Non-Immersive Virtual Reality-Based Therapy Applied in Cardiac Rehabilitation: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
2.2. Literature Search and Databases
2.3. Study Selection: Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Variables
2.6. Risk of Bias and Quality of Evidence Assessments
2.7. Statistical Analyses
3. Results
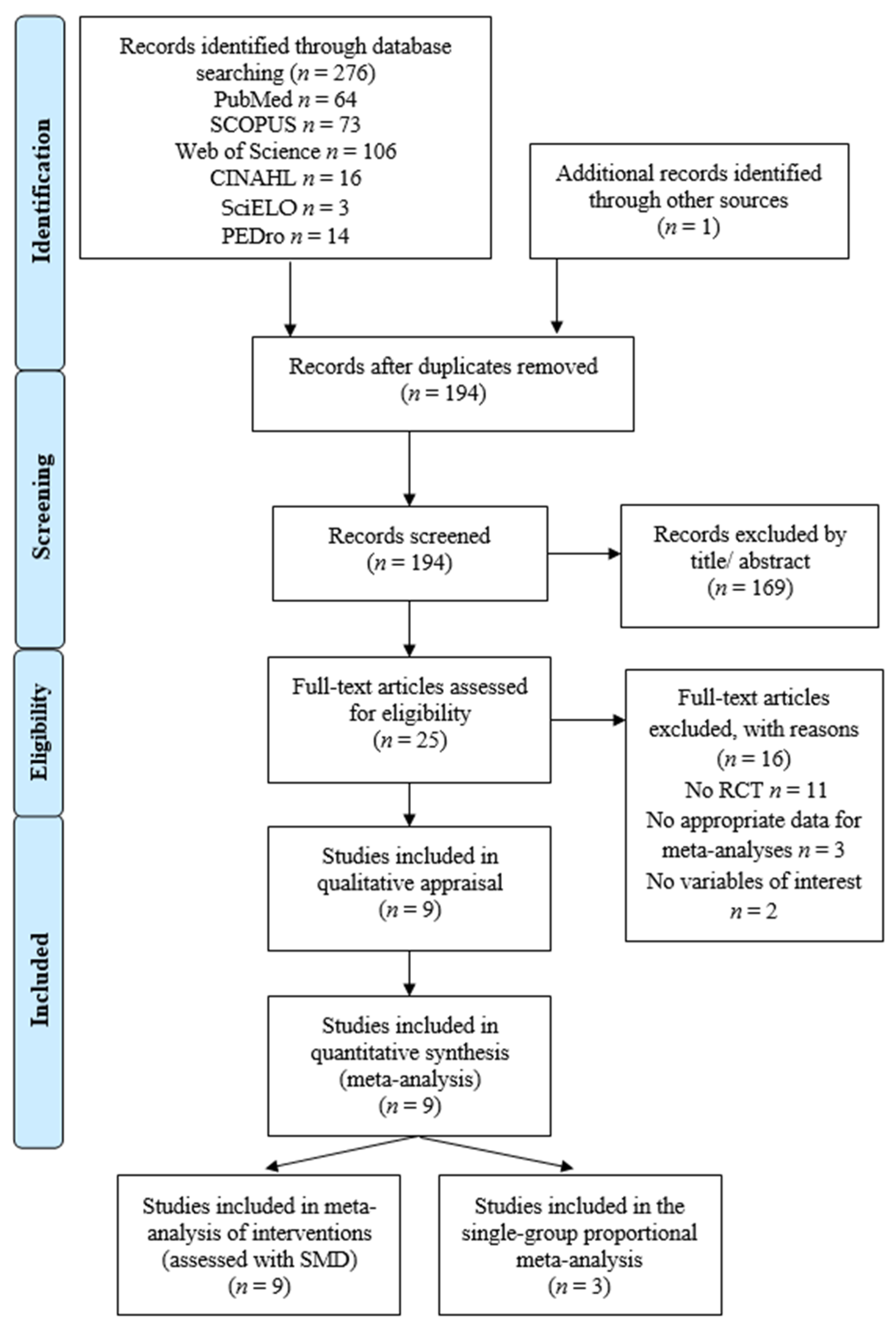
3.1. Study Selection
3.2. Characteristics of the Studies Included in the Review
3.3. Assessment of Risk of Bias in the Studies Included in the Review
3.4. Outcome Synthesis and Measurements
3.4.1. Aerobic Capacity and Cardiovascular Endurance (Physical Function)
3.4.2. Anxiety
3.4.3. Depression
3.4.4. Quality of Life
3.4.5. Proportion of Adverse Events during niVR Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Li, Y.; Zeng, X.; Wang, H.; Yin, P.; Wang, L.; Liu, Y.; Liu, J.; Qi, J.; Ran, S.; et al. Burden of Cardiovascular Diseases in China, 1990–2016. JAMA Cardiol. 2019, 4, 342. [Google Scholar] [CrossRef]
- World Health Organization Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 December 2023).
- Nelson, S.; Whitsel, L.; Khavjou, O.; Phelps, D.; Leib, A. Projections of Cardiovascular Disease Prevalence and Costs; RTI International: Triangle Park, NC, USA, 2016. [Google Scholar]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed]
- den Uijl, I.; ter Hoeve, N.; Sunamura, M.; Stam, H.J.; Boersma, E.; Lenzen, M.J.; Brouwers, R.W.M.; Tenbült-van Limpt, N.C.C.W.; Ista, E.; van den Berg-Emons, R.J.G. Cardiac rehabilitation designed for patients with obesity: OPTICARE XL RCT results on health-related quality of life and psychosocial well-being. Disabil. Rehabil. 2023, 45, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, G. Economic Impacts of Cardiovascular Diseases: An Econometric Evaluation in Turkey. Iran. J. Public Health 2023, 52, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Polsinelli, V.B.; Shah, S.J. Advances in the pharmacotherapy of chronic heart failure with preserved ejection fraction: An ideal opportunity for precision medicine. Expert Opin. Pharmacother. 2017, 18, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Numasawa, Y.; Sawano, M.; Fukuoka, R.; Ejiri, K.; Kuno, T.; Shoji, S.; Kohsaka, S. Antithrombotic Strategy for Patients with Acute Coronary Syndrome: A Perspective from East Asia. J. Clin. Med. 2020, 9, 1963. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Büscher, A.; Wiedmann, F.; L’hoste, Y.; Haefeli, W.E.; Frey, N.; Katus, H.A.; Schmidt, C. Current Drug Treatment Strategies for Atrial Fibrillation and TASK-1 Inhibition as an Emerging Novel Therapy Option. Front. Pharmacol. 2021, 12, 638445. [Google Scholar] [CrossRef]
- Candelaria, D.; Randall, S.; Ladak, L.; Gallagher, R. Health-related quality of life and exercise-based cardiac rehabilitation in contemporary acute coronary syndrome patients: A systematic review and meta-analysis. Qual. Life Res. 2020, 29, 579–592. [Google Scholar] [CrossRef]
- Vassou, C.; Yannakoulia, M.; Cropley, M.; Panagiotakos, D.B. Psychological interventions aiming for changing dietary habits in patients with cardiovascular disease: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2023, 36, 1193–1206. [Google Scholar] [CrossRef]
- Yau, D.K.W.; Underwood, M.J.; Joynt, G.M.; Lee, A. Effect of preparative rehabilitation on recovery after cardiac surgery: A systematic review. Ann. Phys. Rehabil. Med. 2021, 64, 101391. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat. Rev. Cardiol. 2022, 19, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, J.; Troev, T.; Ferreira, A.S.; Tsekoura, D.; Elkova, H.; Kyriopoulos, E.; Ilieva, E. Advanced Role and Field of Competence of the Physical and Rehabilitation Medicine Specialist in Contemporary Cardiac Rehabilitation. Hell. J. Cardiol. 2016, 57, 16–22. [Google Scholar] [CrossRef]
- García-Bravo, S.; Cuesta-Gómez, A.; Campuzano-Ruiz, R.; López-Navas, M.J.; Domínguez-Paniagua, J.; Araújo-Narváez, A.; Barreñada-Copete, E.; García-Bravo, C.; Flórez-García, M.T.; Botas-Rodríguez, J.; et al. Virtual reality and video games in cardiac rehabilitation programs. A systematic review. Disabil. Rehabil. 2021, 43, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Alves da Cruz, M.M.; Ricci-Vitor, A.L.; Bonini Borges, G.L.; Fernanda da Silva, P.; Ribeiro, F.; Marques Vanderlei, L.C. Acute Hemodynamic Effects of Virtual Reality–Based Therapy in Patients of Cardiovascular Rehabilitation: A Cluster Randomized Crossover Trial. Arch. Phys. Med. Rehabil. 2020, 101, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Neubeck, L.; Redfern, J.U.; Fernandez, R.; Briffa, T.; Bauman, A.; Freedman, S. Ben Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 281–289. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, W.; Kim, Y.; Meng, H. Effects of Low- and High-Frequency Cardiac Rehabilitation on Risk Factors, Physical Fitness and Quality of Life in Middle-Aged Women with Coronary Heart Disease. Metabolites 2023, 13, 550. [Google Scholar] [CrossRef]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 175394471987008. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Pena-Gil, C.; Abu-Assi, E.; Raposeiras, S.; van ’t Hof, A.; Meindersma, E.; Bossano Prescott, E.I.; González-Juanatey, J.R. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 2016, 223, 436–443. [Google Scholar] [CrossRef]
- Pio, C.S.d.A.; Chaves, G.; Davies, P.; Taylor, R.; Grace, S. Interventions to Promote Patient Utilization of Cardiac Rehabilitation: Cochrane Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 189. [Google Scholar] [CrossRef]
- Southworth, M.K.; Silva, J.R.; Silva, J.N.A. Use of extended realities in cardiology. Trends Cardiovasc. Med. 2020, 30, 143–148. [Google Scholar] [CrossRef]
- Weiss, P.L.; Kizony, R.; Feintuch, U.; Katz, N. Virtual reality in neurorehabilitation. Textb. Neural Repair Rehabil. 2006, 51, 182–197. [Google Scholar]
- Weiss, P.L.; Keshner, E.A.; Levin, M.F. Virtual Reality Technologies for Health and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Del Rocío Ibancos-Losada, M.; Nieto-Escámez, F.A.; Obrero-Gaitán, E.; Catalina Osuna-Pérez, M.; Godinho, C.; Fernandes, J.B. Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2021, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Zagalaz-Anula, N.; Osuna-Pérez, M.C.; Obrero-Gaitán, E.; Lomas-Vega, R. Nintendo Wii Balance Board therapy for postural control in children with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2021, 63, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, F.; Nasab, N.Z.; Jaberi, A. Comparing the Effects of Virtual Reality and Home Chair-Based Exercises on Balance, Daily Living Activities, and Loneliness Among Older Adults with Balance Disorders. Res. Gerontol. Nurs. 2023, 16, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shimizu, K.; Shiko, Y.; Kawasaki, Y.; Orita, S.; Inage, K.; Shiga, Y.; Suzuki, M.; Sato, M.; Enomoto, K.; et al. Effects of Nintendo Ring Fit Adventure Exergame on Pain and Psychological Factors in Patients with Chronic Low Back Pain. Games Health J. 2021, 10, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Glännfjord, F.; Hemmingsson, H.; Larsson Ranada, Å. Elderly people’s perceptions of using Wii sports bowling—A qualitative study. Scand. J. Occup. Ther. 2017, 24, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.M.; Uchida, P.M.; Barbosa, S.P. The impact of Exergames on emotional experience: A systematic review. Front. Public Health 2023, 11, 1209520. [Google Scholar] [CrossRef]
- Peng, Y.; Vedam, S.; Gao, S.; Balter, P. A new respiratory monitoring and processing system based on Wii remote: Proof of principle. Med. Phys. 2013, 40, 071712. [Google Scholar] [CrossRef]
- Lin, M.; Lee, K. Outdoor Target Positioning Using Wii Remote IR Camera and Signal Modulation. Sensors 2020, 20, 2163. [Google Scholar] [CrossRef]
- Daoud, M.I.; Alhusseini, A.; Ali, M.Z.; Alazrai, R. A Game-Based Rehabilitation System for Upper-Limb Cerebral Palsy: A Feasibility Study. Sensors 2020, 20, 2416. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Aldana, A.C.; Callejas-Cuervo, M.; Bo, A.P.L. Upper Limb Physical Rehabilitation Using Serious Videogames and Motion Capture Systems: A Systematic Review. Sensors 2020, 20, 5989. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Amprimo, G.; Masi, G.; Vismara, L.; Cremascoli, R.; Sinagra, S.; Pettiti, G.; Mauro, A.; Priano, L. Evaluation of Arm Swing Features and Asymmetry during Gait in Parkinson’s Disease Using the Azure Kinect Sensor. Sensors 2022, 22, 6282. [Google Scholar] [CrossRef]
- Darabseh, M.Z.; Aburub, A.; Davies, S. The Effects of Virtual Reality Physiotherapy Interventions on Cardiopulmonary Function and Breathing Control in Cystic Fibrosis: A Systematic Review. Games Health J. 2023, 12, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Buyle, M.; Jung, Y.; Pavlou, M.; Gonzalez, S.C.; Bamiou, D.-E. The role of motivation factors in exergame interventions for fall prevention in older adults: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 903673. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, M.M.A.; Ricci-Vitor, A.L.; Borges, G.L.B.; da Silva, P.F.; Turri-Silva, N.; Takahashi, C.; Grace, S.L.; Vanderlei, L.C.M. A Randomized, Controlled, Crossover Trial of Virtual Reality in Maintenance Cardiovascular Rehabilitation in a Low-Resource Setting: Impact on Adherence, Motivation, and Engagement. Phys. Ther. 2021, 101, pzab071. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Peris, C.; Fuertes-Kenneally, L.; Vetrovsky, T.; Sarabia, J.M.; Climent-Paya, V.; Manresa-Rocamora, A. Effects of Exergaming in Patients with Cardiovascular Disease Compared to Conventional Cardiac Rehabilitation: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 3492. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, L.; Xu, Y.; Zhu, M.; Guan, B.; Ming, W. Effectiveness of virtual reality in cardiac rehabilitation: A systematic review and meta-analysis of randomized controlled trials. Int. J. Nurs. Stud. 2022, 133, 104323. [Google Scholar] [CrossRef]
- Bashir, Z.; Misquith, C.; Shahab, A.; Has, P.; Bukhari, S. The impact of Virtual Reality on Anxiety and Functional Capacity in Cardiac Rehabilitation: A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2023, 48, 101628. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0 [updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Software Version 4; Biostat: Englewood, NJ, USA, 2023. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–421. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Rücker, G.; Schwarzer, G. Beyond the forest plot: The drapery plot. Res. Synth. Methods 2021, 12, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V. Interpreting estimates of treatment effects: Implications for managed care. PT 2008, 33, 700–711. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Shi, L.; Lin, L.; Omboni, S. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.A.B.; de Araújo, G.C.R.; Prado, J.P.; de Aquino, T.N.; da Silva, J.P.; Galdino, G. Effect of a virtual cardiac rehabilitation program on patients with hypertension: A randomized trial. Fisioter. Em Mov. 2021, 34, e34126. [Google Scholar] [CrossRef]
- Cacau, L.d.A.P.; Oliveira, G.U.; Maynard, L.G.; de Araújo Filho, A.A.; da Silva, W.M., Jr.; Cerqueria Neto, M.L.; Antoniolli, A.R.; Santana-Filho, V.J. The use of the virtual reality as intervention tool in the postoperative of cardiac surgery. Rev. Bras. Cir. Cardiovasc. 2013, 28, 281–289. [Google Scholar] [CrossRef] [PubMed]
- García-Bravo, S.; Cano-de-la-Cuerda, R.; Domínguez-Paniagua, J.; Campuzano-Ruiz, R.; Barreñada-Copete, E.; López-Navas, M.J.; Araujo-Narváez, A.; García-Bravo, C.; Florez-Garcia, M.; Botas-Rodríguez, J.; et al. Effects of Virtual Reality on Cardiac Rehabilitation Programs for Ischemic Heart Disease: A Randomized Pilot Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 8472. [Google Scholar] [CrossRef] [PubMed]
- Gulick, V.; Graves, D.; Ames, S.; Krishnamani, P.P. Effect of a Virtual Reality–Enhanced Exercise and Education Intervention on Patient Engagement and Learning in Cardiac Rehabilitation: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e23882. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, T.; Klompstra, L.; Ben Gal, T.; Ben Avraham, B.; Boyne, J.; Bäck, M.; Chialà, O.; Dickstein, K.; Evangelista, L.; Hagenow, A.; et al. Effects of exergaming on exercise capacity in patients with heart failure: Results of an international multicentre randomized controlled trial. Eur. J. Heart Fail. 2021, 23, 114–124. [Google Scholar] [CrossRef]
- Ruivo, J.M.A.d.S.; Karim, K.; O’Shea, R.; Oliveira, R.C.S.; Keary, L.; O’Brien, C.; Gormley, J.P. In-class Active Video Game Supplementation and Adherence to Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2017, 37, 274–278. [Google Scholar] [CrossRef]
- Silva, J.P.L.N.; Novaes, L.F.M.; dos Santos, L.C.R.; Galindo, B.P.; Cavalcante, M.A.; de Araújo, B.C.G.; Pacagnelli, F.L.; Freire, A.P.C.F. Effects of Conventional and Virtual Reality Cardiovascular Rehabilitation in Body Composition and Functional Capacity of Patients with Heart Diseases: Randomized Clinical Trial. Int. J. Cardiovasc. Sci. 2018, 31, 619–629. [Google Scholar] [CrossRef]
- Vieira, Á.; Melo, C.; Machado, J.; Gabriel, J. Virtual reality exercise on a home-based phase III cardiac rehabilitation program, effect on executive function, quality of life and depression, anxiety and stress: A randomized controlled trial. Disabil. Rehabil. Assist. Technol. 2018, 13, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Yuenyongchaiwat, K.; Boonkawee, T.; Pipatsart, P.; Tavonudomgit, W.; Sermsinsaithong, N.; Songsorn, P.; Charususin, N.; Harnmanop, S.; Namdaeng, P.; Kulchanarat, C.; et al. Effects of virtual exercise on cardio-pulmonary performance and depression in cardiac rehabilitation phase I: A randomized control trial. Physiother. Res. Int. 2023, 29, e2066. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Täger, T.; Hanholz, W.; Cebola, R.; Fröhlich, H.; Franke, J.; Doesch, A.; Katus, H.A.; Wians, F.H.; Frankenstein, L. Minimal important difference for 6-minute walk test distances among patients with chronic heart failure. Int. J. Cardiol. 2014, 176, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bojanić, I.; Sund, E.R.; Sletvold, H.; Bjerkeset, O. Prevalence trends of depression and anxiety symptoms in adults with cardiovascular diseases and diabetes 1995–2019: The HUNT studies, Norway. BMC Psychol. 2021, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.G.; Bertolli, E.d.S.; Paiva, L.; Rossi, L.A.; Dantas, R.A.S.; Pompeo, D.A. Anxiety, depression, resilience and self-esteem in individuals with cardiovascular diseases. Rev. Lat. Am. Enfermagem 2016, 24, e2836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Liu, Y.; Zheng, G.; Fan, F.; Tian, H.; Zhang, X. Psychosomatic mechanisms of heart failure symptoms on quality of life in patients with chronic heart failure: A multi-centre cross-sectional study. J. Clin. Nurs. 2023. [Google Scholar] [CrossRef]
- Colder Carras, M.; Van Rooij, A.J.; Spruijt-Metz, D.; Kvedar, J.; Griffiths, M.D.; Carabas, Y.; Labrique, A. Commercial Video Games As Therapy: A New Research Agenda to Unlock the Potential of a Global Pastime. Front. Psychiatry 2018, 8, 300. [Google Scholar] [CrossRef]
- Martínez-Olmos, F.J.; Gómez-Conesa, A.A.; García-Testal, A.; Ortega-Pérez-de-Villar, L.; Valtueña-Gimeno, N.; Gil-Gómez, J.A.; Garcia-Maset, R.; Segura-Ortí, E. An intradialytic non-immersive virtual reality exercise programme: A crossover randomized controlled trial. Nephrol. Dial. Transplant. 2022, 37, 1366–1374. [Google Scholar] [CrossRef]
- Fusco, A.; Tieri, G. Challenges and Perspectives for Clinical Applications of Immersive and Non-Immersive Virtual Reality. J. Clin. Med. 2022, 11, 4540. [Google Scholar] [CrossRef]
- Cowie, M.R.; Lam, C.S.P. Remote monitoring and digital health tools in CVD management. Nat. Rev. Cardiol. 2021, 18, 457–458. [Google Scholar] [CrossRef]

| Database | Search Strategy |
|---|---|
| PubMed | (Cardiac Rehabilitation[mh] OR Cardiac Rehabilitation*[tiab] OR myocardial infarction[mh] or myocardial infarction[tiab] OR heart attack*[tiab]) AND (virtual reality[mh] OR virtual reality[tiab] OR virtual reality exposure therapy[mh] OR non-immersive virtual reality[tiab] OR “Nintendo”[tiab] OR “playstation”[tiab] OR “sony playstation”[tiab] OR “xbox”[tiab] OR Kinect[tiab] OR exergaming[mh] OR exergam*[tiab] OR video games[mh] OR video games[tiab] OR videogam*[tiab] OR “Wii”[tiab]) |
| SCOPUS | (TITLE-ABS-KEY (“cardiac rehabilitation” OR “myocardial infraction” OR “heart attack”) AND TITLE-ABS-KEY (“virtual reality” OR “virtual reality exposure therapy” OR “non-immersive virtual reality” OR “video games” OR “videogames” OR “exergames” OR “exergaming” OR “Wii” OR “Nintendo” OR “playstation” OR “Kinect”)) |
| WOS | (TOPIC(*cardiac rehabilitation* OR *myocardial infraction* OR *heart attack*) AND TOPIC (*virtual reality* OR *virtual reality exposure therapy* OR *non-immersive virtual reality* OR *video games* OR *videogames* OR *exergames* OR *exergaming* OR *Wii* OR *Nintendo* OR *playstation* OR *Kinect*)) |
| CINAHL | (AB(cardiac rehabilitation OR myocardial infraction OR heart attack) AND AB (virtual reality OR virtual reality exposure therapy OR non-immersive virtual reality OR video games OR videogames OR exergames OR exergaming OR Wii OR Nintendo OR playstation OR Kinect)) |
| SciELO | “Cardiac rehabilitation” AND (“virtual reality” OR exergame* OR videogame OR “video game”) |
| PEDro | (1) Cardiac rehabilitation AND virtual reality//(2) Heart attack AND Wii |
| Study | Participants (n; Pathology and Rehabilitation Phase) | NiVR Intervention | Control Intervention | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age | F:M | Intervention Characteristic | n | Age | F:M | Control Characteristic | Variable | Test | ||
| Brandao-Leandro, LA et al., 2021 (Brazil) [61] Setting: Local public health system Funding: yes | 72 patients with controlled arterial hypertension in phase II | 19 | 63.2 ± 10.3 years old | 10:9 | NiVR active videogames using Nintendo Wii (“Hula Hoop”, “Footing” and “Rhythmic Boxeo”) and Xbox 360 (“Run the Word-Broadway”, “Wall Breaker” and “Legs-100%”) during 70 min sessions, with 2 sessions per week over 15 weeks | 20 | 66.5 ± 8.3 years old | 12:8 | Usual care | Functional capacity | 6-MWT |
| 20 | 67.9 ± 6.4 years old | 13:7 | Aerobic exercise on treadmill over 12 weeks, three times per week and 50 min per session | Quality of life (overall, physical, mental, vitality, social) | SF-36 | ||||||
| Cacau, LAP et al., 2013 (Brazil) [62] Setting: Fundaçao de Beneficiencia Hospital Cirujia, Aracaju, Sergipe. Funding: yes | 60 patients with coronary artery bypass/valve replacement in phase I | 30 | 49.2 ± 2.6 years old | 17:13 | Conventional physical therapy (breathing, metabolic and motor exercises and airway clearance techniques). NiVR was employed to perform motor exercises | 30 | 52 ± 2.4 years old | 14:16 | Conventional physical therapy twice a day | Functional capacity | 6-MWT |
| García-Bravo, S et al., 2020 (Spain) [63] Setting: cardiology service of the Hospital Universitario Fundación Alcorcón (Madrid) Funding: none | 20 patients with ischemic heart disease in phase II | 10 | 48.7 ± 9.9 years old | NR | 40 min of aerobic and resistance exercises plus 20 min of niVR using Microsoft Xbox One and Kinect 2. Twice per week over 8 weeks | 31 | 53.7 ± 10.3 years old | NR | Aerobic exercises plus resistance training for 60 min, twice per week over 8 weeks | Functional capacity | 6-MWT |
| Quality of life (overall, physical, mental, vitality, social) | SF-36 | ||||||||||
| Depression | BDI | ||||||||||
| Gulick, V et al., 2021 (United States) [64] Setting: Jefferson Health Methodist CR program, Philadelphia Funding: Yes | 72 patients (mean age of 61 ± 9.9 years old and 20F:52M) with different cardiac diseases in phase II | 41 | - | - | Conventional physical therapy using Bionautica Trails VR System over 12 weeks | 31 | - | - | Conventional physical therapy over 12 weeks | Functional capacity | 6-MWT |
| Jaarsma, T et al., 2021 (Sweden, Italy, Israel, Netherlands, Germany and United States) [65] Setting: NR Funding: yes | 486 patients (33% female population) with heart failure in phase III | 243 | 66 ± 12 years old | - | Nintendo Wii Sports (baseball, bowling, boxing, golf and tennis), five days per week, 30 min per session over one year | 243 | 67 ± 11 years old | - | Usual care plus physical activity | Functional capacity | 6-MWT |
| Ruivo, JMAS et al., 2017 (Ireland) [66] Setting: Kerry General Hospital Funding: none | 32 participants with coronary artery bypass/valve replacement in phase II | 16 | 59.4 ± 11.8 years old | 2:14 | 60 min using Nintendo Wii Sports (boxing and canoeing), twice per week over 6 weeks | 16 | 60.4 ± 8.5 years old | 4:12 | Conventional therapy, twice per week over 6 weeks | Quality of life (overall, physical, mental and social) | MNQ |
| Anxiety | HADS | ||||||||||
| Depression | |||||||||||
| Silva, JPLN et al., 2018 (Brazil) [67] Setting: Physical Therapy Clinic of the Oeste Paulista University (Sao Paulo) Funding: yes | 26 patients with different cardiac diseases in phase II | 14 | 63.2 ± 8.3 years old | 2:12 | 60 min using Microsoft Xbox 360 plus Kinect sensor (Your Shape and Dance Central 3), twice per week over 8 weeks | 12 | 63.8 ± 8.7 years old | 6:6 | Aerobic exercise using treadmill for 30 min and strength training | Functional capacity | |
| Vieira, A et al., 2018 (Portugal) [68] Setting: Hospital Centre of Porto Funding: none | 33 patients with different cardiac diseases in phase III | 11 | 55 ± 9 years old | - | NiVR using Kinect (Kinect-Rehab play project) plus education of control of risk factors in sessions of 60 min, three times per week for 24 weeks | 11 | 59 ± 11.3 years old | Education plus exercise training | Quality of life (overall, physical, mental and social) | MNQ | |
| 11 | 59 ± 5.8 years old | Education plus usual care and daily walks | |||||||||
| Anxiety | DASS-21 | ||||||||||
| Depression | |||||||||||
| Yuenyongchaiwat, K et al., 2023 (Thailand) [69] Setting: University Hospital (Khlong Nueng) Funding: yes | 60 patients with coronary heart or valvular disease | 30 | 63.2 ± 9.6 years old | 14:16 | NiVR using Toucher Software (“Falling Snow”, “Apple Tree” and “Hit the mole”) for 30 min, once a day until hospital discharge | 30 | 64.4 ± 8.7 years old | 11:19 | 30 min of conventional physical therapy, once a day until hospital discharge | Functional capacity | 6-MWT |
| Depression | PHQ-9 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peinado-Rubia, A.B.; Verdejo-Herrero, A.; Obrero-Gaitán, E.; Osuna-Pérez, M.C.; Cortés-Pérez, I.; García-López, H. Non-Immersive Virtual Reality-Based Therapy Applied in Cardiac Rehabilitation: A Systematic Review with Meta-Analysis. Sensors 2024, 24, 903. https://doi.org/10.3390/s24030903
Peinado-Rubia AB, Verdejo-Herrero A, Obrero-Gaitán E, Osuna-Pérez MC, Cortés-Pérez I, García-López H. Non-Immersive Virtual Reality-Based Therapy Applied in Cardiac Rehabilitation: A Systematic Review with Meta-Analysis. Sensors. 2024; 24(3):903. https://doi.org/10.3390/s24030903
Chicago/Turabian StylePeinado-Rubia, Ana Belén, Alberto Verdejo-Herrero, Esteban Obrero-Gaitán, María Catalina Osuna-Pérez, Irene Cortés-Pérez, and Héctor García-López. 2024. "Non-Immersive Virtual Reality-Based Therapy Applied in Cardiac Rehabilitation: A Systematic Review with Meta-Analysis" Sensors 24, no. 3: 903. https://doi.org/10.3390/s24030903
APA StylePeinado-Rubia, A. B., Verdejo-Herrero, A., Obrero-Gaitán, E., Osuna-Pérez, M. C., Cortés-Pérez, I., & García-López, H. (2024). Non-Immersive Virtual Reality-Based Therapy Applied in Cardiac Rehabilitation: A Systematic Review with Meta-Analysis. Sensors, 24(3), 903. https://doi.org/10.3390/s24030903















