Van Langenhove, L. Integrating Wearable Textiles Sensors and IoT for Continuous sEMG Monitoring. Sensors 2024, 24, 1834. [Google Scholar] [CrossRef]
- Hoang, T.T.; Cunio, A.M.; Zhao, S.; Nguyen, T.V.; Peng, S.; Liaw, S.; Barber, T.; Zhang, J.; Farajikhah, S.; Dehghani, F.; et al. Flexible, Wearable Mechano-Acoustic Sensors for Real-Time, Wireless Monitoring of Low Frequency Body Sounds. Adv. Sens. Res. 2024, 3, 2400039. [Google Scholar] [CrossRef]
- Zhu, M.; Yuan, Y.; Yin, H.; Guo, Z.; Wei, X.; Qi, X.; Liu, H.; Dang, Z. Environmental Contamination and Human Exposure of Polychlorinated Biphenyls (PCBs) in China: A review. Sci. Total Environ. 2022, 805, 150270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, B.; Yu, S.; Xiong, J.; Yao, Z.; Hu, B.; Yan, J. Waste-Printed Circuit Board Recycling: Focusing on Preparing Polymer Composites and Geopolymers. ACS Omega 2020, 5, 17850–17856. [Google Scholar] [CrossRef]
- Tan, H.W.; Choong, Y.Y.C.; Kuo, C.N.; Low, H.Y.; Chua, C.K. 3D Printed Electronics: Processes, Materials and Future Trends. Prog. Mater. Sci. 2022, 127, 100945. [Google Scholar] [CrossRef]
- Andersson Ersman, P.; Lassnig, R.; Strandberg, J.; Tu, D.; Keshmiri, V.; Forchheimer, R.; Fabiano, S.; Gustafsson, G.; Berggren, M. All-printed Large-scale Lntegrated Circuits Based on Organic Electrochemical Transistors. Nat. Commun. 2019, 10, 5053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Fu, J.Z.; Gao, Q.; Zhao, P.; He, Y. All-Printed Flexible and Stretchable Electronics with Pressing or Freezing Activatable Liquid-Metal–Silicone Inks. Adv. Funct. Mater. 2019, 30, 1906683. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, X.; Ge, T.; Zhou, J. Fully Printed Electronics on Flexible Substrates: High Gain Amplifiers and DAC. Org. Electron. 2014, 15, 701–710. [Google Scholar] [CrossRef]
- Khan, Y.; Thielens, A.; Muin, S.; Ting, J.; Baumbauer, C.; Arias, A.C. A New Frontier of Printed Electronics: Flexible Hybrid Electronics. Adv. Mater. 2020, 32, e1905279. [Google Scholar] [CrossRef]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.D.; Hart, P.; Welte, R.; Wilson, W.; et al. Flexible Hybrid Electronics: Direct Interfacing of Soft and Hard Electronics for Wearable Health Monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Huang, Z.; Hao, Y.; Li, Y.; Hu, H.; Wang, C.; Nomoto, A.; Pan, T.; Gu, Y.; Chen, Y.; Zhang, T.; et al. Three-dimensional Integrated Stretchable Electronics. Nat. Electron. 2018, 1, 473–480. [Google Scholar] [CrossRef]
- Vanfleteren, J.; Gonzalez, M.; Bossuyt, F.; Hsu, Y.Y.; Vervust, T.; De Wolf, I.; Jablonski, M. Printed Circuit Board Technology Inspired Stretchable Circuits. MRS Bull. 2012, 37, 254–260. [Google Scholar] [CrossRef]
- Zhuang, Q.; Yao, K.; Zhang, C.; Song, X.; Zhou, J.; Zhang, Y.; Huang, Q.; Zhou, Y.; Yu, X.; Zheng, Z. Permeable, Three-dimensional Integrated Electronic Skins with Stretchable Hybrid Liquid Metal Solders. Nat. Electron. 2024, 7, 598–609. [Google Scholar] [CrossRef]
- Ma, X.; Wang, P.; Huang, L.; Ding, R.; Zhou, K.; Shi, Y.; Chen, F.; Zhuang, Q.; Huang, Q.; Lin, Y.; et al. A Monolithically Integrated In-textile Wristband for Wireless Epidermal Biosensing. Sci. Adv. 2023, 9, eadj2763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Low Power Circuits for Smart Flexible ECG Sensors. Ph.D. Thesis, York University, Toronto, ON, Canada, 2019. [Google Scholar]
- Kim, I.; Bhagat, Y.A.; Homer, J.; Lobo, R. Multimodal Analog Front End for Wearable Bio-Sensors. IEEE Sens. J. 2016, 16, 8784–8791. [Google Scholar] [CrossRef]
- Xu, P. Analog Front-End Pre-Amplifiers for Portable Capacitive ECG Monitoring Applications Analysis. Highlights Sci. Eng. Technol. 2023, 72, 361–368. [Google Scholar] [CrossRef]
- Yao, W.; Yan, Y.; Sun, J.; Zhang, Z.; Sun, W.; Huang, W.; Cheng, J.; Zhao, H.; Xie, M.; Sun, Q.; et al. Mechanically Durable Superhydrophobic Strain Sensors with High Biocompatibility and Sensing Performance for Underwater Motion Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 6548–6561. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Joshi, R. Portable Out-of-hospital Electrocardiography: A Review of Current Technologies. J. Arrhythmia 2018, 34, 129–138. [Google Scholar] [CrossRef]
- Bravo-Zanoguera, M.; Cuevas-González, D.; García-Vázquez, J.P.; Avitia, R.L.; Reyna, M.A. Portable ECG System Design Using the AD8232 Microchip and Open-Source Platform. In Proceedings of the 6th International Electronic Conference on Sensors and Applications, Online, 15–30 November 2019; p. 49. [Google Scholar]
- Potvin, J.R. Effects of Muscle Kinematics on Surface EMG Amplitude and Frequency During Fatiguing Dynamic Contractions. J. Appl. Physiol. 1997, 82, 144–151. [Google Scholar] [CrossRef]







| Title | Model | Viscosity | Ag Content | Drying Condition | Manufacturer |
|---|---|---|---|---|---|
| Conductive ink | 8000C | 10,000–12,000 cp | 75 ± 2% | hot air at 120 °C, 15 min | Shenzhen Sunflower Electronic Material Co., Ltd. (Shenzhen, China). |
| Inkjet Height | Inkjet Speed | Dispensing Air Pressure | Needle Diameter |
|---|---|---|---|
| 31.90 mm | 2.00 mm/s | 200.00 KPa | 0.25 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, H.; Li, Z.; Chen, Z.; Gao, Y.; Xuan, F. A Step Forward for Smart Clothes: Printed Fabric-Based Hybrid Electronics for Wearable Health Monitoring. Sensors 2024, 24, 6991. https://doi.org/10.3390/s24216991
Tu H, Li Z, Chen Z, Gao Y, Xuan F. A Step Forward for Smart Clothes: Printed Fabric-Based Hybrid Electronics for Wearable Health Monitoring. Sensors. 2024; 24(21):6991. https://doi.org/10.3390/s24216991
Chicago/Turabian StyleTu, Huating, Zhenglin Li, Zihao Chen, Yang Gao, and Fuzhen Xuan. 2024. "A Step Forward for Smart Clothes: Printed Fabric-Based Hybrid Electronics for Wearable Health Monitoring" Sensors 24, no. 21: 6991. https://doi.org/10.3390/s24216991
APA StyleTu, H., Li, Z., Chen, Z., Gao, Y., & Xuan, F. (2024). A Step Forward for Smart Clothes: Printed Fabric-Based Hybrid Electronics for Wearable Health Monitoring. Sensors, 24(21), 6991. https://doi.org/10.3390/s24216991







