Optrode-Assisted Multiparametric Near-Infrared Spectroscopy for the Analysis of Liquids
Abstract
1. Introduction
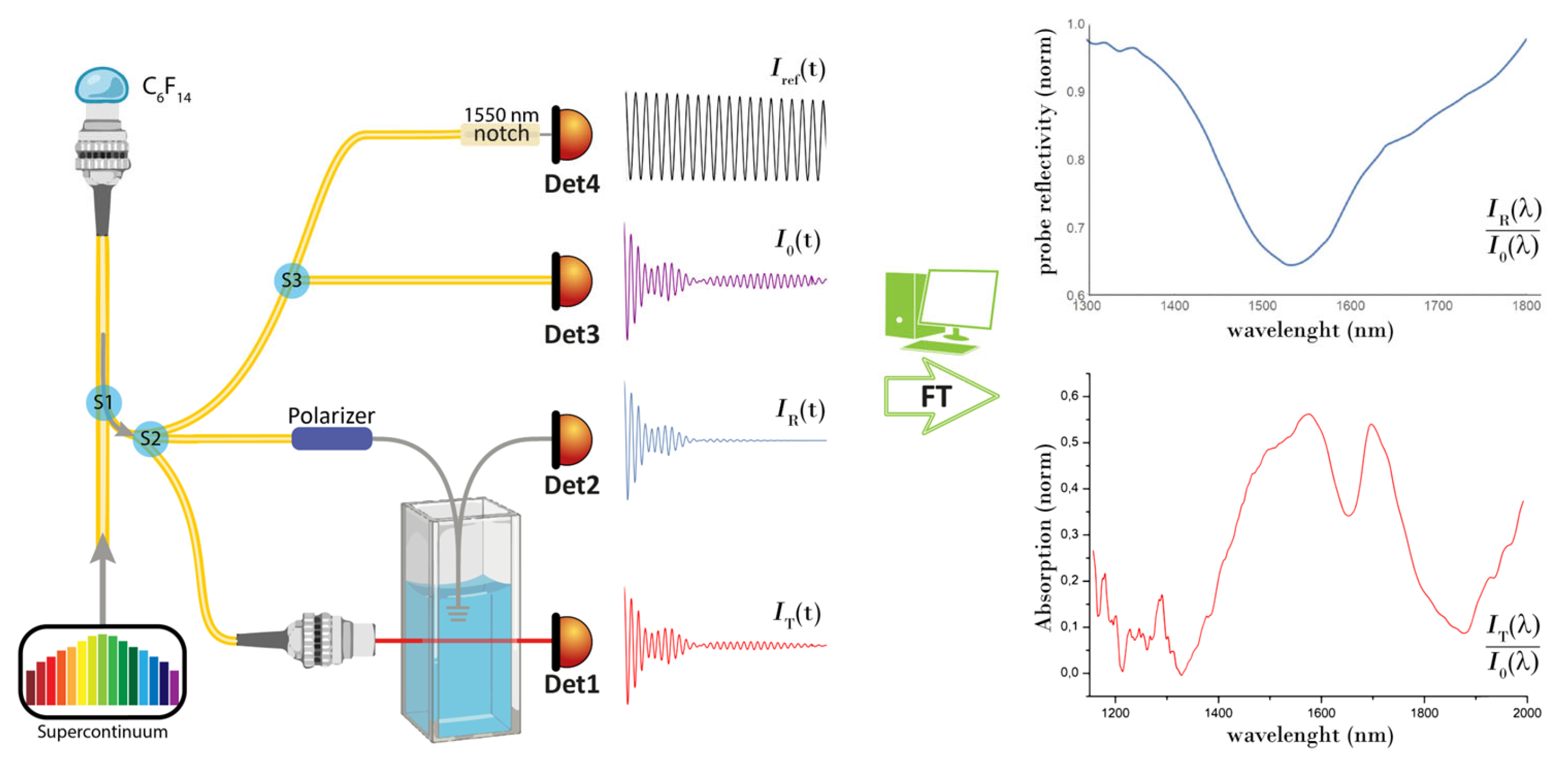
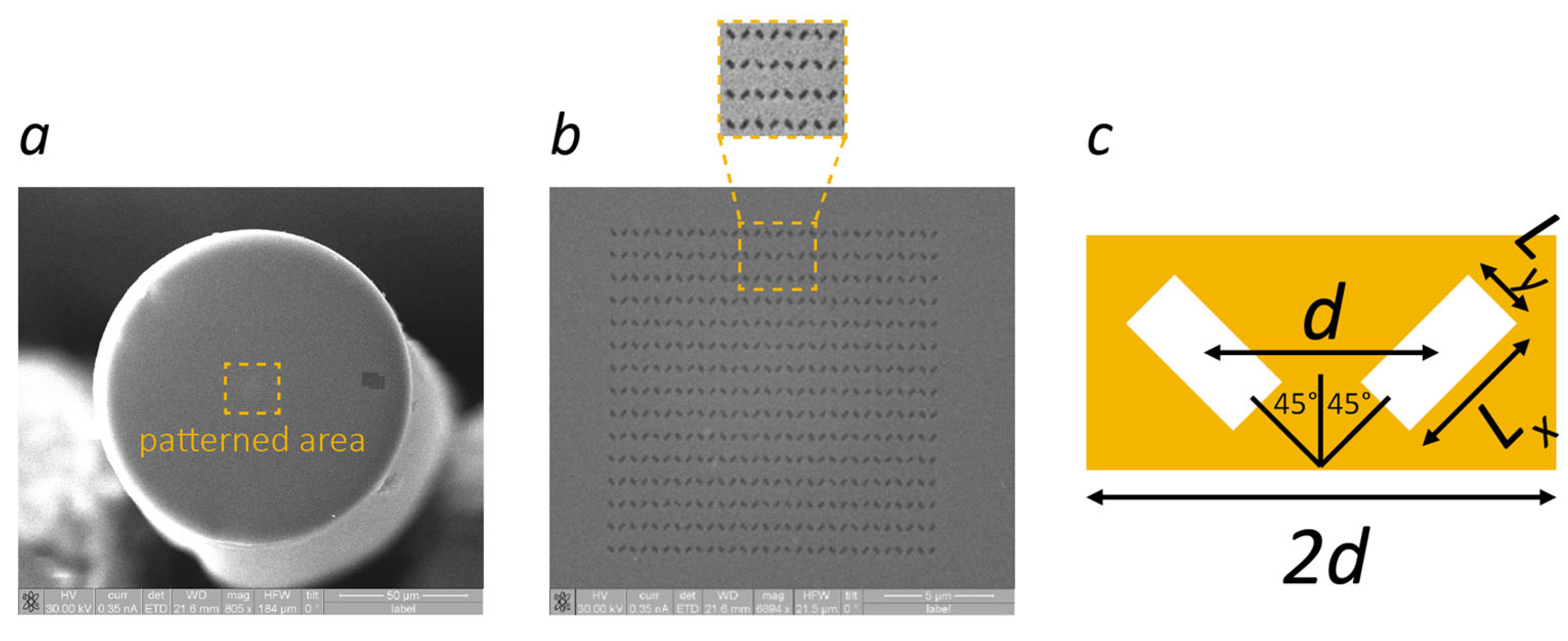
2. Materials and Methods
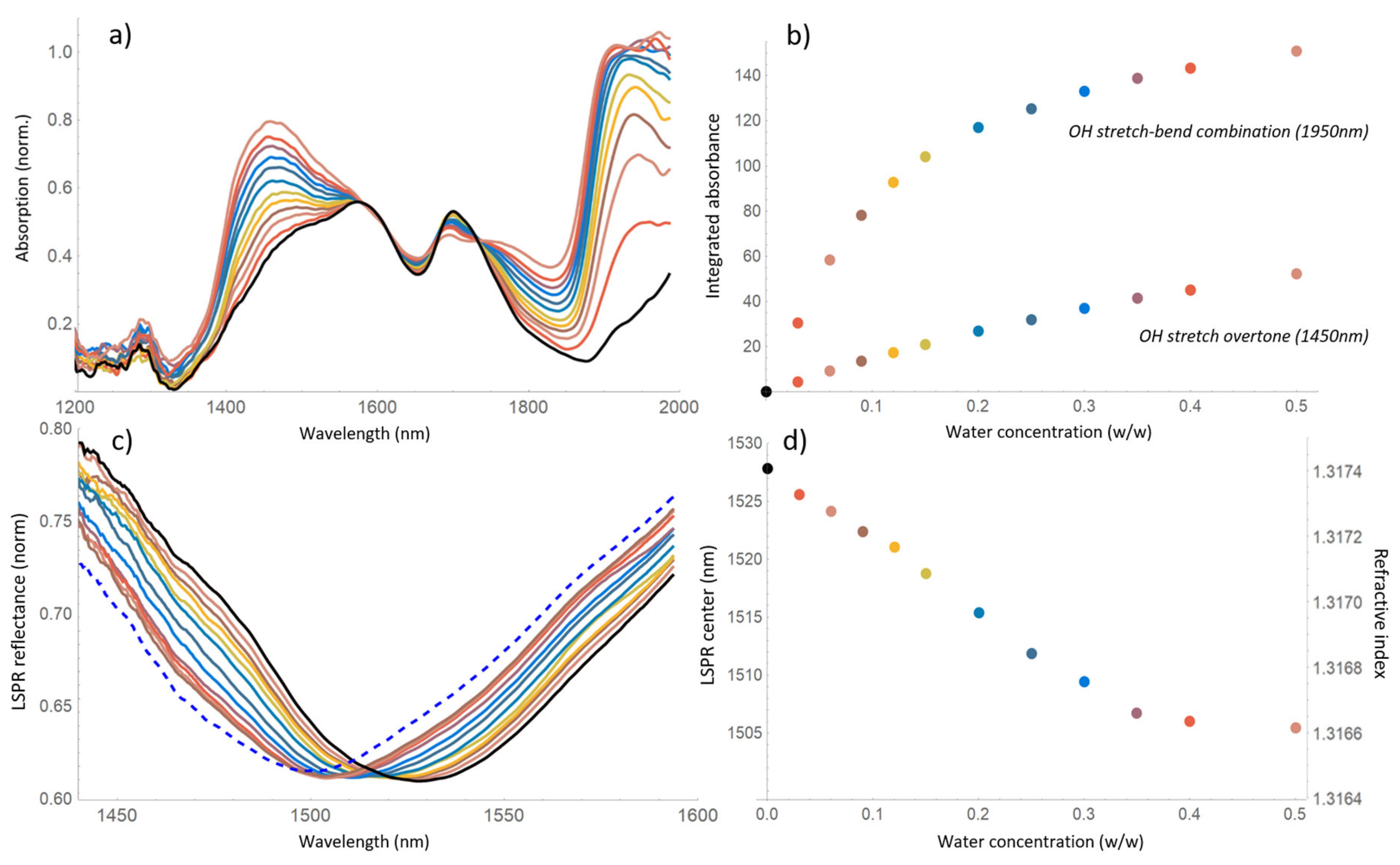
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozaki, Y.; Huck, C.; Tsuchikawa, S.; Engelsen, S.B. Near-Infrared Spectroscopy: Theory, Spectral Analysis, Instrumentation, and Applications; Springer: Singapore, 2021. [Google Scholar]
- Van Kollenburg, G.H.; van Manen, H.J.; Admiraal, N.; Gerretzen, J.; Jansen, J.J. Low-cost handheld NIR spectroscopy for identification of organic solvents and low-level quantification of water contamination. Talanta 2021, 223, 121865. [Google Scholar] [CrossRef] [PubMed]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Shenk, J.; Workman, J.J., Jr.; Westerhaus, M.O. Application of NIR spectroscopy to agricultural products. In Handbook of Near-infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007; pp. 365–404. [Google Scholar]
- Yang, Z.; Albrow-Owen, T.; Cai, W.; Hasan, T. Miniaturization of optical spectrometers. Science 2021, 371, eabe0722. [Google Scholar] [CrossRef] [PubMed]
- Crocombe, R.A. Portable spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef] [PubMed]
- Wiedemair, V.; Langore, D.; Garsleitner, R.; Dillinger, K.; Huck, C. Investigations into the performance of a novel pocket-sized near-infrared spectrometer for cheese analysis. Molecules 2019, 24, 428. [Google Scholar] [CrossRef] [PubMed]
- Bec, K.B.; Grabska, J.; Siesler, H.W.; Huck, C.W. Handheld near-infrared spectrometers: Where are we heading? NIR News 2020, 31, 28–35. [Google Scholar] [CrossRef]
- Ballabio, D.; Todeschini, R. Multivariate classification for qualitative analysis. In Infrared Spectroscopy for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2009; pp. 83–104. [Google Scholar]
- Amirvaresi, A.; Parastar, H. Miniaturized NIR spectroscopy and chemometrics: A smart combination to solve food authentication challenges. Front. Anal. Sci. 2023, 3, 1118590. [Google Scholar] [CrossRef]
- Wang, X.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2015–2019). Anal. Chem. 2019, 92, 397–430. [Google Scholar] [CrossRef]
- Klantsataya, E.; Jia, P.; Ebendorff-Heidepriem, H.; Monro, T.M.; François, A. Plasmonic Fiber Optic Refractometric Sensors: From Conventional Architectures to Recent Design Trends. Sensors 2017, 17, 12. [Google Scholar] [CrossRef]
- Malara, P.; Crescitelli, A.; Di Meo, V.; Giorgini, A.; Avino, S.; Esposito, E.; Ricciardi, A.; Cusano, A.; Rendina, I.; De Natale, P.; et al. Resonant enhancement of plasmonic nanostructured fiber optic sensors. Sens. Actuators B Chem. 2018, 273, 1587–1592. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, W.; Qu, J.; Cheng, Z.; Peng, G.D.; Yu, C. Optical fiber-integrated metasurfaces: An emerging platform for multiple optical applications. Nanomaterials 2022, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Snelders, D.; MacKenzie, F.V.; Boersma, A.; Peeters, R. Zeolites as coatings for Fiber Bragg Grating chemical sensors for extreme conditions. Sens. Actuators B Chem. 2016, 235, 698–706. [Google Scholar] [CrossRef]
- Wang, D.H.-C.; Hwang, S.S.H.; Maunder, S.A.; Blenman, N.G.; Arkwright, J.W. Optical fiber Bragg grating based chemical sensor. In Proceedings of the SENSORS, 2012 IEEE, Taipei, Taiwan, 28–31 October 2012; pp. 1–3. [Google Scholar]
- Singh, Y.; Sadhu, A.; Raghuwanshi, S.K. Development and Experimental Analysis of Titanium Dioxide (TiO2) Coated Etched Fiber Bragg Grating Sensor for Chemical Sensing. IEEE Sens. J. 2020, 20, 8528–8534. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit ofdetection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, A.; Del Villar, I.; Zubiate, P.; Zamarreño, C.R. A comprehensive review of optical fiber refractometers: Toward a standard comparative criterion. Laser Photonics Rev. 2019, 13, 1900094. [Google Scholar] [CrossRef]
- Malara, P.; Giorgini, A.; Avino, S.; Di Sarno, V.; Aiello, R.; Maddaloni, P.; De Natale, P.; Gagliardi, G. A self-operating broadband spectrometer on a droplet. Nat. Commun. 2020, 11, 2263. [Google Scholar] [CrossRef]
- Capezzuto, M.; D’Ambrosio, D.; Avino, S.; Giorgini, A.; Gagliardi, G.; Malara, P. Ultra-broadband high-resolution microdroplet spectrometers for the near infrared. Opt. Lett. 2022, 47, 102–105. [Google Scholar] [CrossRef]
- Principe, M.; Consales, M.; Micco, A.; Crescitelli, A.; Castaldi, G.; Esposito, E.; La Ferrara, V.; Cutolo, A.; Galdi, V.; Cusano, A. Optical fiber meta-tips. Light Sci. Appl. 2017, 6, 16226. [Google Scholar] [CrossRef]
- Consales, M.; Quero, G.; Spaziani, S.; Principe, M.; Micco, A.; Galdi, V.; Cutolo, A.; Cusano, A. Metasurface-Enhanced Lab-on-Fiber Biosensors. Laser Photonics Rev. 2020, 14, 2000180. [Google Scholar] [CrossRef]
- Saunders, J.E.; Sanders, C.; Chen, H.; Loock, H.-P. Refractive indices of common solvents and solutions at 1550 nm. Appl. Opt. AO 2016, 55, 947–953. [Google Scholar] [CrossRef]
- Herráez, J.V.; Belda, R. Refractive Indices, Densities and Excess Molar Volumes of Monoalcohols + Water. J. Solut. Chem. 2006, 35, 1315–1328. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Trung, L.C.; Cuong, N.D.; Quang, H.D.; Khoa, D.X.; Van Phu, N.; Van Lanh, C.; Vinh, N.T.; Thuy, D.T.; Thuan, B.D. Measuring the refractive index of a methanol—Water mixture according to the wavelength. Photonics Lett. Pol. 2021, 13, 10–12. [Google Scholar] [CrossRef]
- Reis, J.C.R.; Lampreia, I.M.S.; Santos, Â.F.S.; Moita, M.L.C.J.; Douhéret, G. Refractive Index of Liquid Mixtures: Theory and Experiment. ChemPhysChem 2010, 11, 3722–3733. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Barras, A.; Mishyn, V.; Heyte, S.; Heuson, E.; Oubaha, H.; Sandu, G.; Melinte, S.; Boukherroub, R.; Szunerits, S. Plasmon-Driven Electrochemical Methanol Oxidation on Gold Nanohole Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 50426–50432. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Chakroborty, S.; Pal, K.; Barik, A.; Priyadarsini Mishra, N.; Kralj, S. Recent Advances in Plasmonic Enhanced Nanocatalyst for Oxidation of Alcohol. Top. Catal. 2023, 1–11. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Q.; Zheng, W.; Cui, X. Highly Ordered Periodic Au/TiO2 Hetero-Nanostructures for Plasmon-Induced Enhancement of the Activity and Stability for Ethanol Electro-oxidation. ACS Appl. Mater. Interfaces 2016, 8, 5273–5279. [Google Scholar] [CrossRef]
- Li, Y.; Shen, F.; Hu, L.; Lang, Z.; Liu, Q.; Cai, F.; Fu, L. A Stare-Down Video-Rate High-Throughput Hyperspectral Imaging System and Its Applications in Biological Sample Sensing. IEEE Sens. J. 2023, 23, 23629–23637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delli Santi, M.G.; Castrignano, S.; Capezzuto, M.; Consales, M.; Vaiano, P.; Cusano, A.; Gagliardi, G.; Malara, P. Optrode-Assisted Multiparametric Near-Infrared Spectroscopy for the Analysis of Liquids. Sensors 2024, 24, 729. https://doi.org/10.3390/s24030729
Delli Santi MG, Castrignano S, Capezzuto M, Consales M, Vaiano P, Cusano A, Gagliardi G, Malara P. Optrode-Assisted Multiparametric Near-Infrared Spectroscopy for the Analysis of Liquids. Sensors. 2024; 24(3):729. https://doi.org/10.3390/s24030729
Chicago/Turabian StyleDelli Santi, Maria Giulia, Salvatore Castrignano, Marialuisa Capezzuto, Marco Consales, Patrizio Vaiano, Andrea Cusano, Gianluca Gagliardi, and Pietro Malara. 2024. "Optrode-Assisted Multiparametric Near-Infrared Spectroscopy for the Analysis of Liquids" Sensors 24, no. 3: 729. https://doi.org/10.3390/s24030729
APA StyleDelli Santi, M. G., Castrignano, S., Capezzuto, M., Consales, M., Vaiano, P., Cusano, A., Gagliardi, G., & Malara, P. (2024). Optrode-Assisted Multiparametric Near-Infrared Spectroscopy for the Analysis of Liquids. Sensors, 24(3), 729. https://doi.org/10.3390/s24030729








