Novel Conductive Polymer Composite PEDOT:PSS/Bovine Serum Albumin for Microbial Bioelectrochemical Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Microorganism Cultivation
2.3. Biosensor Preparation
2.4. Instrumentation
2.5. MFC Characterization
2.6. Clark-Type Oxygen Electrode Measurements
2.7. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Assessment of Bioelectrochemical Properties of Bacteria in the Composite Composition
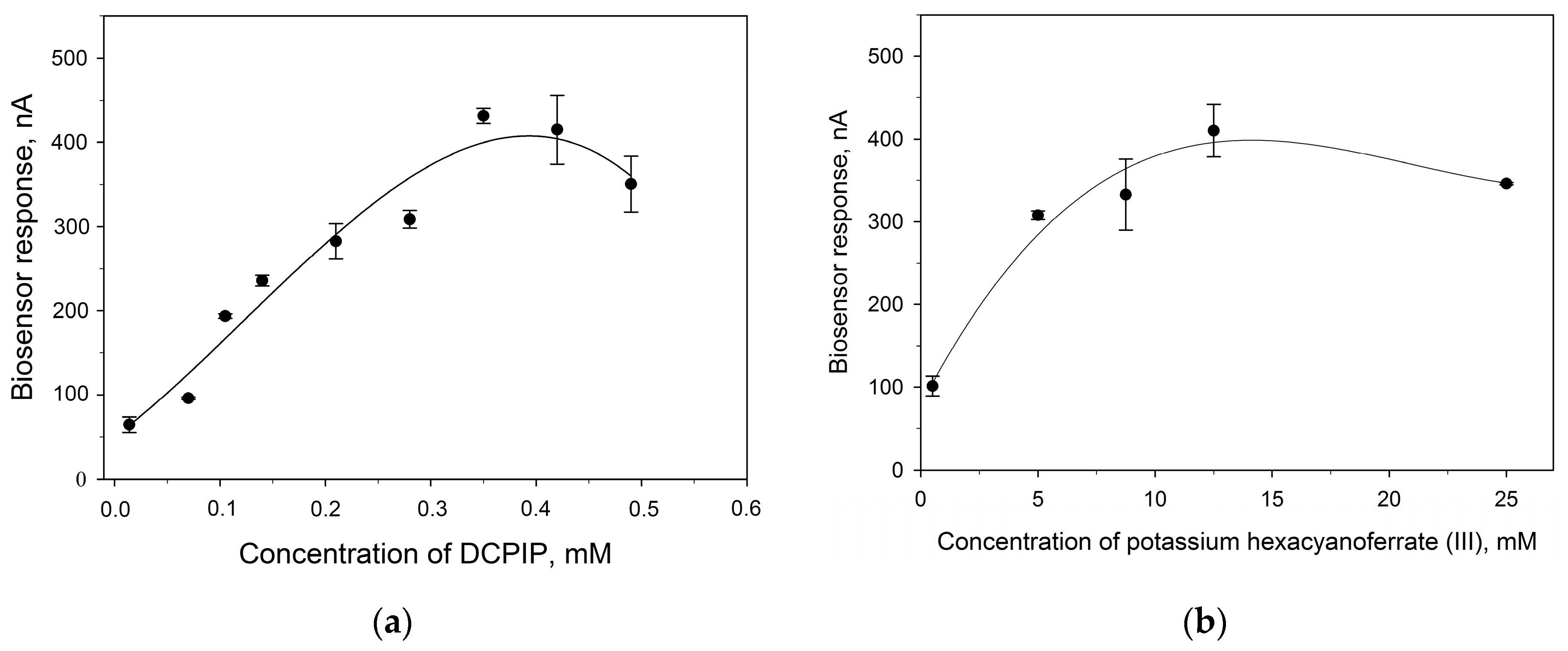
3.2. Evaluation of Heterogeneous Electron Transfer
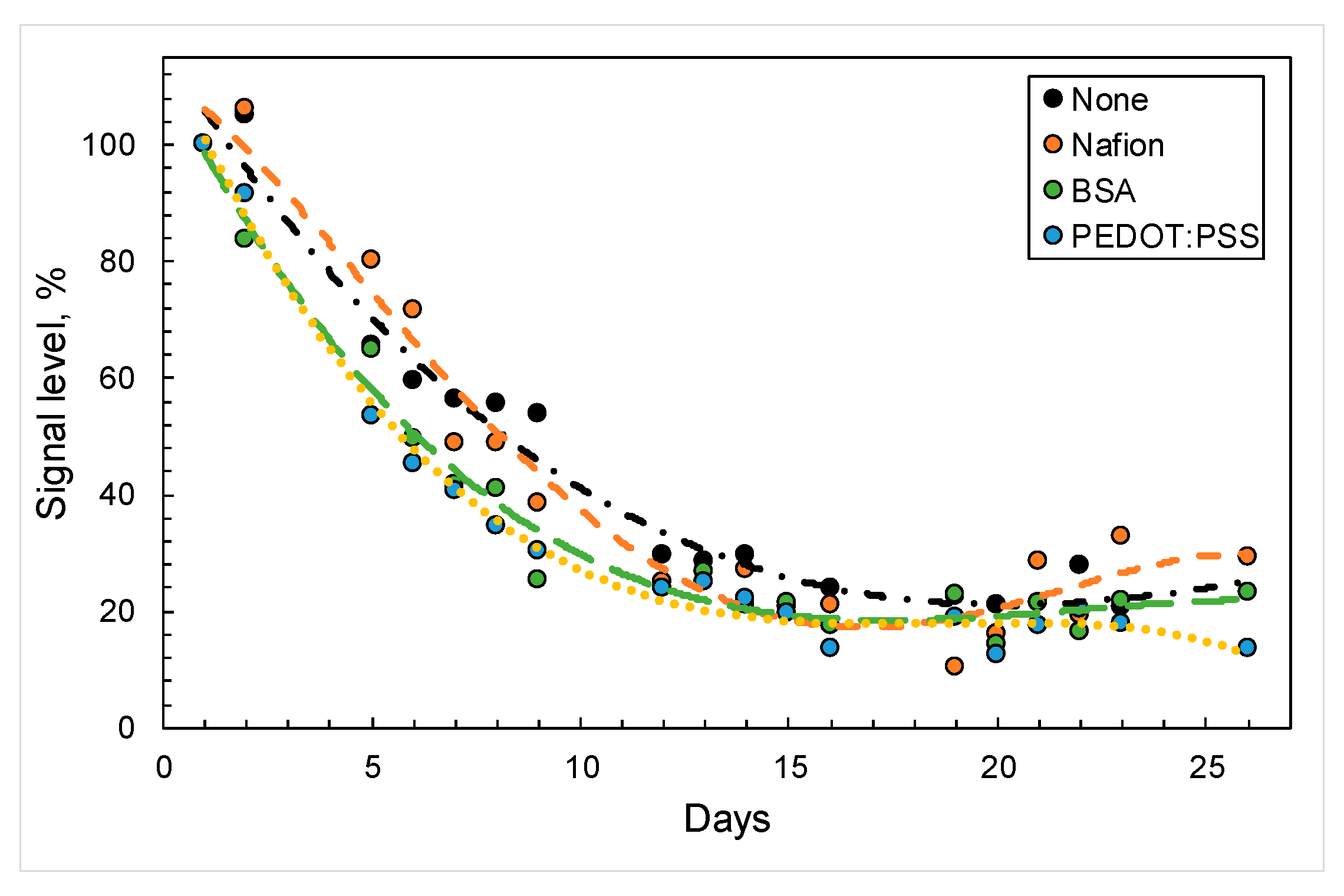
3.3. Analytical Characteristics of Biosensors
3.4. Practical Application of Composite as a Basis for an MFC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Simoska, O.; Lim, K.; Grattieri, M.; Yuan, M.; Dong, F.; Lee, Y.S.; Beaver, K.; Weliwatte, S.; Gaffney, E.M.; et al. Fundamentals, Applications, and Future Directions of Bioelectrocatalysis. Chem. Rev. 2020, 120, 12903–12993. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.E.; Lu, T.K. Microbes as Biosensors. Annu. Rev. Microbiol. 2020, 74, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liao, C.; Zhong, Z.; Liu, S.; Li, M.; Wang, X. Design, Optimization and Application of a Highly Sensitive Microbial Electrolytic Cell-Based BOD Biosensor. Environ. Res. 2023, 216, 114533. [Google Scholar] [CrossRef]
- Yang, W.; Wei, X.; Fraiwan, A.; Coogan, C.G.; Lee, H.; Choi, S. Fast and Sensitive Water Quality Assessment: A μL-Scale Microbial Fuel Cell-Based Biosensor Integrated with an Air-Bubble Trap and Electrochemical Sensing Functionality. Sens. Actuators B Chem. 2016, 226, 191–195. [Google Scholar] [CrossRef]
- Ma, Z.; Meliana, C.; Munawaroh, H.S.H.; Karaman, C.; Karimi-Maleh, H.; Low, S.S.; Show, P.L. Recent Advances in the Analytical Strategies of Microbial Biosensor for Detection of Pollutants. Chemosphere 2022, 306, 135515. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.-L.; Ho, L.-N.; Ong, S.-A.; Guo, K.; Liew, Y.-M.; Oon, Y.-S.; Thor, S.-H.; Tan, S.-M.; Teoh, T.-P. Primary insights into the effects of organic pollutants and carbon-based cathode materials in a double chambered microbial fuel cell integrated electrocatalytic process. J. Water Process Eng. 2021, 44, 102358. [Google Scholar] [CrossRef]
- Ali Yaqoob, A.; Al-Zaqri, N.; Suriaty Yaakop, A.; Umar, K. Potato Waste as an Effective Source of Electron Generation and Bioremediation of Pollutant through Benthic Microbial Fuel Cell. Sustain. Energy Technol. Assess. 2022, 53, 102560. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero-Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local Fruit Wastes Driven Benthic Microbial Fuel Cell: A Sustainable Approach to Toxic Metal Removal and Bioelectricity Generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Conventional Vegetable Waste: A Potential Source for the High Performance of Benthic Microbial Fuel Cells. Biomass Convers. Bioref. 2023, 1–13. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Bioelectricity Generation by Microbial Degradation of Banana Peel Waste Biomass in a Dual-Chamber S. Cerevisiae-Based Microbial Fuel Cell. Biomass Bioenergy 2023, 168, 106677. [Google Scholar] [CrossRef]
- Zafar, H.; Peleato, N.; Roberts, D. A Comparison of Reactor Configuration Using a Fruit Waste Fed Two-Stage Anaerobic up-Flow Leachate Reactor Microbial Fuel Cell and a Single-Stage Microbial Fuel Cell. Bioresour. Technol. 2023, 374, 128778. [Google Scholar] [CrossRef]
- Kuznetsova, L.S.; Arlyapov, V.A.; Plekhanova, Y.V.; Tarasov, S.E.; Kharkova, A.S.; Saverina, E.A.; Reshetilov, A.N. Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers 2023, 15, 3783. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.H.; Dam, M.S.; Bujna, E.; Rezessy-Szabo, J.; Farkas, C.; Vi, V.N.H.; Csernus, O.; Nguyen, V.D.; Gathergood, N.; Friedrich, L.; et al. In Situ Fabrication of Electrically Conducting Bacterial Cellulose-Polyaniline-Titanium-Dioxide Composites with the Immobilization of Shewanella Xiamenensis and Its Application as Bioanode in Microbial Fuel Cell. Fuel 2021, 285, 119259. [Google Scholar] [CrossRef]
- Zinovicius, A.; Rozene, J.; Merkelis, T.; Bruzaite, I.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Evaluation of a Yeast–Polypyrrole Biocomposite Used in Microbial Fuel Cells. Sensors 2022, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, B.A.; El-Taweel, G.E.; Naha, S.; Goswami, P. Bacterial Community Structure of Electrogenic Biofilm Developed on Modified Graphite Anode in Microbial Fuel Cell. Sci. Rep. 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Borin, G.P.; de Melo, R.R.; Crespim, E.; Sato, H.H.; Contesini, F.J. An Overview on Polymer Gels Applied to Enzyme and Cell Immobilization. In Polymer Gels: Science and Fundamentals; Thakur, V.K., Thakur, M.K., Eds.; Gels Horizons: From Science to Smart Materials; Springer: Singapore, 2018; pp. 63–86. ISBN 978-981-10-6086-1. [Google Scholar]
- Geetanjali; Dhillon, S.K.; Kundu, P.P. Development of Polypyrrole Nanotube Coated with Chitosan and Nickel Oxide as a Biocompatible Anode to Enhance the Power Generation in Microbial Fuel Cell. J. Power Sources 2022, 539, 231595. [Google Scholar] [CrossRef]
- Berggren, M.; Crispin, X.; Fabiano, S.; Jonsson, M.P.; Simon, D.T.; Stavrinidou, E.; Tybrandt, K.; Zozoulenko, I. Ion Electron–Coupled Functionality in Materials and Devices Based on Conjugated Polymers. Adv. Mater. 2019, 31, 1805813. [Google Scholar] [CrossRef] [PubMed]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in Understanding Structure and Transport Properties of PEDOT-Based Materials: A Critical Review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Zajdel, T.J.; Baruch, M.; Méhes, G.; Stavrinidou, E.; Berggren, M.; Maharbiz, M.M.; Simon, D.T.; Ajo-Franklin, C.M. PEDOT:PSS-Based Multilayer Bacterial-Composite Films for Bioelectronics. Sci. Rep. 2018, 8, 15293. [Google Scholar] [CrossRef]
- Salar-Garcia, M.J.; Montilla, F.; Quijada, C.; Morallon, E.; Ieropoulos, I. Improving the Power Performance of Urine-Fed Microbial Fuel Cells Using PEDOT-PSS Modified Anodes. Appl. Energy 2020, 278, 115528. [Google Scholar] [CrossRef]
- Rajendran, J.; Shetty, B.H.; Ganapathy, D.; Murugan, P.; Atchudan, R.; Umapathy, D.; Khosla, A.; Sundramoorthy, A.K. Thermally Expanded Graphite Incorporated with PEDOT:PSS Based Anode for Microbial Fuel Cells with High Bioelectricity Production. J. Electrochem. Soc. 2022, 169, 017515. [Google Scholar] [CrossRef]
- Gupta, S.; Datt, R.; Mishra, A.; Tsoi, W.C.; Patra, A.; Bober, P. Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sulfonate) in Antibacterial, Tissue Engineering and Biosensors Applications: Progress, Challenges and Perspectives. J. Appl. Polym. Sci. 2022, 139, e52663. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Z.; Wang, Y.; Fei, Z.; Cao, J. Changing the Activities and Structures of Bovine Serum Albumin Bound to Graphene Oxide. Appl. Surf. Sci. 2018, 427, 1019–1029. [Google Scholar] [CrossRef]
- Xu, F.; Ren, S.; Gu, Y. A Novel Conductive Poly(3,4-Ethylenedioxythiophene)-BSA Film for the Construction of a Durable HRP Biosensor Modified with NanoAu Particles. Sensors 2016, 16, 374. [Google Scholar] [CrossRef]
- Brîncoveanu, O.; Ioanid, A.; Iftimie, S.; AL-Zanganawee, J.; Enachescu, M.; Antohe, S. Study of physical properties of BSA:PEDOT-PSS thin film obtained by spin-coating. Dig. J. Nanomater. Biostruct. 2016, 11, 833–843. [Google Scholar]
- Brîncoveanu, O.; Ioanid, A.; Mesterca, R.; Pantazi, A.; Moise, C.; Enachescu, M.; Iftimie, S.; Antohe, S. Glucose detection using BSA:PEDOT-PSS as bioactive solute and solid bioactive layer deposited by spin coating. Rom. Rep. Phys. 2019, 71, 603. [Google Scholar]
- Gorshenev, V.N. Microwave-assisted and thermal stepwise expansion of oxidized graphites. Russ. J. Phys. Chem. B 2011, 5, 780–786. [Google Scholar] [CrossRef]
- Plekhanova, Y.; Tarasov, S.; Reshetilov, A. Use of PEDOT:PSS/Graphene/Nafion Composite in Biosensors Based on Acetic Acid Bacteria. Biosensors 2021, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Maruthapandi, M.; Saravanan, A.; Gupta, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial Activities of Conducting Polymers and Their Composites. Macromol 2022, 2, 78–99. [Google Scholar] [CrossRef]
- Krishnaveni, P.; Ganesh, V. Electron Transfer Studies of a Conventional Redox Probe in Human Sweat and Saliva Bio-Mimicking Conditions. Sci. Rep. 2021, 11, 7663. [Google Scholar] [CrossRef] [PubMed]
- Reshetilov, A.; Alferov, S.; Tomashevskaya, L.; Ponamoreva, O. Testing of Bacteria Gluconobacter Oxydans and Electron Transport Mediators Composition for Application in Biofuel Cell. Electroanalysis 2006, 18, 2030–2034. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Tahir, Z.; Arshad, M.; Khalil, S. Redox Protective Potential of Fruits and Vegetables: A Review. J. Coast. Life Med. 2015, 3, 663–668. [Google Scholar] [CrossRef]
- Kirubaharan, C.J.; Kumar, G.G.; Sha, C.; Zhou, D.; Yang, H.; Nahm, K.S.; Raj, B.S.; Zhang, Y.; Yong, Y.-C. Facile Fabrication of Au@polyaniline Core-Shell Nanocomposite as Efficient Anodic Catalyst for Microbial Fuel Cells. Electrochim. Acta 2019, 328, 135136. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Pannipara, M.; Al-Sehemi, A.G.; Kumar, G.G. PEDOT/NiFe2O4 Nanocomposites on Biochar as a Free-Standing Anode for High-Performance and Durable Microbial Fuel Cells. New J. Chem. 2019, 43, 7743–7750. [Google Scholar] [CrossRef]
- Das, S.; Calay, R.K. Experimental Study of Power Generation and COD Removal Efficiency by Air Cathode Microbial Fuel Cell Using Shewanella baltica 20. Energies 2022, 15, 4152. [Google Scholar] [CrossRef]
- Žalnėravičius, R.; Paškevičius, A.; Samukaitė-Bubnienė, U.; Ramanavičius, S.; Vilkienė, M.; Mockevičienė, I.; Ramanavičius, A. Microbial Fuel Cell Based on Nitrogen-Fixing Rhizobium Anhuiense Bacteria. Biosensors 2022, 12, 113. [Google Scholar] [CrossRef]
- Mulyono, T.; Nasifatul, S.M. The Effect of Addition of Vegetable Waste on Microbial Fuel Cell Performance. J. Phys. 2020, 1825, 012073. [Google Scholar] [CrossRef]
- Rojas-Villacorta, W.; Rojas-Flores, S.; Benites, S.M.; Nazario-Naveda, R.; Romero, C.V.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F.; Murga-Torres, E. Preliminary Study of Bioelectricity Generation Using Lettuce Waste as Substrate by Microbial Fuel Cells. Sustainability 2023, 15, 10339. [Google Scholar] [CrossRef]
- Tarasov, S.; Plekhanova, Y.; Kashin, V.; Gotovtsev, P.; Signore, M.A.; Francioso, L.; Kolesov, V.; Reshetilov, A. Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment. Biosensors 2022, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.; Dhungana, P.; Sreerama, L.; Bhatt, P.; Prajapati, B.; Poudel, P.; Kandel, P.; Khadka, M.; Parajuli, A.; Joshi, J. The Use of Microbial Fuel Cell for Efficient Treatment of Cauliflower Waste and Generation of Electricity. Int. J. Sustain. Energy 2023, 42, 304–317. [Google Scholar] [CrossRef]






| Ratio of PEDOT:PSS and BSA in the Composition | RS *, Ohm | С **, F | Rct ***, Ohm |
|---|---|---|---|
| 3:1 | 2700 | 2.33 × 10−8 | 7.97 × 107 |
| 3:1 с with glucose | 2354 | 2.36 × 10−8 | 6.26 × 107 |
| 2:1 | 1555 | 1.23 × 10−8 | 3.71 × 107 |
| 2:1 with glucose | 1359 | 1.73 × 10−8 | 2.16 × 108 |
| 1:1 | 582 | 1.33 × 10−6 | 1.20 × 104 |
| 1:1 with glucose | 322 | 5.20 × 10−5 | 1.16 × 104 |
| 1:2 | 534 | 4.40×10−5 | 1.12 × 106 |
| 1:2 with glucose | 512 | 4.50 × 10−5 | 2.28 × 105 |
| 1:3 | 480 | 6.60 × 10−5 | 3.73 × 105 |
| 1:3 with glucose | 471 | 7.20 × 10−5 | 1.31 × 105 |
| Electrode Modification | Mediator | n | a | ks |
|---|---|---|---|---|
| TEG/PEDOT:PSS/BSA/G. oxydans/Nafion | DCPIP | 2 | 0.0240 | 0.0168 |
| TEG/PEDOT:PSS/BSA/G. oxydans/Nafion | HCF | 1 | 0.0164 | 0.0065 |
| PEDOT:PSS/BSA/G. oxydans/Nafion | DCPIP | 2 | 0.0247 | 0.0152 |
| PEDOT:PSS/BSA/G. oxydans/Nafion | HCF | 1 | 0.0097 | 0.0038 |
| Composite | Printed Electrode Not Modified by TEG | Printed Electrode Modified by TEG |
|---|---|---|
| Parameter | ||
| Hill coefficient | 1.60 ± 0.38 | 2.41 ± 0.38 |
| Apparent Michaelis constant | 3.08 ± 0.61 | 3.13 ± 0.25 |
| Biosensor sensitivity, µA × mM −1 × cm−2 | 1.48 | 2.81 |
| Linear detection range, mmol | 0.25–3.76 | 0.57–4.62 |
| Detection range, mmol | 0.02–10 | 0.02–10 |
| Minimum detectable concentration, mmol | 0.02 | 0.02 |
| Imax, nА | 661 | 1035 |
| Electrode Material | Anode Modification | Biocatalyst | Carbon Source | Maximum Voltage, mV | Power Density, mW/m2 | Reference |
|---|---|---|---|---|---|---|
| Carbon cloth | Au@polyaniline | Escherichia coli | Glucose | 640 | 804 | [37] |
| Biochar | PEDOT/NiFe2O4 | Microbial consortium | Glucose | 690 | 1200 | [38] |
| Carbon felt | - | Shewanella baltica | Artificial wastewater | 190 | 12 | [39] |
| Graphite rod | - | Microbial consortium | Potato waste | 112 | current density—36.84 mA/m2 | [7] |
| Graphite rod | - | Microbial consortium | Fruit waste | 180 | 0.71 | [8] |
| Carbon felt | oxidation | Rhizobium anhuiense | Glucose | 683 | 4.93 | [40] |
| Graphite felt cloth | heated at 110 °C | Microbial soil consortium | Vegetable waste | 305 | 89 | [41] |
| Copper | - | Microbial consortium | Lettuce waste | 959 | 378 | [42] |
| Carbon veil | PEDOT:PSS | Microbial consortium | Human urine | 640 | 61 | [21] |
| Graphite rod | PEDOT:PSS/Graphene/Nafion | Gluconobacter oxydans | Municipal wastewater | 480 | 65 | [43] |
| Graphite felt | MWCNT/polyaniline | Microbial consortium | Cauliflower leaf waste | 681 | 10.1 (W/m3) | [44] |
| Thermally expanded graphite | PEDOT:PSS/BSA/Nafion | Gluconobacter oxydans | Vegetable waste | 440 | 40 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasov, S.E.; Plekhanova, Y.V.; Bykov, A.G.; Kadison, K.V.; Medvedeva, A.S.; Reshetilov, A.N.; Arlyapov, V.A. Novel Conductive Polymer Composite PEDOT:PSS/Bovine Serum Albumin for Microbial Bioelectrochemical Devices. Sensors 2024, 24, 905. https://doi.org/10.3390/s24030905
Tarasov SE, Plekhanova YV, Bykov AG, Kadison KV, Medvedeva AS, Reshetilov AN, Arlyapov VA. Novel Conductive Polymer Composite PEDOT:PSS/Bovine Serum Albumin for Microbial Bioelectrochemical Devices. Sensors. 2024; 24(3):905. https://doi.org/10.3390/s24030905
Chicago/Turabian StyleTarasov, Sergei E., Yulia V. Plekhanova, Aleksandr G. Bykov, Konstantin V. Kadison, Anastasia S. Medvedeva, Anatoly N. Reshetilov, and Vyacheslav A. Arlyapov. 2024. "Novel Conductive Polymer Composite PEDOT:PSS/Bovine Serum Albumin for Microbial Bioelectrochemical Devices" Sensors 24, no. 3: 905. https://doi.org/10.3390/s24030905
APA StyleTarasov, S. E., Plekhanova, Y. V., Bykov, A. G., Kadison, K. V., Medvedeva, A. S., Reshetilov, A. N., & Arlyapov, V. A. (2024). Novel Conductive Polymer Composite PEDOT:PSS/Bovine Serum Albumin for Microbial Bioelectrochemical Devices. Sensors, 24(3), 905. https://doi.org/10.3390/s24030905







