Sensor-Based Assessment of Time-of-Day-Dependent Physiological Responses and Physical Performances during a Walking Football Match in Higher-Weight Men
Abstract
1. Introduction
2. Materials and Methods
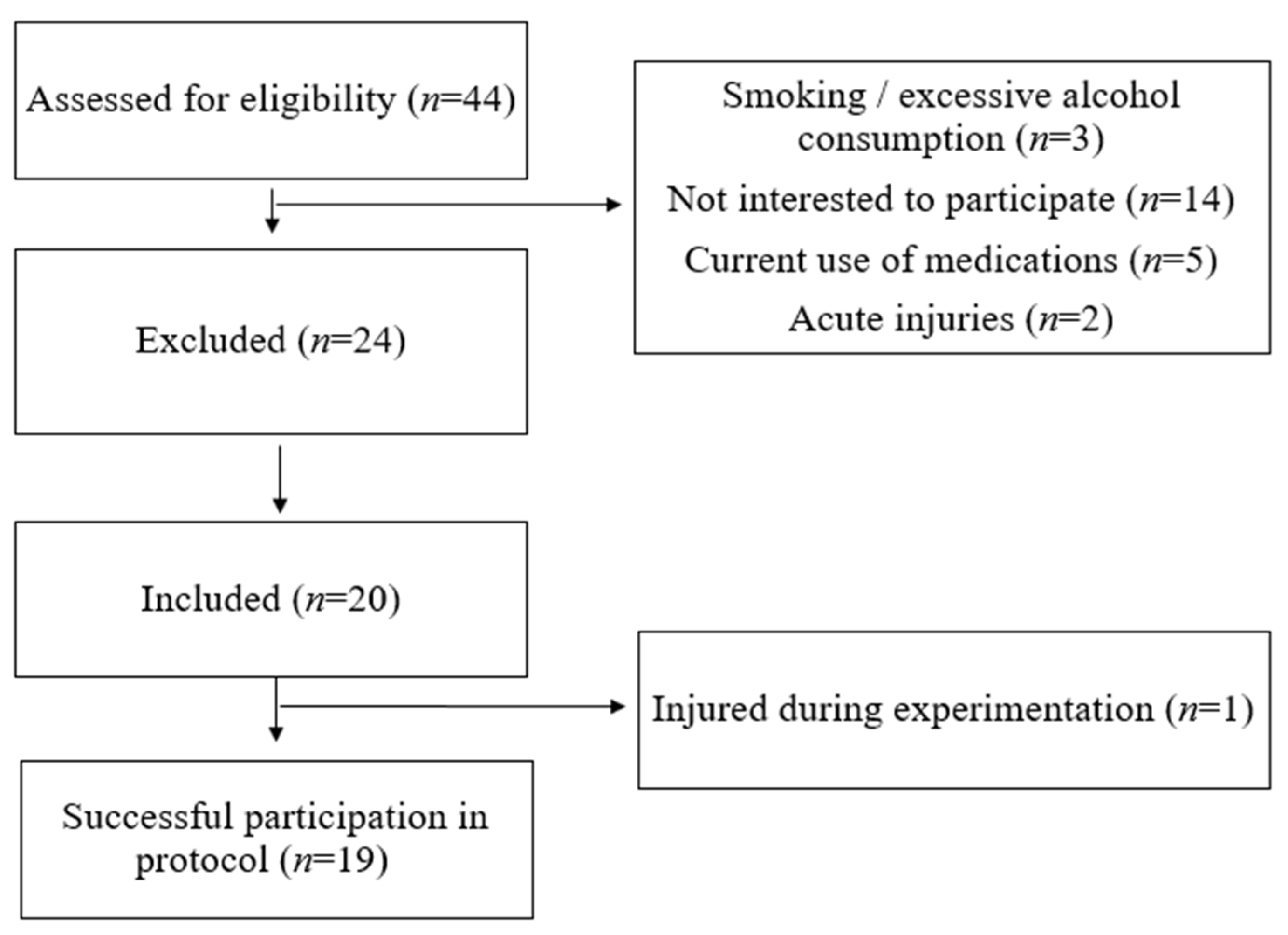
2.1. Participants
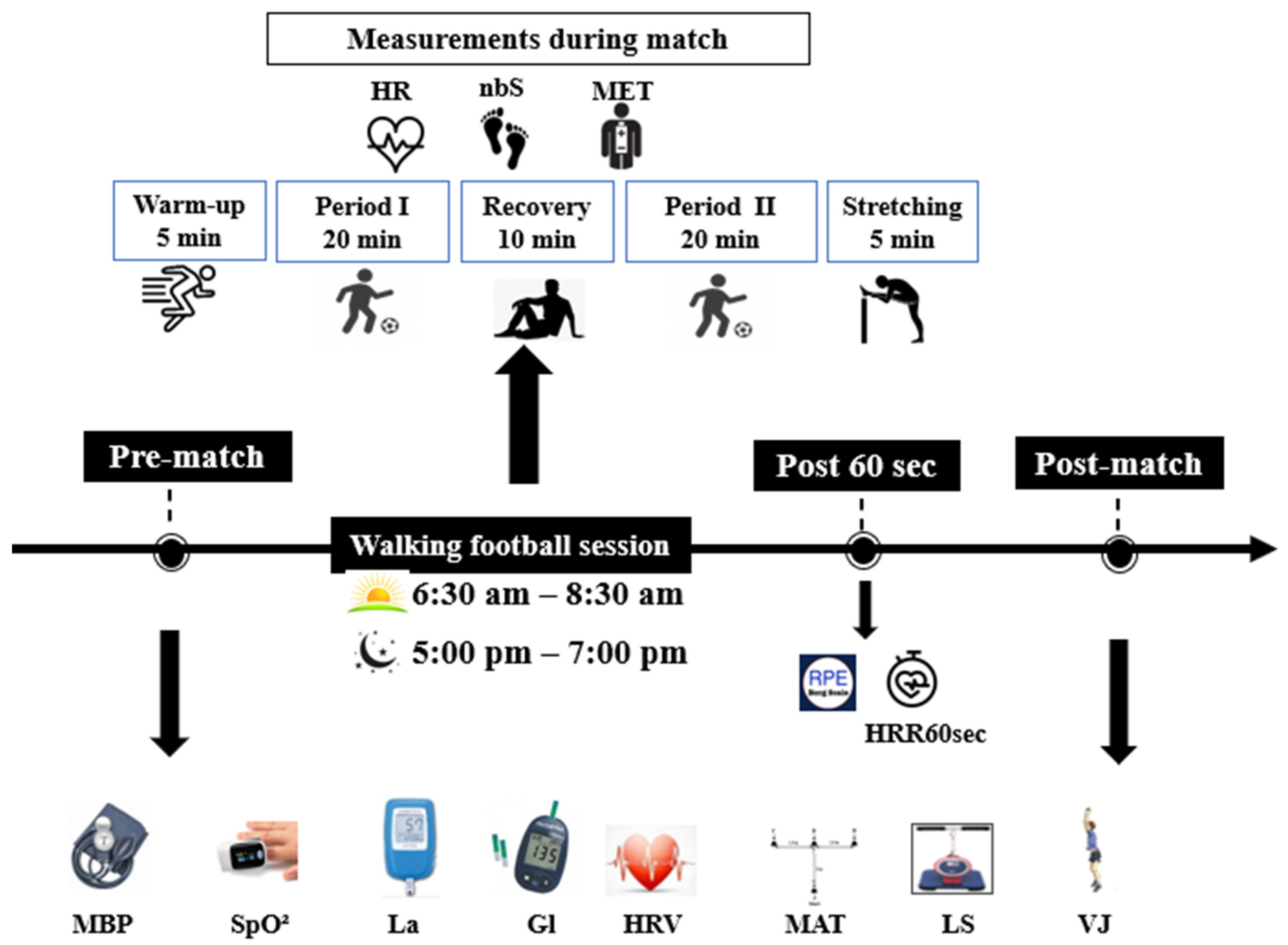
2.2. Experimental Design
2.3. Measurements
- Body Composition
- Blood Pressure (BP)
- Heart Rate Variability (HRV)
- Oxygen Saturation (SpO2)
- Blood Lactate (La) and Glycemia (Gl) Levels
- Modified Agility T-Test (MAT)
- Vertical Jump Height (VJ)
- Lumbar Strength (LS)
- Felt Arousal Scale (FAS)
- Match Parameters
- Heart Rate (HR)
- Rating of Perceived Exertion (RPE)
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
3.1. HRV
- Time domain
- Frequency domain
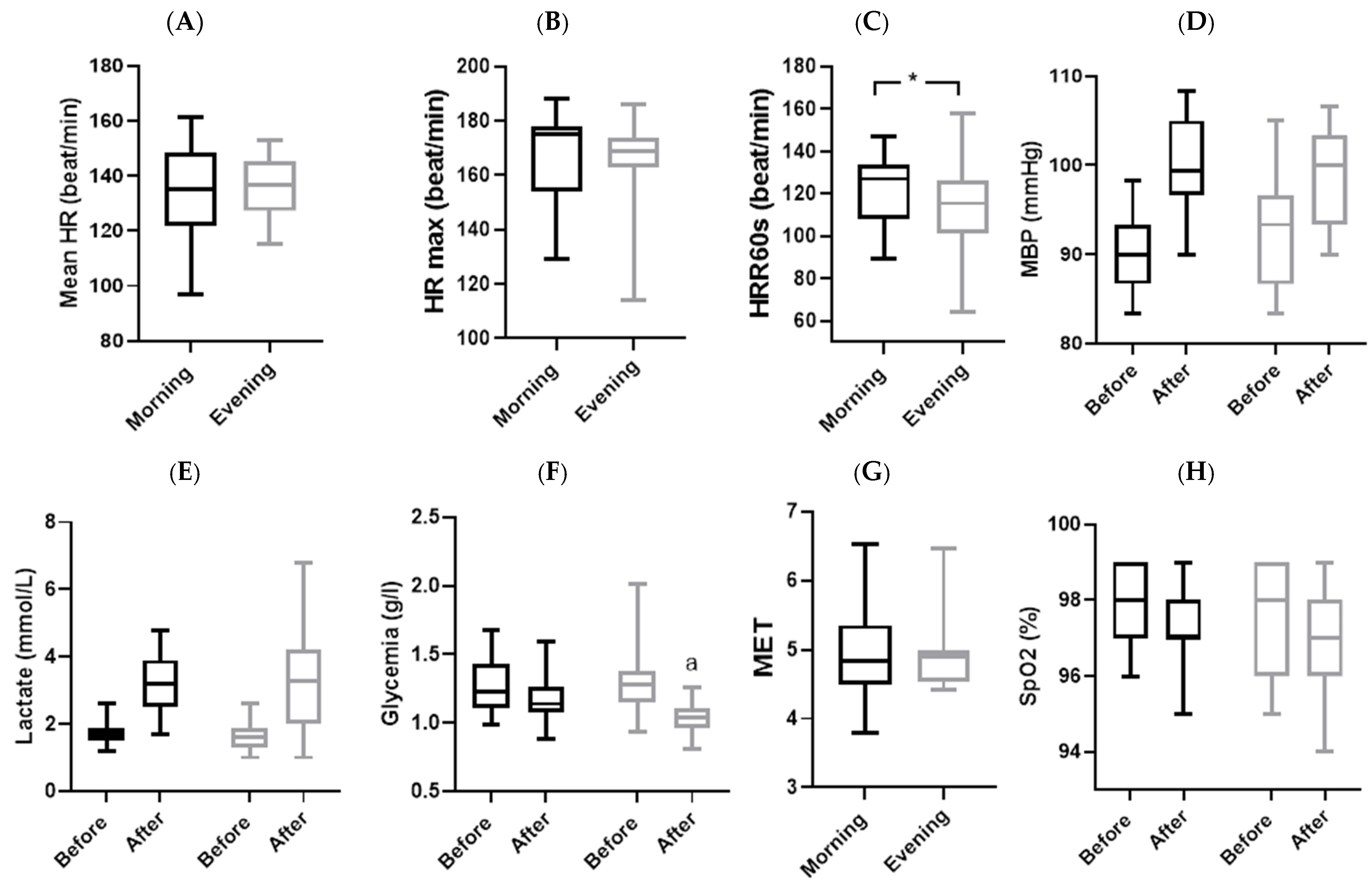
3.2. Physiological Parameters
3.3. Physical Parameters
3.4. Psychological State
3.5. Match Parameters
4. Discussion
4.1. Physiological Parameters
4.2. Physical Parameters
4.3. Match Parameters
4.4. RPE
4.5. Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Winkler, S.; Hebestreit, A.; Ahrens, W. Physical activity and obesity. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2012, 55, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.E.; Briss, P.A.; Goodman, R.A.; Bowman, B.A. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014, 384, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Landolfo, C.; Niebauer, J.; Ozemek, C.; Arena, R.; Lavie, C.J. Promoting Physical Activity and Exercise: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1622–1639. [Google Scholar] [CrossRef]
- Kohl, H.W., 3rd; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; Lancet Physical Activity Series Working, G. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Stallknecht, B.M.; Sorensen, T.I.A.; Kilpelainen, T.O.; Hansen, T. Evidence for shared genetics between physical activity, sedentary behaviour and adiposity-related traits. Obes. Rev. 2021, 22, e13182. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.F.; Baade, P.D.; Green, A.C.; Jordan, S.J.; Kendall, B.J.; Neale, R.E.; Olsen, C.M.; Youlden, D.R.; Webb, P.M.; Whiteman, D.C. The impact of changing the prevalence of overweight/obesity and physical inactivity in Australia: An estimate of the proportion of potentially avoidable cancers 2013–2037. Int. J. Cancer 2019, 144, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé Publique. Rapport de L’enquête National THES-2016; Ministère de la Santé Publique: Kinshasa, Democratic Republic of the Congo, 2016. [Google Scholar]
- Mota, M.C.; Silva, C.M.; Balieiro, L.C.T.; Fahmy, W.M.; Crispim, C.A. Social jetlag and metabolic control in non-communicable chronic diseases: A study addressing different obesity statuses. Sci. Rep. 2017, 7, 6358. [Google Scholar] [CrossRef] [PubMed]
- Serin, Y.; Acar Tek, N. Effect of Circadian Rhythm on Metabolic Processes and the Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Tahara, Y.; Hitosugi, M.; Shibata, S. Impairment of Circadian Rhythms in Peripheral Clocks by Constant Light Is Partially Reversed by Scheduled Feeding or Exercise. J. Biol. Rhythm. 2015, 30, 533–542. [Google Scholar] [CrossRef]
- Lewis, P.; Korf, H.W.; Kuffer, L.; Groß, J.V.; Erren, T.C. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: A systematic review. BMJ Open Sport Exerc. Med. 2018, 4, e000443. [Google Scholar] [CrossRef]
- Schroeder, A.M.; Truong, D.; Loh, D.H.; Jordan, M.C.; Roos, K.P.; Colwell, C.S. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J. Physiol. 2012, 590, 6213–6226. [Google Scholar] [CrossRef]
- Bilski, J.; Jaworek, J.; Pokorski, J.; Nitecki, J.; Nitecka, E.; Pokorska, J.; Mazur-Bialy, A.; Szklarczyk, J. Effects of time of day and the wingate test on appetite perceptions, food intake and plasma levels of adipokines. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2016, 67, 667–676. [Google Scholar]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.İ.; Saygın, Ö.; Özel Türkcü, Ü. Assessment of acute aerobic exercise in the morning versus evening on asprosin, spexin, lipocalin-2, and insulin level in overweight/obese versus normal weight adult men. Chronobiol. Int. 2020, 37, 1252–1268. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Verheyden, B.; Aubert, A.E.; Fagard, R.H. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J. Hum. Hypertens. 2010, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Krustrup, P.; Nielsen, J.J.; Krustrup, B.R.; Christensen, J.F.; Pedersen, H.; Randers, M.B.; Aagaard, P.; Petersen, A.-M.; Nybo, L.; Bangsbo, J. Recreational soccer is an effective health-promoting activity for untrained men. Br. J. Sports Med. 2009, 43, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Junge, A.; Dvorak, J.; Krustrup, P. Executive summary: Football for health—Prevention and treatment of non-communicable diseases across the lifespan through football. Scand. J. Med. Sci. Sports 2014, 24, 147–150. [Google Scholar] [CrossRef]
- Andersen, T.R.; Schmidt, J.F.; Pedersen, M.T.; Krustrup, P.; Bangsbo, J. The Effects of 52 Weeks of Soccer or Resistance Training on Body Composition and Muscle Function in +65-Year-Old Healthy Males—A Randomized Controlled Trial. PLoS ONE 2016, 11, e0148236. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.T.; Vorup, J.; Nistrup, A.; Wikman, J.M.; Alstrøm, J.M.; Melcher, P.S.; Pfister, G.U.; Bangsbo, J. Effect of team sports and resistance training on physical function, quality of life, and motivation in older adults. Scand. J. Med. Sci. Sports 2017, 27, 852–864. [Google Scholar] [CrossRef]
- Ferguson, R. Walking Soccer/Football Has Arrived|50+ World—50+ World. 2019. Available online: https://50plusworld.com/walking-soccer-football-has-arrived/ (accessed on 15 October 2023).
- Salle, D.D.A.; Newton, R.U.; Heil, D.P. The Walking Cadence Threshold Associated With A Moderate Metabolic Intensity During Competitive Walking Football: 2554. Med. Sci. Sports Exerc. 2023, 55, 844. [Google Scholar] [CrossRef]
- Harper, L.D.; Field, A.; Corr, L.D.; Naughton, R.J. The Physiological, Physical, and Biomechanical Demands of Walking Football: Implications for Exercise Prescription and Future Research in Older Adults. J. Aging Phys. Act. 2019, 28, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.T.; Bruce-Low, S.; Sammut, L. The impact of 12 weeks walking football on health and fitness in males over 50 years of age. BMJ Open Sport Exerc. Med. 2015, 1, bmjsem-2015-000048. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, N.; Hidouri, S.; Ghram, A.; Ammar, A.; Masmoudi, L.; Driss, T.; Knechtle, B.; Weiss, K.; Hammouda, O.; Chlif, M. Effects of Walking Football During Ramadan Fasting on Heart Rate Variability and Physical Fitness in Healthy Middle-Aged Males. Am. J. Mens. Health 2022, 16, 15579883221103418. [Google Scholar] [CrossRef]
- Lamont, E.; Harris, J.; McDonald, G.; Kerin, T.; Dickens, G.L. Qualitative investigation of the role of collaborative football and walking football groups in mental health recovery. Ment. Health Phys. Act. 2017, 12, 116–123. [Google Scholar] [CrossRef]
- Reddy, P.; Dias, I.; Holland, C.; Campbell, N.; Nagar, I.; Connolly, L.; Krustrup, P.; Hubball, H. Walking football as sustainable exercise for older adults—A pilot investigation. Eur. J. Sport Sci. 2017, 17, 638–645. [Google Scholar] [CrossRef] [PubMed]
- McEwan, G.; Buchan, D.; Cowan, D.; Arthur, R.; Sanderson, M.; Macrae, E. Recruiting Older Men to Walking Football: A Pilot Feasibility Study. Explore 2019, 15, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Bjornholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef]
- Moholdt, T.; Parr, E.B.; Devlin, B.L.; Debik, J.; Giskeodegard, G.; Hawley, J.A. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: A randomised trial. Diabetologia 2021, 64, 2061–2076. [Google Scholar] [CrossRef]
- Kim, H.-K.; Radak, Z.; Takahashi, M.; Inami, T.; Shibata, S. Chrono-exercise: Time-of-day-dependent physiological responses to exercise. Sports Med. Health Sci. 2023, 5, 50–58. [Google Scholar] [CrossRef]
- Feng, H.; Yang, L.; Liang, Y.Y.; Ai, S.; Liu, Y.; Liu, Y.; Jin, X.; Lei, B.; Wang, J.; Zheng, N.; et al. Associations of timing of physical activity with all-cause and cause-specific mortality in a prospective cohort study. Nat. Commun. 2023, 14, 930. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Strain, T.; Wijndaele, K.; Dempsey, P.C.; Sharp, S.J.; Pearce, M.; Jeon, J.; Lindsay, T.; Wareham, N.; Brage, S. Wearable-device-measured physical activity and future health risk. Nat. Med. 2020, 26, 1385–1391. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Coats, A.J.; Conway, J.; Isea, J.E.; Pannarale, G.; Sleight, P.; Somers, V.K. Systemic and forearm vascular resistance changes after upright bicycle exercise in man. J. Physiol. 1989, 413, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ravier, G. Quantifying internal workload during training drills in handball players: Comparison between heart rate and perceived exertion based methods. Mov. Sport Sci./Sci. Mot. 2022, 118, 15–22. [Google Scholar] [CrossRef]
- Lipponen, J.A.; Tarvainen, M.P. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J. Med. Eng. Technol. 2019, 43, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lipponen, J.A.; Tarvainen, M.P. Accuracy of Kubios HRV Software Respiratory Rate Estimation Algorithms. 2 July 2022. Available online: https://cinc.org/2022/Program/accepted/134_Preprint.pdf (accessed on 3 October 2023).
- Crotty, N.M.; Boland, M.; Mahony, N.; Donne, B.; Fleming, N. Reliability and Validity of the Lactate Pro 2 Analyzer. Meas. Phys. Educ. Exerc. Sci. 2021, 25, 202–211. [Google Scholar] [CrossRef]
- Pariente Rodrigo, E.; Deib-Morgan, K.; Garcia de Diego, O.; Garcia-Velasco, P.; Sgaramella, G.A.; Garcia Gonzalez, I. Accuracy and reliability between glucose meters: A study under normal clinical practice conditions. Semergen 2017, 43, 20–27. [Google Scholar] [CrossRef]
- Feldman, B. Electrochemistry Encyclopedia—Electrochemical Blood Glucose Test. 2009. Available online: https://knowledge.electrochem.org/encycl/art-g01-glucose.htm (accessed on 19 October 2023).
- Sassi, R.H.; Dardouri, W.; Yahmed, M.H.; Gmada, N.; Mahfoudhi, M.E.; Gharbi, Z. Relative and absolute reliability of a modified agility T-test and its relationship with vertical jump and straight sprint. J. Strength Cond. Res. 2009, 23, 1644–1651. [Google Scholar] [CrossRef]
- Chow, G.C.-C.; Kong, Y.-H.; Pun, W.-Y. The Concurrent Validity and Test-Retest Reliability of Possible Remote Assessments for Measuring Countermovement Jump: My Jump 2, HomeCourt & Takei Vertical Jump Meter. Appl. Sci. 2023, 13, 2142. [Google Scholar] [CrossRef]
- Farias, D.L.; Teixeira, T.G.; Madrid, B.; Pinho, D.; Boullosa, D.A.; Prestes, J. Reliability of vertical jump performance evaluated with contact mat in elderly women. Clin. Physiol. Funct. Imaging 2013, 33, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Mizuta, C.; Yamada, Y.; Okayama, Y.; Nakamura, E. Constructing an index of physical fitness age for Japanese elderly based on 7-year longitudinal data: Sex differences in estimated physical fitness age. Age 2012, 34, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.H.; Özkaya, İ.; Karakaş, Ç.S.; Taşkıran, Y.; Esformes, J.I. Validity and Reliability of Isometric Muscle Strength using the Powrlink Portable Device. Int. J. Strength Cond. 2023, 3, 237. [Google Scholar] [CrossRef]
- Svebak, S.; Murgatroyd, S. Metamotivational dominance: A multimethod validation of reversal theory constructs. J. Personal. Soc. Psychol. 1985, 48, 107–116. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Torres-Luque, G.; Marin, P.J.; Ruiz, J.R.; Lucia, A.; Garatachea, N. Intermonitor variability of GT3X accelerometer. Int. J. Sports Med. 2012, 33, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Shariat, A.; Cleland, J.A.; Danaee, M.; Alizadeh, R.; Sangelaji, B.; Kargarfard, M.; Ansari, N.N.; Sepehr, F.H.; Tamrin, S.B.M. Borg CR-10 scale as a new approach to monitoring office exercise training. Work 2018, 60, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.; Pecanha, T.; Tinucci, T.; Silva-Junior, N.; Costa, L.; Forjaz, C. Time of day affects heart rate recovery and variability after maximal exercise in pre-hypertensive men. Chronobiol. Int. 2015, 32, 1385–1390. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P. Physical Activity Guidelines for Americans From the US Department of Health and Human Services. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005263. [Google Scholar] [CrossRef]
- Sessa, F.; Anna, V.; Messina, G.; Cibelli, G.; Monda, V.; Marsala, G.; Ruberto, M.; Biondi, A.; Cascio, O.; Bertozzi, G.; et al. Heart rate variability as predictive factor for sudden cardiac death. Aging 2018, 10, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Fidan-Yaylali, G.; Yaylali, Y.T.; Erdogan, C.; Can, B.; Senol, H.; Gedik-Topcu, B.; Topsakal, S. The Association between Central Adiposity and Autonomic Dysfunction in Obesity. Med. Princ. Pract. 2016, 25, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Salinas, A.; Brito, C.; Arenas Sanchez, G.; Peiret Villacura, L.; Molina Sotomayor, E.; Cigarroa Cuevas, I.; Gonzalez-Jurado, J.A. Autonomic function and its relationship with central obesity and hemodynamic variables in obese and overweight adults. Nutr. Hosp. 2022, 39, 320–328. [Google Scholar] [CrossRef]
- Hautala, A.J.; Rankinen, T.; Kiviniemi, A.M.; Makikallio, T.H.; Huikuri, H.V.; Bouchard, C.; Tulppo, M.P. Heart rate recovery after maximal exercise is associated with acetylcholine receptor M2 (CHRM2) gene polymorphism. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H459–H466. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Han, H.; Aguiar, E.J.; Barreira, T.V.; Schuna, J.M., Jr.; Kang, M.; Rowe, D.A. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: A narrative review. Br. J. Sports Med. 2018, 52, 776–788. [Google Scholar] [CrossRef]
- Prodel, E.; Pecanha, T.; Silva, L.P.D.; Paula, R.B.; Martinez, D.G.; Lima, J.R.P.; Laterza, M.C. Different times of day do not change heart rate variability recovery after light exercise in sedentary subjects: 24 hours Holter monitoring. Chronobiol. Int. 2017, 34, 1354–1365. [Google Scholar] [CrossRef]
- Pecanha, T.; Prodel, E.; Bartels, R.; Nasario-Junior, O.; Paula, R.B.; Silva, L.P.; Laterza, M.C.; Lima, J.R. 24-h cardiac autonomic profile after exercise in sedentary subjects. Int. J. Sports Med. 2014, 35, 245–252. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac Parasympathetic Reactivation Following Exercise: Implications for Training Prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]
- Michael, S.; Jay, O.; Halaki, M.; Graham, K.; Davis, G.M. Submaximal exercise intensity modulates acute post-exercise heart rate variability. Eur. J. Appl. Physiol. 2016, 116, 697–706. [Google Scholar] [CrossRef]
- Jones, H.; George, K.; Edwards, B.; Atkinson, G. Effects of time of day on post-exercise blood pressure: Circadian or sleep-related influences? Chronobiol. Int. 2008, 25, 987–998. [Google Scholar] [CrossRef]
- Nóbrega, T.K.S.d.; Moura, J.S., Jr.; Brito, A.d.F.; Gonçalves, M.C.R.; Martins, C.d.O.; Silva, A.S. Caminhada/corrida ou uma partida de futebol recreacional apresentam efetividade semelhante na indução de hipotensão pós-exercício. Rev. Bras. De Med. Do Esporte 2013, 19, 31–34. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Zechman, F.W.; Smith, R.E. Circadian variation in human peripheral blood flow levels and exercise responses. J. Appl. Physiol. 1968, 25, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Pritchard, C.; George, K.; Edwards, B.; Atkinson, G. The acute post-exercise response of blood pressure varies with time of day. Eur. J. Appl. Physiol. 2008, 104, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.E.; Fontana, K.E.; Porto, L.G.G.; Junqueira, L.F. Post-exercise heart-rate recovery correlates to resting heart-rate variability in healthy men. Clin. Auton. Res. 2016, 26, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hu, K.; Evoniuk, H.; Kelly, E.E.; Malhotra, A.; Hilton, M.F.; Shea, S.A. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. USA 2010, 107, 20541–20546. [Google Scholar] [CrossRef]
- Qian, J.; Scheer, F.A.; Hu, K.; Shea, S.A. The circadian system modulates the rate of recovery of systolic blood pressure after exercise in humans. Sleep 2020, 43, zsz253. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Barak, O.F.; Ovcin, Z.B.; Jakovljevic, D.G.; Lozanov-Crvenkovic, Z.; Brodie, D.A.; Grujic, N.G. Heart rate recovery after submaximal exercise in four different recovery protocols in male athletes and non-athletes. J. Sports Sci. Med. 2011, 10, 369–375. [Google Scholar]
- Jeng, C.; Chang, W.Y.; Chen, S.R.; Tseng, I.J. Effects of arm exercise on serum glucose response in type 2 DM patients. J. Nurs. Res. 2002, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Jeng, C.; Ku, C.T.; Huang, W.H. Establishment of a predictive model of serum glucose changes under different exercise intensities and durations among patients with type 2 diabetes mellitus. J. Nurs. Res. 2003, 11, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the Effects of Exercise, Training and Gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, N.J.; Parker, B.L.; Chaudhuri, R.; Fisher-Wellman, K.H.; Kleinert, M.; Humphrey, S.J.; Yang, P.; Holliday, M.; Trefely, S.; Fazakerley, D.J.; et al. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015, 22, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Ellingsgaard, H.; Ehses, J.A.; Hammar, E.B.; Van Lommel, L.; Quintens, R.; Martens, G.; Kerr-Conte, J.; Pattou, F.; Berney, T.; Pipeleers, D.; et al. Interleukin-6 regulates pancreatic α-cell mass expansion. Proc. Natl. Acad. Sci. USA 2008, 105, 13163–13168. [Google Scholar] [CrossRef] [PubMed]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Furuhashi, S.; Takahashi, M.; Chijiki, H.; Nanba, T.; Inami, T.; Radak, Z.; Sakamoto, S.; Shibata, S. Late-afternoon endurance exercise is more effective than morning endurance exercise at improving 24-h glucose and blood lipid levels. Front. Endocrinol. 2022, 13, 957239. [Google Scholar] [CrossRef]
- Knaier, R.; Qian, J.; Roth, R.; Infanger, D.; Notter, T.; Wang, W.; Cajochen, C.; Scheer, F.A.J.L. Diurnal Variation in Maximum Endurance and Maximum Strength Performance: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2022, 54, 169–180. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Brashear, M.M.; Johnson, W.D.; Katzmarzyk, P.T. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 60. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Rowland, T.; Unnithan, V.; Barker, P.; Lindley, M.; Roche, D.; Garrard, M. Time-of-Day Effect on Cardiac Responses to Progressive Exercise. Chronobiol. Int. 2011, 28, 611–616. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports, M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]

| Sex | Male |
| Age | 44.89 ± 6.51 |
| Height (cm) | 173.16 ± 4.31 |
| Weight (kg) | 100.16 ± 13.47 |
| BMI (kg/m2) | 33.16 ± 4.75 |
| Fat mass (kg) | 33.79 ± 10.19 |
| Lean mass (kg) | 66.37 ± 4.67 |
| Body water (kg) | 47.55 ± 6.99 |
| Parameters | Unit | Description | Indication |
|---|---|---|---|
| Time domain measurements | |||
| RMSSD | milliseconds: ms | Root mean square of the successive differences of the R-R intervals | Contribution of variations at high frequencies, which are in turn associated with vagal activity |
| SDNN | milliseconds: ms | Standard deviation of NN intervals | Participation of all rhythmic components responsible for variability, being related to contributions from both branches of the autonomic nervous system |
| Frequency domain measurements | |||
| LF | normalized units: n.u | Relative power of the low-frequency band (0.04–0.15 Hertz) in normal units | Baroreceptors activity during resting conditions and parasympathetic and sympathetic nervous systems activity |
| HF | normalized units: n.u | Relative power of the high-frequency band (0.15–0.4 Hertz) in normal units. | Parasympathetic activity |
| LF/HF ratio | — | Ratio of LF to HF power | Sympathetic–parasympathetic balance |
| Means ± SD | Interaction Time of Day × Match | ||||||
|---|---|---|---|---|---|---|---|
| Before | After | Δ | F/Z | p-Value | ηp2/ Cohen’s d | ||
| Mean HR (beat/min) | Morning | 74.4 ± 5.28 | 86.8 ± 8.71 | 12.39 ± 10.29 | 1.825 | 0.194 | 0.092 |
| Evening | 76.42 ± 5.78 | 93.27 ± 10.17 | 16.85 ± 12.09 | ||||
| Mean RR (ms) | Morning | 810.1 ± 54.8 | 698 ± 71.5 | −112.1 ± 89.4 | 0.847 | 0.370 | 0.045 |
| Evening | 789.4 ± 59.4 | 651 ± 75.3 | −138.4 ± 97.4 | ||||
| SDNN (ms) * | Morning | 38.79 ± 23.43 | 30.63 ± 11.64 | −8.16 ± 27.89 | 0.644 | 0.520 | 0.148 |
| Evening | 37.51 ± 19.68 | 23.68 ± 10.02 | −13.83 ± 17.01 | ||||
| RMSSD (ms) * | Morning | 32.01 ± 22.03 | 19.68 ± 7.95 | −12.33 ± 24.87 | 1.248 | 0.212 | 0.286 |
| Evening | 32.14 ± 23.6 | 16.89 ± 10.7 | −15.25 ± 22.35 | ||||
| LF (n.u.) * | Morning | 59.97 ± 20.88 | 72.71 ± 15.71 | 12.73 ± 22.79 | 1.327 | 0.184 | 0.304 |
| Evening | 65.27 ± 17.45 | 68.9 ± 17.29 | 3.63 ± 20.11 | ||||
| HF (n.u.) * | Morning | 39.97 ± 20.86 | 27.24 ± 15.66 | −12.73 ± 22.76 | 1.248 | 0.212 | 0.286 |
| Evening | 34.67 ± 17.41 | 31.06 ± 17.27 | −3.61 ± 20.03 | ||||
| LF/HF_ratio * | Morning | 2.46 ± 2.2 | 4.09 ± 3.08 | 1.63 ± 4.03 | 0.885 | 0.375 | 0.203 |
| Evening | 3.03 ± 2.8 | 3.38 ± 2.5 | 0.36 ± 3.34 | ||||
| Means ± SD | Interaction TOD × Match | ||||
|---|---|---|---|---|---|
| Parameters | TOD | Δ | F/Z | p-Value | ηp2/ Cohen’s d |
| MBP (mmHg) | Morning | 9.88 ± 5.33 | 9.275 | 0.007 | 0.340 |
| Evening | 5.61 ± 6.94 | ||||
| SpO2 (%) * | Morning | −0.74 ± 1.24 | 0.659 | 0.510 | 0.151 |
| Evening | −1.11 ± 1.91 | ||||
| Lactate (mmol/L) | Morning | 1.48 ± 0.95 | 0.310 | 0.585 | 0.017 |
| Evening | 1.63 ± 1.13 | ||||
| Glycemia (g/L) | Morning | −0.08 ± 0.2 | 4.960 | 0.039 | 0.216 |
| Evening | −0.27 ± 0.29 | ||||
| MAT (s) * | Morning | 0.08 ± 0.48 | 1.087 | 0.277 | 0.249 |
| Evening | −0.17 ± 0.84 | ||||
| LS (Kg) | Morning | −0.71 ± 21.87 | 0.003 | 0.955 | 0.000 |
| Evening | −0.71 ± 13.08 | ||||
| VJ (cm) | Morning | 2 ± 3.42 | 0.018 | 0.894 | 0.001 |
| Evening | 1.84 ± 4.52 | ||||
| Means ± SD | Interaction Time of Day × Match | ||||||
|---|---|---|---|---|---|---|---|
| Before | After | Δ | Z | p-Value | Cohen’s d | ||
| FAS | Morning | 3.32 ± 0.95 | 2.89 ± 0.74 | 0.42 ± 0.84 | 0.104 | 0.91 | 0.00 |
| Evening | 3.37 ± 0.96 | 2.95 ± 1.22 | 0.42 ± 1.02 | ||||
| Parameters | t/Z | p | Confidence Interval −95% | Confidence Interval 95% | Cohen’s d |
|---|---|---|---|---|---|
| RPE | −2.22 | 0.04 | −2.05 | −0.06 | 0.47 |
| nbS | −0.63 | 0.54 | −281.95 | 153.06 | 0.17 |
| MET * | 1.33 | 0.18 | - | - | 0.08 |
| Mean HR (beat/min) | −0.86 | 0.40 | −9.43 | 3.96 | 0.19 |
| HR max * (beat/min) | 0.52 | 0.60 | - | - | 0.03 |
| HRR60s * (beat/min) | 2.29 | 0.048 | 0.073 | 16.091 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidouri, S.; Driss, T.; Tagougui, S.; Kammoun, N.; Chtourou, H.; Hammouda, O. Sensor-Based Assessment of Time-of-Day-Dependent Physiological Responses and Physical Performances during a Walking Football Match in Higher-Weight Men. Sensors 2024, 24, 909. https://doi.org/10.3390/s24030909
Hidouri S, Driss T, Tagougui S, Kammoun N, Chtourou H, Hammouda O. Sensor-Based Assessment of Time-of-Day-Dependent Physiological Responses and Physical Performances during a Walking Football Match in Higher-Weight Men. Sensors. 2024; 24(3):909. https://doi.org/10.3390/s24030909
Chicago/Turabian StyleHidouri, Sami, Tarak Driss, Sémah Tagougui, Noureddine Kammoun, Hamdi Chtourou, and Omar Hammouda. 2024. "Sensor-Based Assessment of Time-of-Day-Dependent Physiological Responses and Physical Performances during a Walking Football Match in Higher-Weight Men" Sensors 24, no. 3: 909. https://doi.org/10.3390/s24030909
APA StyleHidouri, S., Driss, T., Tagougui, S., Kammoun, N., Chtourou, H., & Hammouda, O. (2024). Sensor-Based Assessment of Time-of-Day-Dependent Physiological Responses and Physical Performances during a Walking Football Match in Higher-Weight Men. Sensors, 24(3), 909. https://doi.org/10.3390/s24030909







