Characterisation of a Silicon Photomultiplier Based Oncological Brachytherapy Fibre Dosimeter
Abstract
1. Introduction
| 125I | 192Ir | 60Co | |
|---|---|---|---|
| BT-treatment kind | LDR-BT | HDR-BT | HDR-BT |
| Activity | ∼15 MBq | ∼100 GBq | ∼100 GBq |
| Half-life | 59.4 days | 73.8 days | 1925.3 days |
| Mean -ray energy | 35.5 keV | 380 keV | 1250 keV |
| Abs. length (50%) | 2.2 cm | 6.4 cm | 11 cm |
| Abs. length (90%) | 7.5 cm | 21 cm | 37 cm |
2. The ORIGIN System
- Dose sensitivity is the capability of the dosimeter to single out a dose signal from the background fluctuations at a given distance.
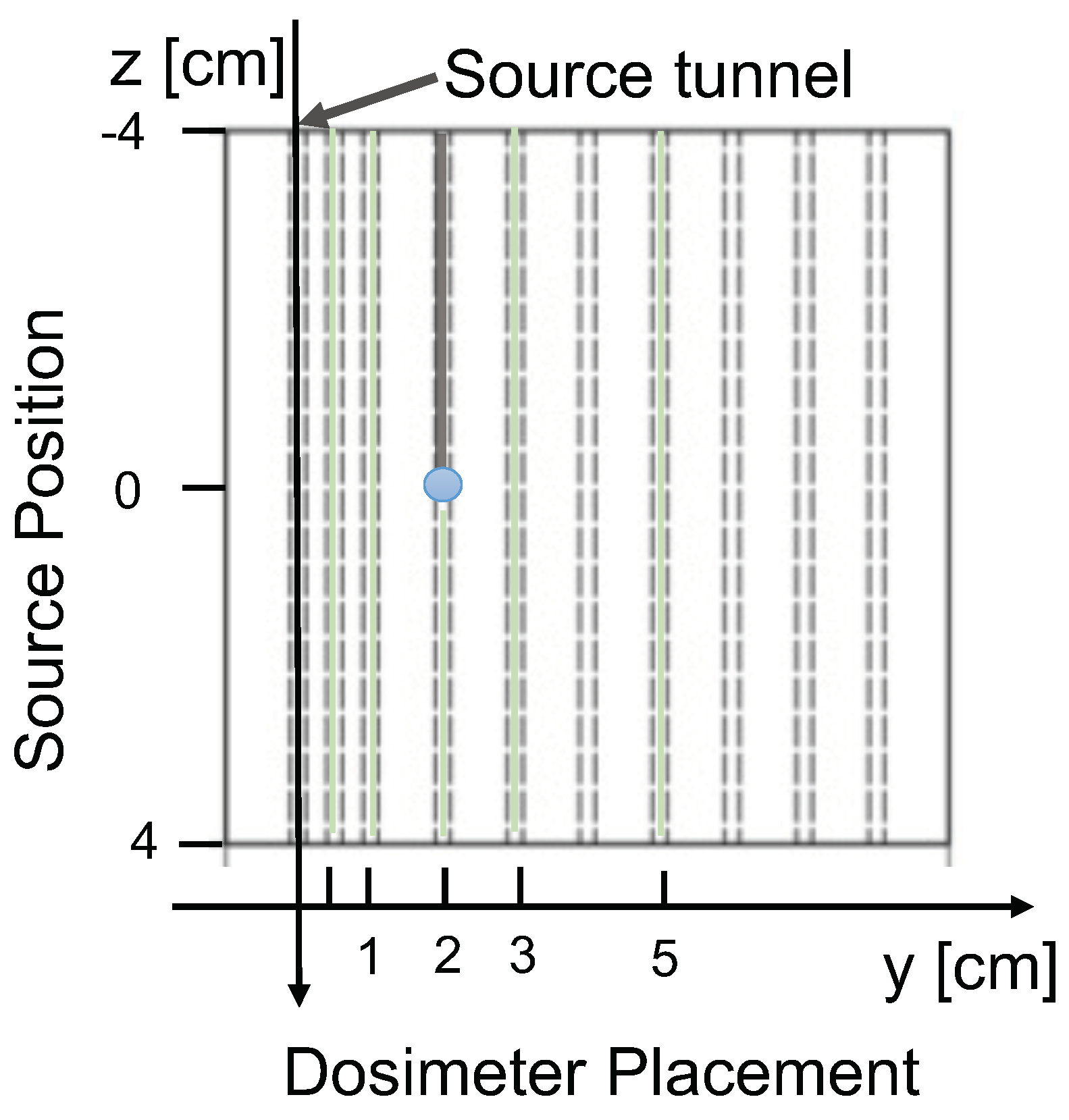
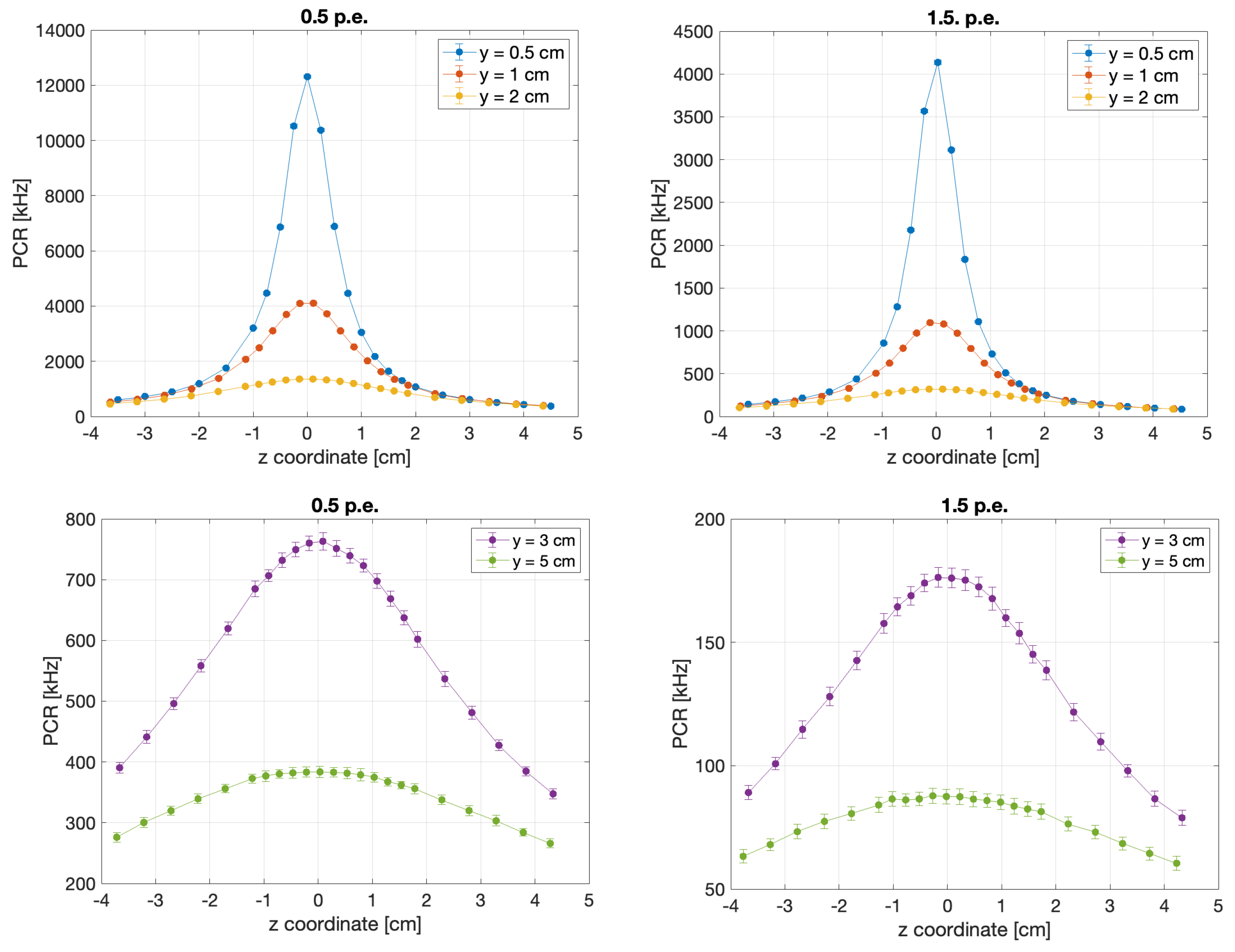
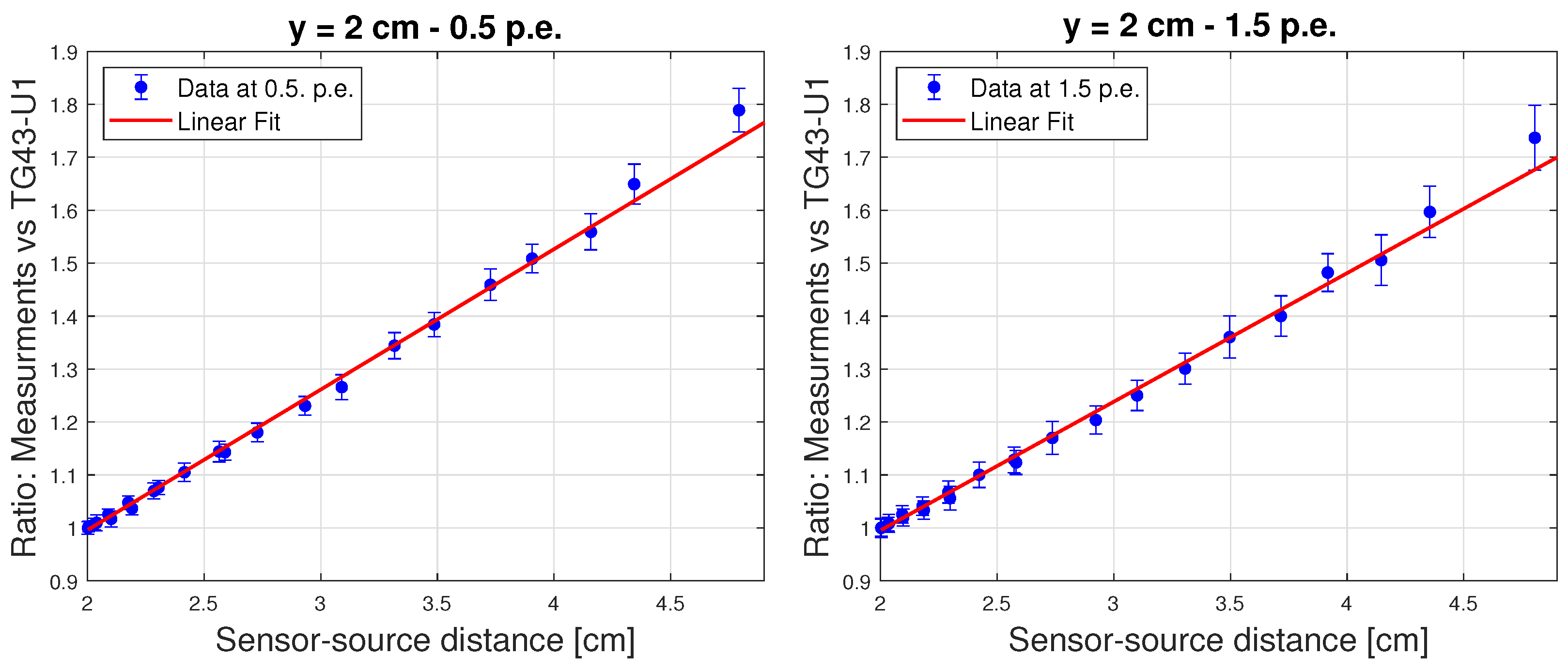
- Spatial resolution, , is the minimum displacement of the source at a selected distance (r), at any angle, for which the variation in the background-subtracted signal is three times its uncertainty.
- Statistical precision, , is the ratio between the counting uncertainty () and the signal counts () for the selected measurement time window and at the chosen distance
3. Laboratory Characterisation
- Minimum Detectable Rate (MDR), defined as the minimum Photon Counting Rate (PCR) exceeding 3 times the Poisson fluctuations of the DCR in the defined measurement time window:This parameter is retained as a measurement of the minimum detectable dose rate above the DCR background. The time windows () are 0.1 s (HDR) and 0.5 s (LDR).
- Linearity Range, constrained by the MDR (lower limit) and event pile-up (upper limit). To obtain the measurements a current scan was performed from 0.05 mA to 1 mA in increments of 0.05 mA up to 0.3 mA and increments of 0.1 mA thereafter. To avoid systematic errors that could be introduced by the afterglow and the memory effect of the sensor [21], the DCR was measured after every irradiation and subtracted to obtain the PCR. The linearity range is measured by including measurements as frequencies increase until the exceeds 2.7 (corresponding to ≈90% Confidence Level), where is the sum of the normalised squared differences between measurements and predictions and d.f.e. is the number of degrees of freedom.
- Sensitivity, defined as the minimum variation of the X-ray current (I) that induces a change in PCR exceeding three standard deviations of a single measurement (). In the linear regime, it may be written asand the sensitivity is evaluated from the slope of the linear trend.
4. Pre-Clinical Trial of the Prototype Dosimetric System
4.1. High Dose Rate Measurements
4.2. Pre-Clinical Campaign: LDR
5. Conclusions
- The accuracy of the point-to-point DCR subtraction is confirmed by the value of the offset in the linear fit, statistically compatible with zero;
- The difference in the sensitivity between the two HAMAMATSU SiPM can be traced to the different Photon Detection Efficiency. In fact, the ratio between the two values is , compatible with the PDE ratio (1.25, as of the data sheet and Table 2).
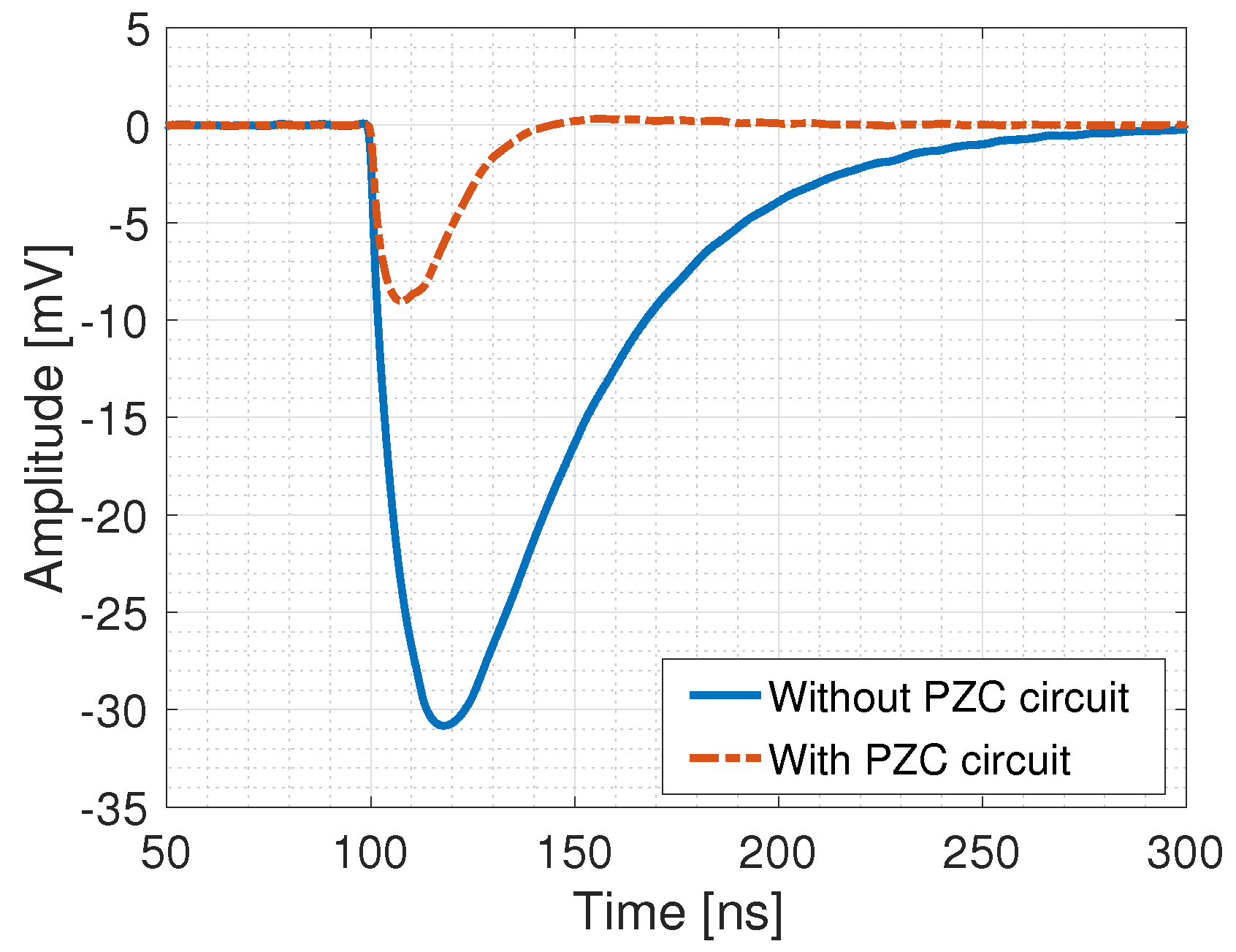
- The upper limit in the linearity range, once the DCR is accounted for, corresponds for all the detectors to a counting rate of ≈4 MHz (corresponding to a pile-up slightly above 10%), as expected by the comparable development time after the PZC and the discriminator logical signal duration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Khan, F.; Gibbons, J. Khan’s the Physics of Radiation Therapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R. Basic Radiotherapy Physics and Biology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Thomas, G.M. Better Radiation Therapy for Cervix Cancer Would Save Lives. Int. J. Radiat.-Oncol.-Biol.-Phys. 2014, 89, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.W.; Grimm, P.; Yaxley, J.; Jagavkar, R.; Ng, M.; Lawrentschuk, N. Brachytherapy: State-of-the-art radiotherapy in prostate cancer. BJU Int. 2015, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- ICRU. Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology; Tech. Rep. 38; International Commission on Radiation Units and Measurements Inc. (ICRU): Bethesda, MD, USA, 1985. [Google Scholar]
- Koukourakis, G.; Kelekis, N.; Armonis, V.; Kouloulias, V. Brachytherapy for prostate cancer: A systematic review. Adv. Urol. 2009, 2009, 327945. [Google Scholar] [CrossRef] [PubMed]
- Delishaj, D.; Barcellini, A.; D’Amico, R.; Ursino, S.; Pasqualetti, F.; Fumagalli, I.C.; Soatti, C.P. Vaginal toxicity after high-dose-rate endovaginal brachytherapy: 20 years of results. J. Contemp. Brachytherapy 2018, 10, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.; Drummond, F.J.; Donnelly, C.; O’Leary, E.; Sharp, L.; Kinnear, H.R. Patient-reported ‘ever had’ and ‘current’ long-term physical symptoms after prostate cancer treatments. BJU Int. 2015, 116, 397–406. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, S.; McCarthy, D.; Woulfe, P.; Grattan, M.W.D.; Hounsell, A.R.; Sporea, D.; Mihai, L.; Vata, I.; Leen, G.; Lewis, E. A review of recent advances in optical fiber sensors for in-vivo dosimetry during radiotherapy. Br. J. Radiol. 2015, 88, 20140702. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Nakano, T.; Law, S.; Elsey, J.; McKenzie, D.R.; Suchowerska, N. In vivo dosimeters for hdr brachytherapy: A comparison of a diamond detector, mosfet, tld, and scintillation detector. Med. Phys. 2007, 34, 1759–1765. [Google Scholar] [CrossRef]
- Linares Rosales, H.M.; Johansen, J.G.; Kertzscher, G.; Tanderup, K.; Beaulieu, L.; Beddar, S. 3D source tracking and error detection in HDR using two independent scintillator dosimetry systems. Med. Phys. 2021, 48, 2095. [Google Scholar] [CrossRef]
- Kim, J.; Koo, J.; Choi, S.H.; Lee, S.S.; Kim, D.W. A preliminary study on a real-time dose monitoring system based on scintillating fibers for brachytherapy. Nucl. Instrum. Methods A 2019, 929, 50–56. [Google Scholar] [CrossRef]
- Houlihan, O.A.; Workman, G.; Hounsell, A.R.; Prise, K.M.; Jain, S. In vivo dosimetry in pelvic brachytherapy. Br. J. Radiol. 2022, 95, 20220046. [Google Scholar] [CrossRef]
- Seymour, E.; Downes, S.J.; Fogarty, G.B.; Izard, M.A.; Metcalfe, P. In vivo real-time dosimetric verification in high dose rate prostate brachytherapy. Med. Phys. 2011, 38, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Rivard, M.J.; Coursey, B.M.; DeWerd, L.A.; Hanson, W.F.; Huq, M.S.; Ibbott, G.S.; Mitch, M.G.; Nath, R.; Williamson, J.F. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med. Phys. 2004, 31, 633–674. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, M.; Sadeghi, M.; Richeson, D.; Gholami, S. Determination of TG-43 dosimetric parameters for I-125, Ir-192, and Co-60 brachytherapy sources using the Monte Carlo simulation. Radiat. Phys. Eng. 2022, 3, 7–15. [Google Scholar] [CrossRef]
- Mostaghimi, H.; Mehdizadeh, A.R.; Darvish, L.; Akbari, S.; Rezaei, H. Mathematical formulation of 125I seed dosimetry parameters and heterogeneity correction in lung permanent implant brachytherapy. J. Can. Res. Ther. 2017, 13, 436–441. [Google Scholar] [CrossRef]
- Impiombato, D.; Giarrusso, S.; Mineo, T.; Catalano, O.; Gargano, C.; La Rosa, G.; Russo, F.; Sottile, G.; Billotta, S.; Bonanno, G.; et al. Characterization and performance of the ASIC (CITIROC) front-end of the ASTRI camera. NIM A 2015, 794, 185–192. [Google Scholar] [CrossRef]
- Woulfe, P.; Sullivan, F.J.; Kam, W.; O’Keeffe, S. Optical fiber dosimeter for real-time in-vivo dose monitoring during LDR brachytherapy. Biomed. Opt. Express 2020, 11, 4027–4036. [Google Scholar] [CrossRef] [PubMed]
- Kertzscher, G.; Beddar, S. Inorganic scintillation detectors based on Eu- activated phosphors for 192Ir brachytherapy. Phys. Med. Biol. 2017, 62, 5046. [Google Scholar] [CrossRef]
- Kertzscher, G.; Beddar, S. Inorganic scintillation detectors for 192Ir brachytherapy. Phys. Med. Biol. 2019, 64, 225018. [Google Scholar] [CrossRef]
- Cometti, S.; Gierej, A.; Giaz, A.; Lomazzi, S.; Baghdasaryan, T.; Van Erps, J.; Berghmans, F.; Santoro, R.; Caccia, M.; O’Keeffe, S. Characterization of scintillating materials in use for brachytherapy fiber based dosimeters. Nucl. Instrum. Methods A 2022, 1042, 167083. [Google Scholar] [CrossRef]
- Pittet, P.; Jalade, P.; Balosso, J.; Gindraux, L.; Guiral, P.; Wang, R.; Chaikh, A.; Gaudu, A.; Ribouton, J.; Rousseau, J.; et al. Dosimetry systems based on Gallium Nitride probe for radiotherapy, brachytherapy and interventional radiology. IRBM 2015, 35, 92–100. [Google Scholar] [CrossRef]
- Taylor, R.E.P.; Rogers, D.W.O. More accurate fitting of I125 and Pd103 radial dose functions. Med. Phys. 2008, 35, 4242–4250. [Google Scholar] [CrossRef]
- Meigooni, A.S.; Meli, J.A.; Nath, R. Influence of the variation of energy spectra with depth in the dosimetry of 192Ir using LiF TLD. Phys. Med. Biol. 1988, 33, 1159. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Q.; Wang, Q.; Li, Y.; Perks, R.M.; Zhao, L. Advances on inorganic scintillator-based optic fiber dosimeters. EJNMMI Phys. 2020, 7, 1–23. [Google Scholar] [CrossRef]
- Martyn, M.; Kam, W.; Woulfe, P.; O’Keeffe, S. Water Phantom Characterization of a Novel Optical Fiber Sensor for LDR Brachytherapy. IEEE Sens. J. 2023, 23, 1146–1156. [Google Scholar] [CrossRef]




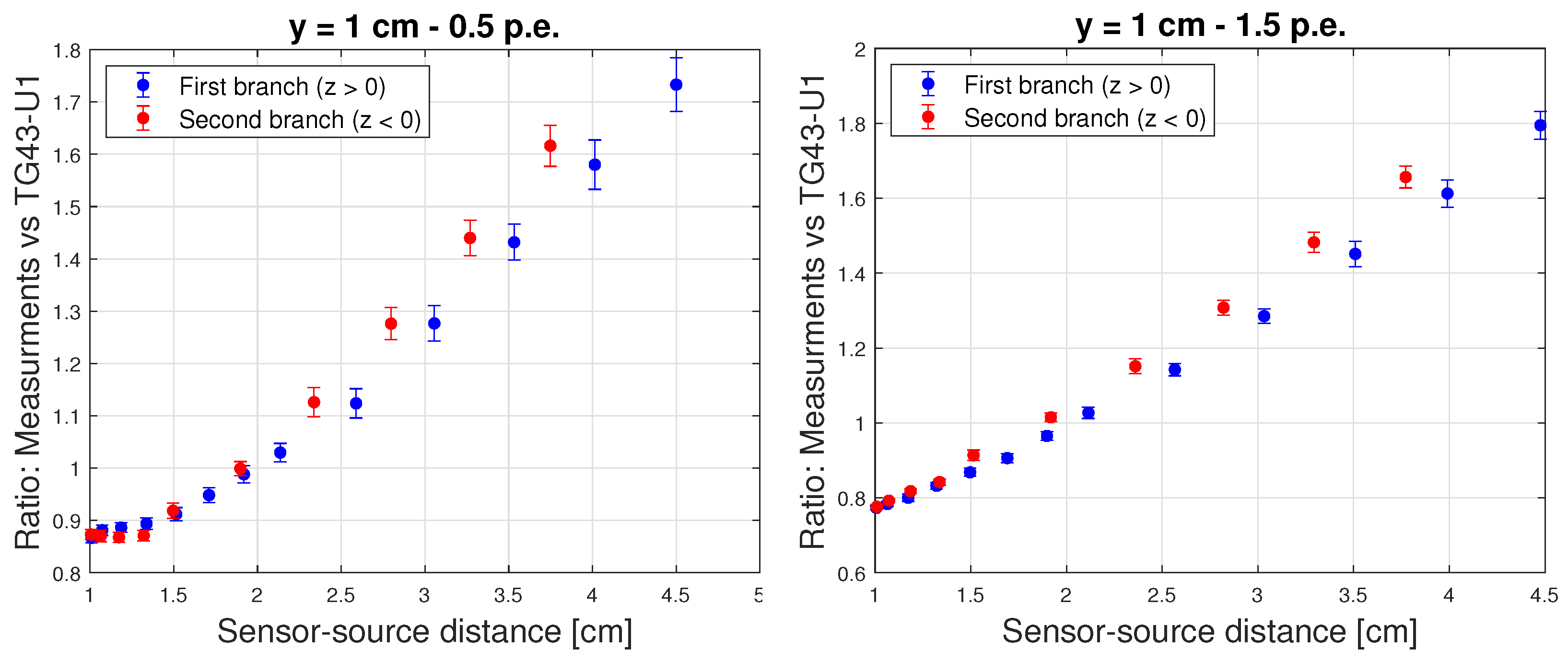
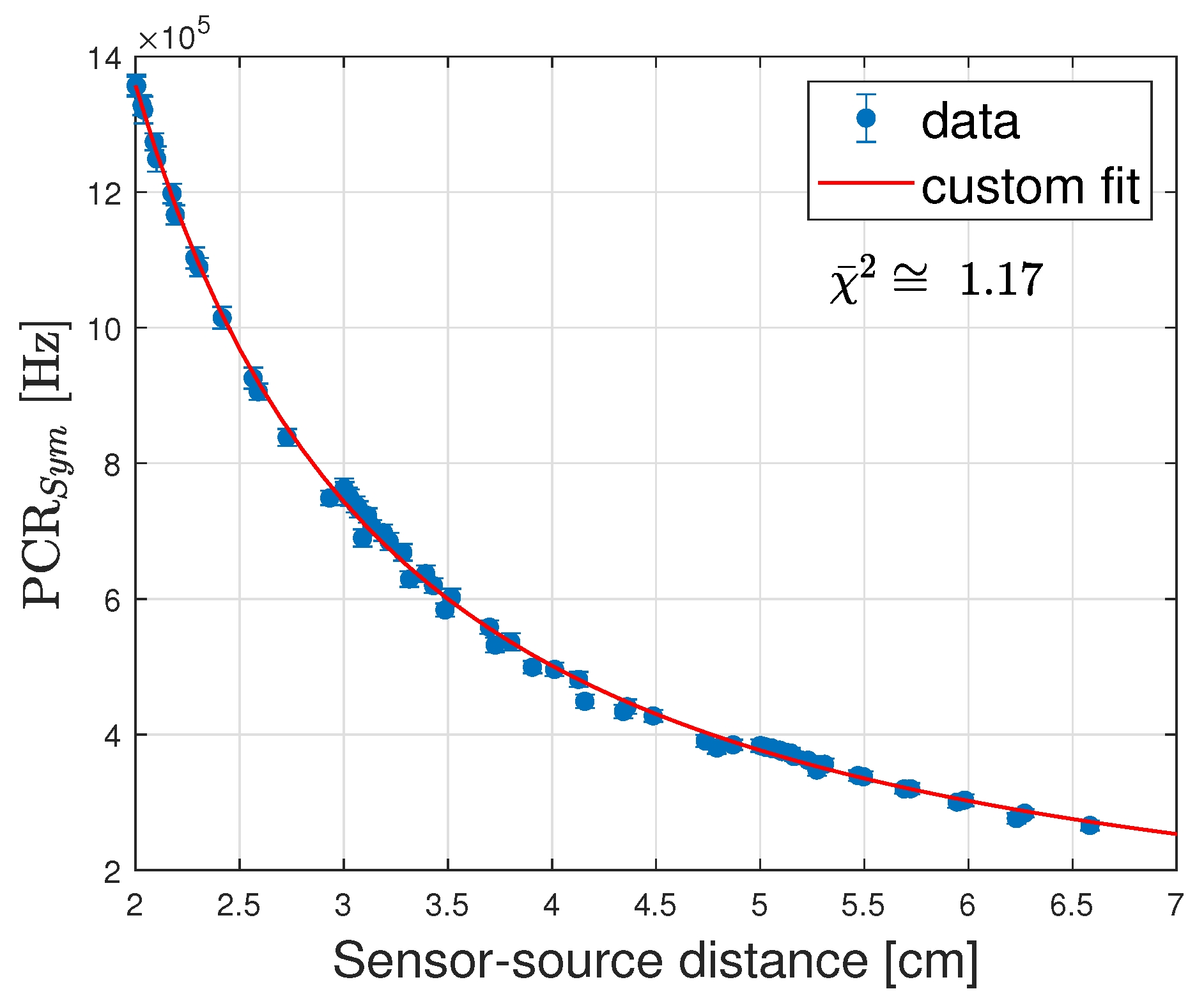


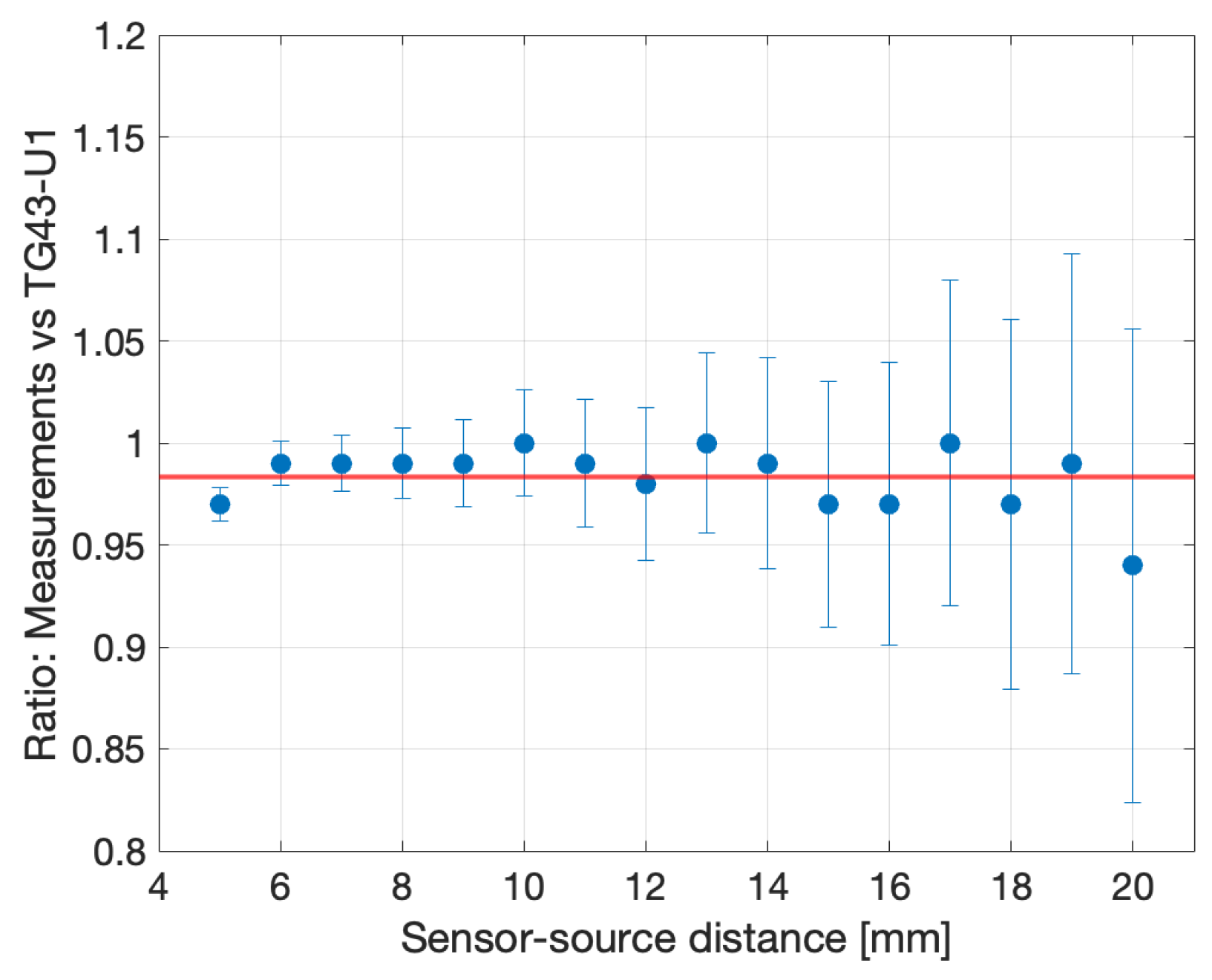

| LDR | HDR | |
|---|---|---|
| Sensitivity to Dose | up to 3 cm | up to 10 cm |
| Spatial Resolution | 3 mm @ 3 cm | 1 mm @ 5 cm |
| Dose Rate Range | 0.4–2 Gy/h | >12 Gy/h |
| Statistical Precision | 5% in 0.5 s @ 3 cm | 5% in 0.1 s @10 cm |
| PM1125 | PM3325 | S13360-1350 | S13360-1375 | |
|---|---|---|---|---|
| Sensitive area [mm2] | 1 × 1 | 3 × 3 | 1.3 × 1.3 | 1.3 × 1.3 |
| Cell pitch m | 25 | 25 | 50 | 75 |
| PDE at 420 nm | 45% | 45% | 40% | 50% |
| PDE at 620 nm | 17% | 17% | 24% | 30% |
| DCR [kHz]@ 0.5 p.e. | 125 | 1125 | 90 | 90 |
| Optical cross-talk | 26% | 26% | 3% | 7% |
| Breakdown Voltage [V] | 24 | 24 | 53 | 53 |
| Operational Voltage [V] | + 5 | + 5 | + 3 | + 3 |
| Gain at operational voltage | 1.7 | 1.7 | 1.7 | 4.0 |
| PM1125 | PM3325 | S13360-1350 | S13360-1375 | |
|---|---|---|---|---|
| MDR in 0.1 s [kHz] | 3.1 ± 0.2 | 10.5 ± 0.3 | 3.0 ± 0.2 | 2.8 ± 0.2 |
| MDR in 0.5 s [kHz] | 1.4 ± 0.05 | 4.7 ± 0.1 | 1.4 ± 0.05 | 1.2 ± 0.05 |
| Linearity upper limit (PCR) | 0.6 mA | 0.25 mA | 0.3 mA | 0.25 mA |
| 4.04 MHz | 3.09 MHz | 4.31 MHz | 4.43 MHz | |
| Sensitivity (m) [kHz/mA] | 6710 ± 31 | 12,438 ± 448 | 14,203 ± 354 | 18,187 ± 378 |
| PM1125 | PM3325 | S13360-1350 | S13360-1375 | |
|---|---|---|---|---|
| MDR in 0.1 s [kHz] | 1.5 ± 0.1 | 5.5 ± 0.2 | 0.49 ± 0.07 | 0.47 ± 0.07 |
| MDR in 0.5 s [kHz] | 0.68 ± 0.04 | 2.5 ± 0.07 | 0.22 ± 0.002 | 0.21 ± 0.002 |
| Linearity upper limit (PCR) | 1 mA | 1 mA | 1 mA | 1 mA |
| Sensitivity (m) [kHz/mA] | 1713 ± 22 | 3192 ± 31 | 354 ± 3 | 533 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccia, M.; Giaz, A.; Galoppo, M.; Santoro, R.; Martyn, M.; Bianchi, C.; Novario, R.; Woulfe, P.; O’Keeffe, S. Characterisation of a Silicon Photomultiplier Based Oncological Brachytherapy Fibre Dosimeter. Sensors 2024, 24, 910. https://doi.org/10.3390/s24030910
Caccia M, Giaz A, Galoppo M, Santoro R, Martyn M, Bianchi C, Novario R, Woulfe P, O’Keeffe S. Characterisation of a Silicon Photomultiplier Based Oncological Brachytherapy Fibre Dosimeter. Sensors. 2024; 24(3):910. https://doi.org/10.3390/s24030910
Chicago/Turabian StyleCaccia, Massimo, Agnese Giaz, Marco Galoppo, Romualdo Santoro, Micheal Martyn, Carla Bianchi, Raffaele Novario, Peter Woulfe, and Sinead O’Keeffe. 2024. "Characterisation of a Silicon Photomultiplier Based Oncological Brachytherapy Fibre Dosimeter" Sensors 24, no. 3: 910. https://doi.org/10.3390/s24030910
APA StyleCaccia, M., Giaz, A., Galoppo, M., Santoro, R., Martyn, M., Bianchi, C., Novario, R., Woulfe, P., & O’Keeffe, S. (2024). Characterisation of a Silicon Photomultiplier Based Oncological Brachytherapy Fibre Dosimeter. Sensors, 24(3), 910. https://doi.org/10.3390/s24030910







