Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF
Abstract
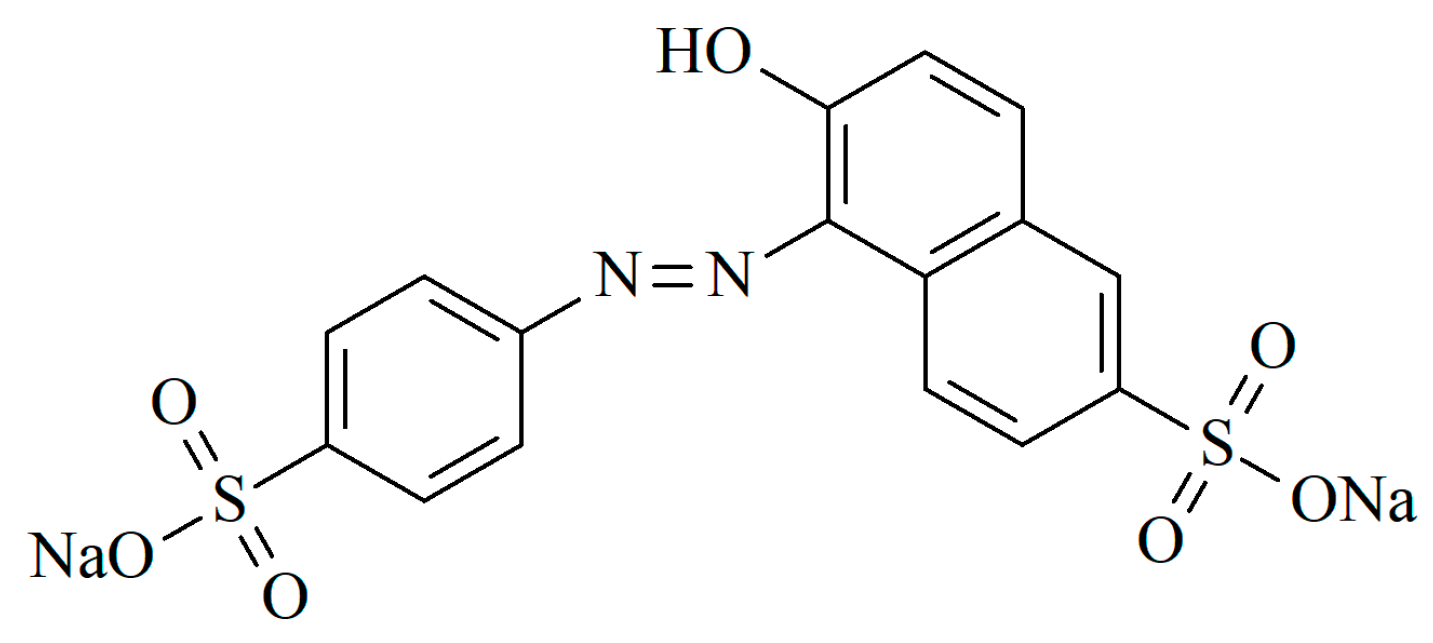
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Procedures
2.3.1. Modification of the Electrode Surface with Metal Dioxide NPs Dispersion
2.3.2. Voltammetric and Chronoamperometric Measurements
2.3.3. Electrochemical Impedance Spectroscopy
2.3.4. Soft Drinks Analysis
2.3.5. Statistical Treatment of the Data
3. Results and Discussion
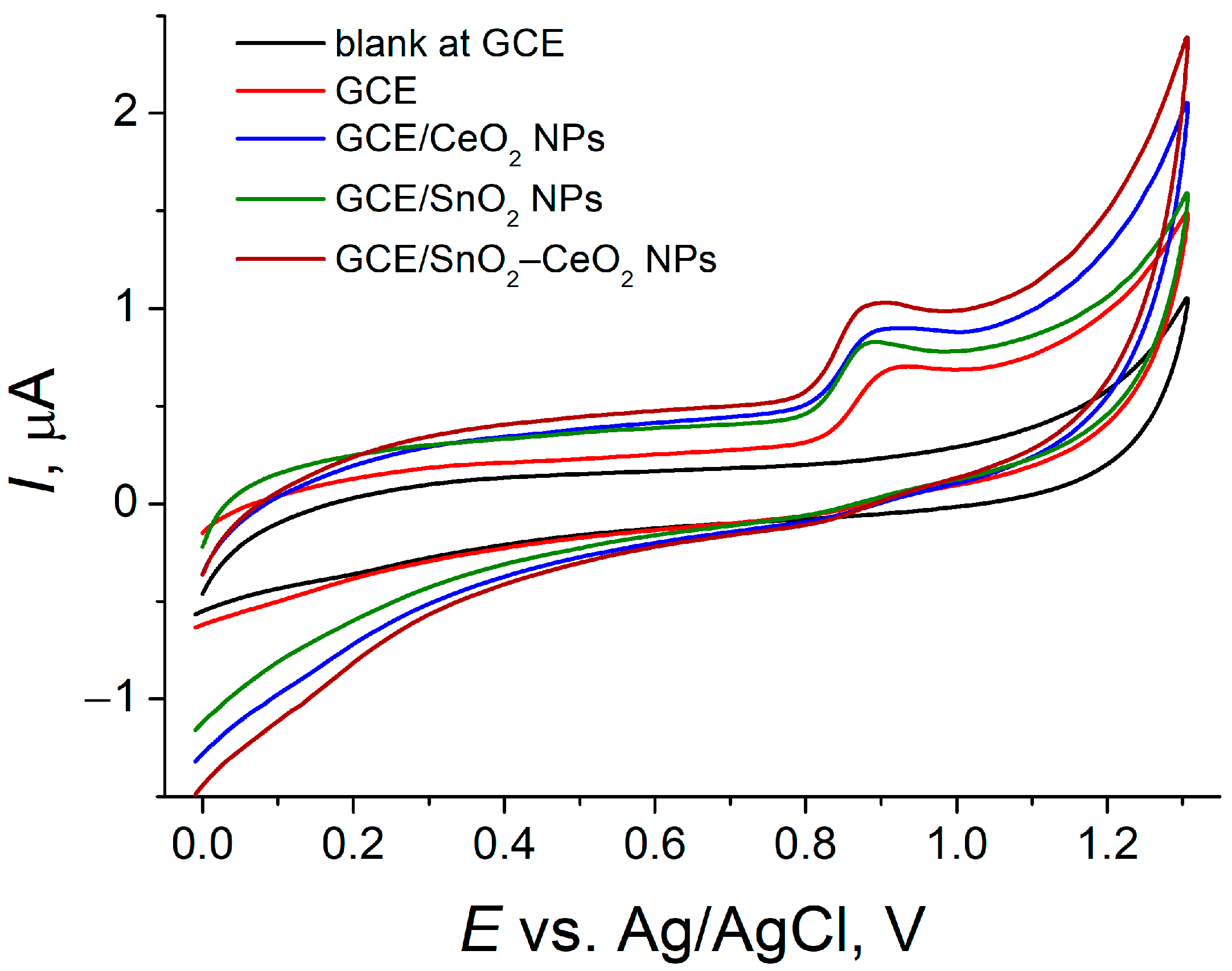
3.1. Voltammetric Behavior of SY FCF on Bare and MO2-NPs-Modified GCE
3.2. Characterization of the Electrodes
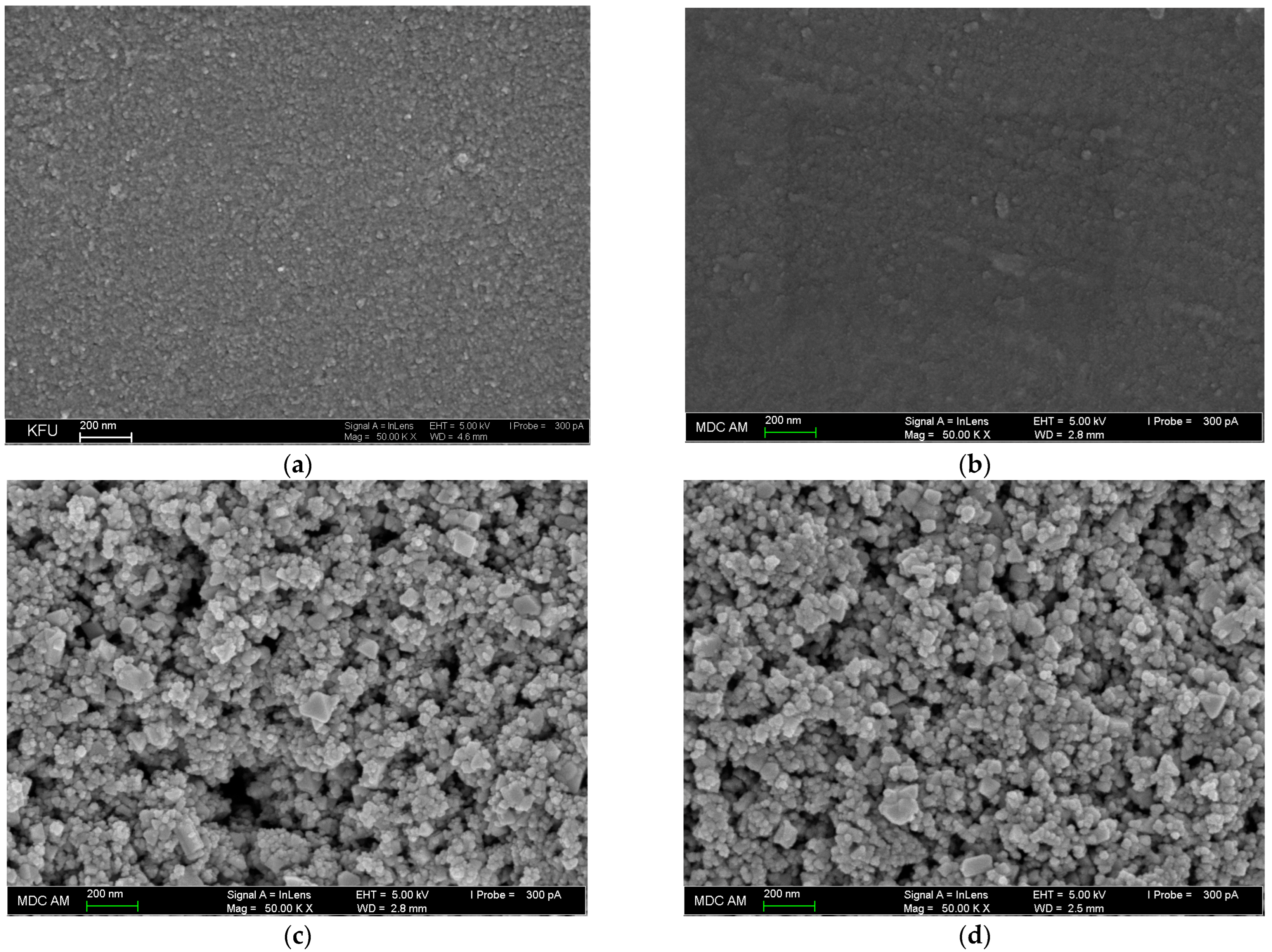
3.2.1. Field Emission Scanning Electron Microscopy
3.2.2. Evaluation of the Electroactive Surface Area and Electron Transfer Properties
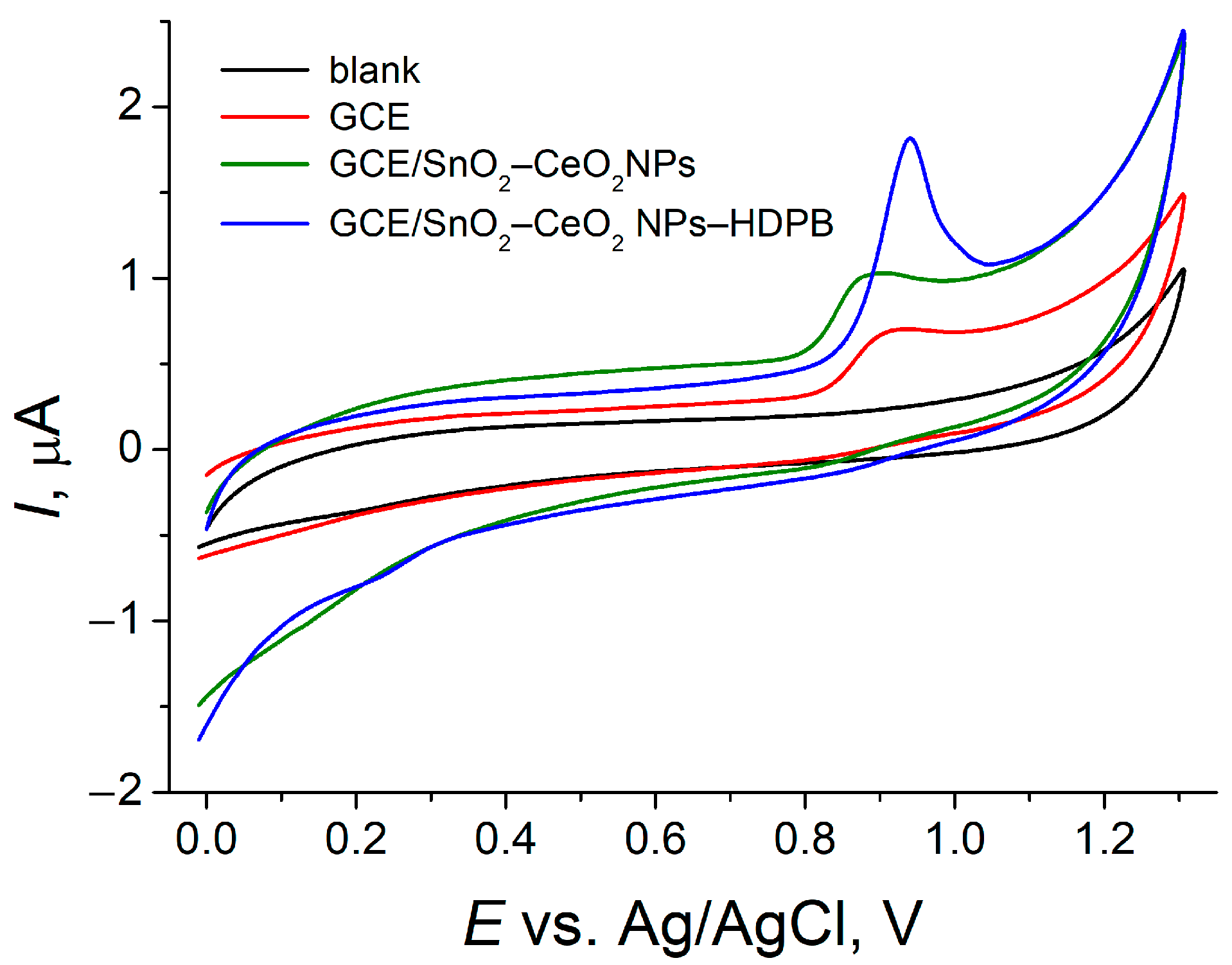
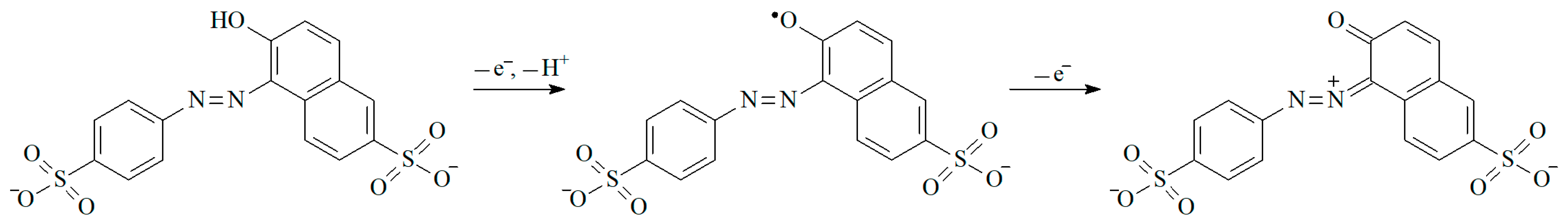
3.3. Electrooxidation of SY FCF at the GCE/SnO2–CeO2 NPs–HDPB
3.3.1. Effect of the Supporting Electrolyte pH
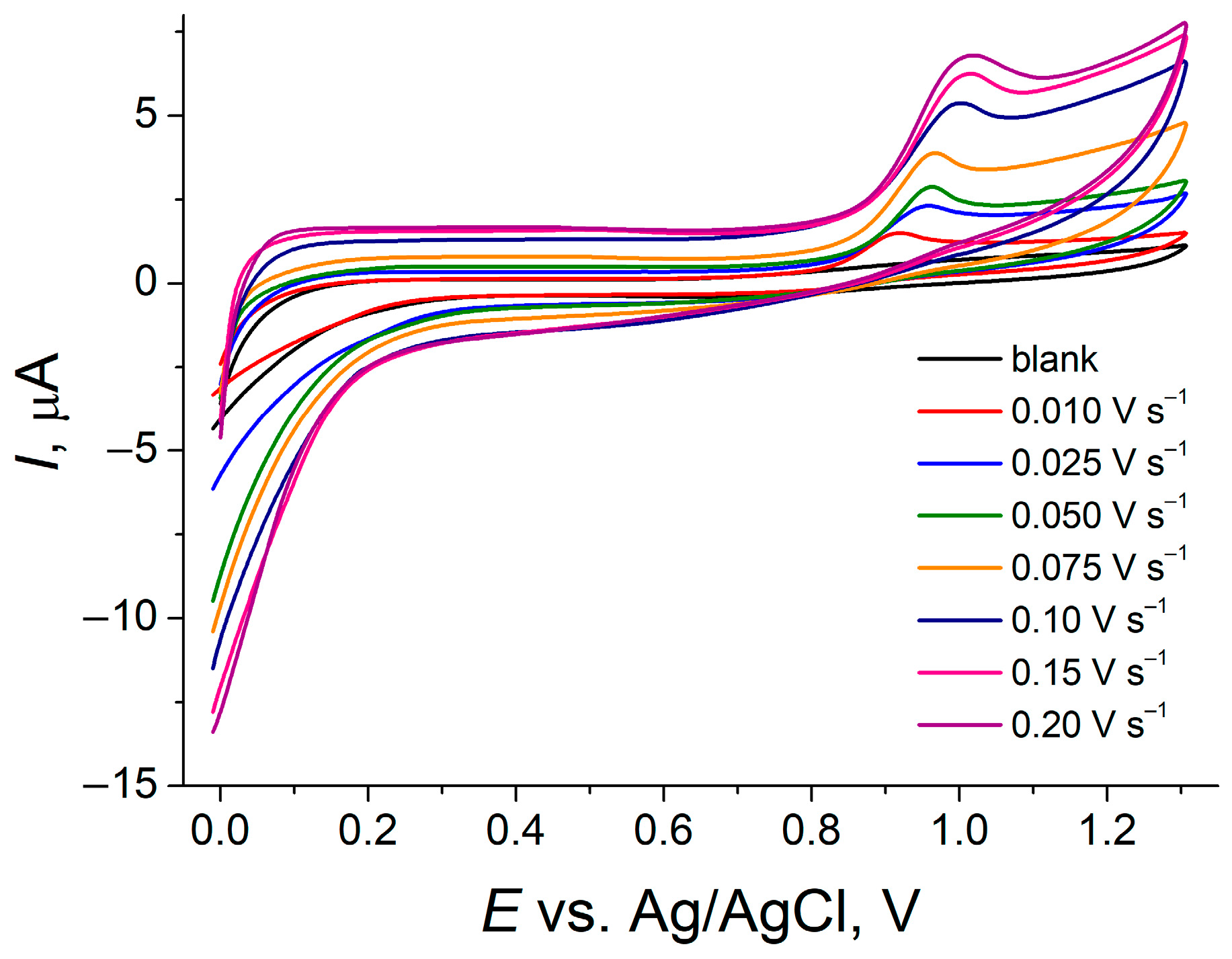
3.3.2. Effect of the Potential Scan Rate
3.4. Analytical Performance of the Developed Voltammetric Sensor
3.4.1. Optimization of Differential Pulse Voltammetry Conditions
3.4.2. Analytical Characteristics of SY FCF
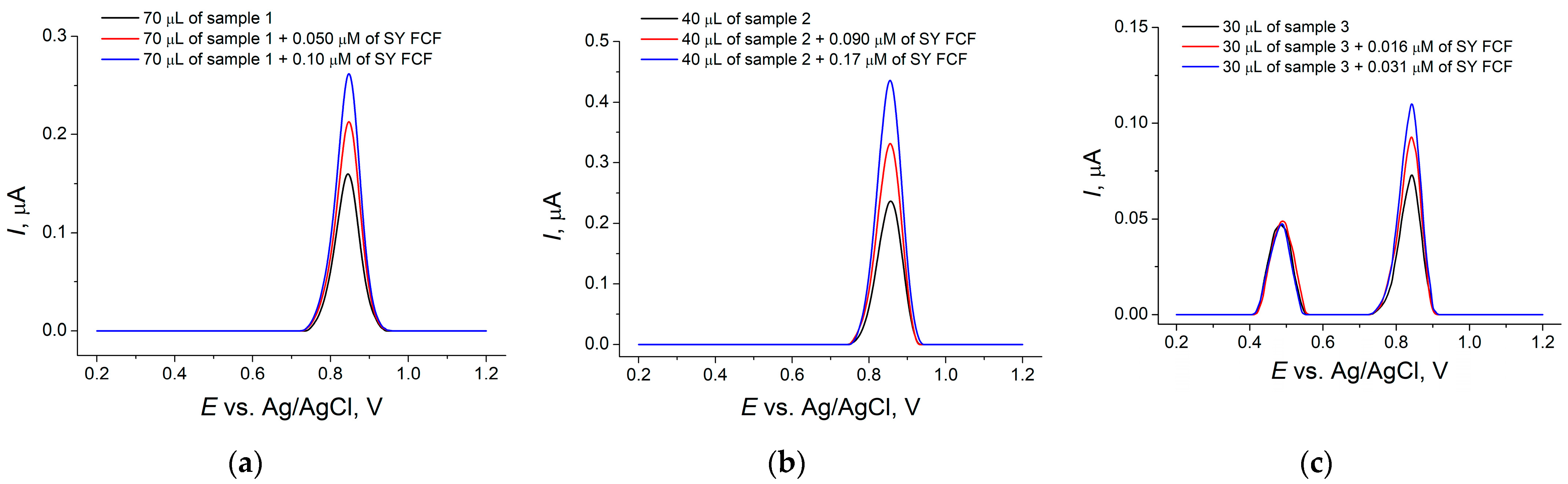
3.4.3. Soft Drinks Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subburaj, M.; Faseela, P.M. Synthetic Food Colours; Scholars’ Press: London, UK, 2016; 80p. [Google Scholar]
- Chung, K.T. Azo dyes and human health. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Matsyura, O.; Besh, L.; Besh, O.; Troyanovska, O.; Slyuzar, Z. Hypersensitivity reactions to food additives in pediatric practice: Two clinical cases. Georgian Med. News 2020, 307, 91–95. [Google Scholar]
- Rovina, K.; Prabakaran, P.P.; Siddiquee, S.; Shaarani, S.M. Methods for the analysis of Sunset Yellow FCF (E110) in food and beverage products—A review. TrAC Trends Anal. Chem. 2016, 85, 47–56. [Google Scholar] [CrossRef]
- EFSA. Reconsideration of the temporary ADI and refined exposure assessment for Sunset Yellow FCF (E 110). EFSA J. 2014, 12, 3765. [Google Scholar] [CrossRef]
- Ntrallou, K.; Gika, H.; Tsochatzis, E. Analytical and sample preparation techniques for the determination of food colorants in food matrices. Foods 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Rovina, K.; Acung, L.A.; Siddiquee, S.; Akanda, J.H.; Shaarani, S.M. Extraction and analytical methods for determination of Sunset Yellow (E110)—A review. Food Anal. Methods 2017, 10, 773–787. [Google Scholar] [CrossRef]
- Islam, A.; Sarker, M.; Haque Khan, S.; Hossain, M.I.; Abedin, M.Z.; Zubair, M.A.; Bari, L. Determination of sunset yellow in different brands of orange jellies of bangladesh by HPLC. Ital. J. Food Sci. 2019, 31, 184–194. [Google Scholar] [CrossRef]
- Alp, H.; Başkan, D.; Yaşar, A.; Yaylı, N.; Ocak, Ü.; Ocak, M. Simultaneous determination of Sunset Yellow FCF, Allura Red AC, Quinoline Yellow WS, and Tartrazine in food samples by RP-HPLC. J. Chem. 2018, 2018, 6486250. [Google Scholar] [CrossRef]
- Oymak, T.; Dural, E. Determination of sunset yellow, allura red, and fast green using a novel magnetic nanoadsorbent modified with Elaeagnus angustifolia based on magnetic solid-phase extraction by HPLC. Braz. J. Pharm. Sci. 2022, 58, e20884. [Google Scholar] [CrossRef]
- Périat, A.; Bieri, S.; Mottier, N. SWATH-MS screening strategy for the determination of food dyes in spices by UHPLC-HRMS. Food Chem. X 2019, 1, 100009. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Kuo, C.-H.; Shih, D.Y.-C. Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J. Food Drug Anal. 2015, 23, 453–462. [Google Scholar] [CrossRef]
- Soponar, F.; Mot, A.C.; Sabu, C. Quantitative determination of some food dyes using digital processing of images obtained by thin-layer chromatography. J. Chromatogr. A 2008, 1188, 295–300. [Google Scholar] [CrossRef]
- De Andrade, F.I.; Guedes, M.I.F.; Vieira, Í.G.; Mendes, F.N.P.; Rodrigues, P.A.S.; Maia, C.S.C.; Ávila, M.M.M.; Ribeiro, L.d.M. Determination of synthetic food dyes in commercial soft drinks by TLC and ion-pair HPLC. Food Chem. 2014, 157, 193–198. [Google Scholar] [CrossRef]
- Liu, F.-J.; Liu, C.-T.; Li, W.; Tang, A.-N. Dispersive solid-phase micro- extraction and capillary electrophoresis separation of food colorants in beverages using diamino moiety functionalized silica nanoparticles as both extractant and pseudostationary phase. Talanta 2015, 132, 366–372. [Google Scholar] [CrossRef]
- Heidarizadi, E.; Tabaraki, R. Simultaneous spectrophotometric determination of synthetic dyes in food samples after cloud point extraction using multiple response optimizations. Talanta 2016, 148, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Hashem, E.Y.; Saleh, M.S.; Al-Salahi, N.O.A.; Youssef, A.K. Advanced spectrophotometric analysis of Sunset Yellow Dye E110 in commercial food samples. Food Anal. Methods 2017, 10, 865–875. [Google Scholar] [CrossRef]
- Soleymani, B.; Zargar, B.; Rastegarzadeh, S. Separation and pre-concentration of Sunset Yellow in beverages and effervescent vitamin C tablets by a new flotation technique prior to spectrophotometric determination. Food Addit. Contam. Part A 2019, 36, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, S.; Tan, Z.; Liu, Y. Nondestructive detection of sunset yellow in cream based on near-infrared spectroscopy and interval random forest. Spectrochim. Acta Part A 2020, 242, 118718. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Meng, M.; Xue, H.; Zhang, T.; Yin, Y.; Xi, R. Development of a polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of sunset yellow FCF in food samples. Talanta 2012, 99, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, F.; Dias, A.C.P.; Zhang, X. Development of quantum dot-linked immunosorbent assay (QLISA) and ELISA for the detection of sunset yellow in foods and beverages. Food Chem. 2022, 385, 132648. [Google Scholar] [CrossRef]
- State, R.G.; van Staden, J.K.F.; Stefan-van Staden, R.-I. Review—Recent trends on the electrochemical sensors used for the determination of Tartrazine and Sunset Yellow FCF from food and beverage products. J. Electrochem. Soc. 2022, 169, 017509. [Google Scholar] [CrossRef]
- Agnihotri, A.S.; Varghese, A.; Nidhin, M. Transition metal oxides in electrochemical and bio sensing: A state-of-art review. Appl. Surf. Sci. Adv. 2021, 4, 100072. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Gimadutdinova, L.; Antonova, T.; Grigoreva, I.; Yakupova, E. The analytical capabilities of electrochemical sensors based on transition metal oxide nanomaterials. Eng. Proc. 2023, 48, 13. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-oxide based nanomaterials: Synthesis, characterization and their applications in electrical and electrochemical sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Ziganshina, E.; Romashkina, S.; Budnikov, H. Highly sensitive amperometric sensor for eugenol quantification based on CeO2 nanoparticles and surfactants. Electroanalysis 2017, 29, 1197–1204. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Yakupova, E.; Davletshin, R. Voltammetric determination of hesperidin on the electrode modified with SnO2 nanoparticles and surfactants. Electroanalysis 2021, 33, 2417–2427. [Google Scholar] [CrossRef]
- Pwavodi, P.C.; Ozyurt, V.H.; Asir, S.; Ozsoz, M. Electrochemical sensor for determination of various phenolic compounds in wine samples using Fe3O4 nanoparticles modified carbon paste electrode. Micromachines 2021, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Ziganshina, E.; Nguyen Cong, P.; Budnikov, H. Voltammetric determination of thymol in oregano using CeO2-modified electrode in Brij® 35 micellar medium. Food Anal. Meth. 2017, 10, 129–136. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Wang, S.; Kang, P.; Chen, X.; Cuo, Z.; Huang, X.-J. Electrochemical monitoring of persistent toxic substances using metal oxide and its composite nanomaterials: Design, preparation, and application. TrAC Trends Anal. Chem. 2019, 119, 115636. [Google Scholar] [CrossRef]
- Veerapandi, G.; Govindan, R.; Sekar, C. Quick and accurate determination of hazardous phenolic compounds using CaCu2O3 nanorods based electrochemical sensor. Chemosphere 2023, 313, 137370. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Fakude, C.T.; Mabuba, N.; Peleyeju, G.M.; Arotiba, O.A. The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 2019, 554, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kamble, B.B.; Garadkar, K.M.; Sharma, K.K.; Kamble, P.; Tayade, S.N.; Ajalkar, B.D. Determination of 4-nitrophenol using MoO3 loaded glassy carbon electrode via electrochemical sensing approach. J. Electrochem. Sci. Eng. 2021, 11, 143–159. [Google Scholar] [CrossRef]
- de Carvalho, R.C.; Betts, A.J.; Cassidy, J.F. Diclofenac determination using CeO2 nanoparticle modified screen-printed electrodes—A study of background correction. Microchem. J. 2020, 158, 105258. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Erkmen, C.; Kurbanoglu, S.; Ozkan, S.A.; Uslu, B. Electrochemical analysis for pharmaceuticals by the advantages of metal oxide nanomaterials. Curr. Anal. Chem. 2021, 17, 1322–1339. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Large surface vanadium pentoxide nanosheet modified screen-printed electrode for nanomolar diclofenac determination. Electrochim. Acta 2022, 428, 140919. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Gimadutdinova, L. Cerium(IV) and iron(III) oxides nanoparticles based voltammetric sensor for the sensitive and selective determination of lipoic acid. Sensors 2021, 21, 7639. [Google Scholar] [CrossRef]
- Lavanya, N.; Fazio, E.; Neri, F.; Bonavita, A.; Leonardi, S.G.; Neri, G.; Sekar, C. Simultaneous electrochemical determination of epinephrine and uric acid in the presence of ascorbic acid using SnO2/graphene nanocomposite modified glassy carbon electrode. Sens. Actuators B Chem. 2015, 221, 1412–1422. [Google Scholar] [CrossRef]
- Nor, N.M.; Razak, K.A.; Lockman, Z. Physical and electrochemical properties of iron oxide nanoparticles-modified electrode for amperometric glucose detection. Electrochim. Acta 2017, 248, 160–168. [Google Scholar] [CrossRef]
- Bas, S.Z.; Cummins, C.; Borah, D.; Ozmen, M.; Morris, M.A. Electrochemical sensing of hydrogen peroxide using block copolymer templated iron oxide nanopatterns. Anal. Chem. 2018, 90, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Ganesamurthi, J.; Shanmugam, R.; Chen, T.-W.; Chen, S.-M.; Balamurugan, M.; Gan, Z.-W.; Siddiqui, M.R.; Wabaidur, S.M.; Ajmal Ali, M. NiO/ZnO binary metal oxide based electrochemical sensor for the evaluation of hazardous flavonoid in biological and vegetable samples. Colloids Surf. A Physicochem. Eng. 2022, 647, 129077. [Google Scholar] [CrossRef]
- Babulal, S.M.; Chen, T.-W.; Akilarasan, M.; Lou, B.-S.; Al-onazi, W.A.; Al-Mohaimeed, A.M.; Elshikh, M.S. One-pot synthesis of hetero-structured binary metal oxide electrocatalyst for the potential detection of nifedipine in biological and environmental samples. Mater. Today Chem. 2022, 26, 101132. [Google Scholar] [CrossRef]
- Gimadutdinova, L.; Ziyatdinova, G.; Davletshin, R. Selective voltammetric sensor for the simultaneous quantification of Tartrazine and Brilliant Blue FCF. Sensors 2023, 23, 1094. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Voltammetric determination of tartrazine on an electrode modified with cerium dioxide nanoparticles and cetyltriphenylphosphonium bromide. J. Anal. Chem. 2022, 77, 664–670. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Baghelani, Y.-M.; Mousazadeh, F.; Rahimi, S.; Mohammad-Hassani, M. Electrochemical determination of amaranth in food samples by using modified electrode. J. Electrochem. Sci. Eng. 2022, 12, 1165–1177. [Google Scholar] [CrossRef]
- Marquez-Mariño, K.; Penagos-Llanos, J.; García-Beltrán, O.; Nagles, E.; Hurtado, J.J. Development of a novel electrochemical sensor based on a carbon paste electrode decorated with Nd2O3 for the simultaneous detection of tartrazine and sunset yellow. Electroanalysis 2018, 30, 2760–2767. [Google Scholar] [CrossRef]
- Penagos-Llanos, J.; García-Beltrán, O.; Nagles, E.; Hurtado, J.J. A new electrochemical method to detect sunset yellow, tartrazine and thiomersal in a pharmaceutical dose using a carbon paste electrode decorated with molybdenum oxide. Electroanalysis 2020, 32, 2174–2182. [Google Scholar] [CrossRef]
- Ya, Y.; Jiang, C.; Li, T.; Liao, J.; Fan, Y.; Wei, Y.; Yan, F.; Xie, L. A zinc oxide nanoflower-based electrochemical sensor for trace detection of Sunset Yellow. Sensors 2017, 17, 545. [Google Scholar] [CrossRef] [PubMed]
- Nagles, E.; Ceroni, M.; Hurtado, J. Simultaneous detection of Tartrazine-Sunset Yellow in food samples using bioxide/carbon paste microcomposite with lanthanum and titanium. J. Electrochem. Sci. Technol. 2020, 11, 421–429. [Google Scholar] [CrossRef]
- Khanfar, M.F.; Abu-Nameh, E.S.M.; Azizi, N.A.; Zurayk, R.A.; Khalaf, A.; Saket, M.M.; Alnuman, N. Electrochemical determination of Sunset Yellow and Tartrazine at carbon electrodes modified by Fe-Zr oxide. Jordan J. Chem. (JJC) 2020, 15, 119–126. [Google Scholar]
- Beitollahi, H.; Tajik, S.; Dourandish, Z.; Garkani Nejad, F. Simple preparation and characterization of hierarchical flower-like NiCo2O4 nanoplates: Applications for Sunset Yellow electrochemical analysis. Biosensors 2022, 12, 912. [Google Scholar] [CrossRef]
- Ding, Z.; Deng, P.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; He, Q. A novel modified electrode for detection of the food colorant sunset yellow based on nanohybrid of MnO2 nanorods-decorated electrochemically reduced graphene oxide. Molecules 2019, 24, 1178. [Google Scholar] [CrossRef] [PubMed]
- Baytak, A.K.; Akbaş, E.; Aslanoglu, M. A novel voltammetric platform based on dysprosium oxide for the sensitive determination of sunset yellow in the presence of tartrazine. Anal. Chim. Acta 2019, 1087, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Baytak, A.K.; Aslanoglu, M. Praseodymium doped dysprosium oxide-carbon nanofibers based voltammetric platform for the simultaneous determination of sunset yellow and tartrazine. Electroanalysis 2023, 35, e202200136. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar] [CrossRef]
- Arvand, M.; Parhizi, Y.; Mirfathi, S.H. Simultaneous voltammetric determination of synthetic colorants in foods using a magnetic core-shell Fe3O4/SiO2/MWCNTs nanocomposite modified carbon paste electrode. Food Anal. Methods 2016, 9, 863–875. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Yin, K.; Pang, H. An electrochemical sensor for Sunset Yellow detection based on Cu@Cu2O-BNPC formed by modified porous carbon. ACS Omega 2022, 7, 32068–32077. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Electrochemical fabrication of a novel ZnO/cysteic acid nanocomposite modified electrode and its application to simultaneous determination of sunset yellow and tartrazine. Food Chem. 2017, 227, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014; 367p. [Google Scholar] [CrossRef]
- de Sá, F.P.; Cunha, B.N.; Nunes, L.M. Effect of pH on the adsorption of Sunset Yellow FCF food dye into a layered double hydroxide (CaAl-LDH-NO3). Chem. Eng. J. 2013, 215–216, 122–127. [Google Scholar] [CrossRef]
- Boran, F.; Çetinkaya, S.; Şahin, M. Effect of surfactant types on the size of tin oxide nanoparticles. Acta. Phys. Pol. A 2017, 132, 546–548. [Google Scholar] [CrossRef]
- Gnanam, S.; Rajendran, V. Influence of various surfactants on size, morphology, and optical properties of CeO2 nanostructures via facile hydrothermal route. J. Nanoparticles 2013, 2013, 839391. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; 864p. [Google Scholar]
- Ziyatdinova, G.K.; Antonova, T.S.; Mubarakova, L.R.; Budnikov, H.C. An amperometric sensor based on tin dioxide and cetylpyridinium bromide nanoparticles for the determination of vanillin. J. Anal. Chem. 2018, 73, 801–808. [Google Scholar] [CrossRef]
- Randviir, E.P. A cross examination of electron transfer rate constants for carbon screen-printed electrodes using electrochemical impedance spectroscopy and cyclic voltammetry. Electrochim. Acta 2018, 286, 179–186. [Google Scholar] [CrossRef]
- König, J. 2—Food colour additives of synthetic origin. In Colour Additives for Foods and Beverages; Scotter, M.J., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 35–60. [Google Scholar] [CrossRef]
- Chebotarev, A.; Koicheva, A.; Bevziuk, K.; Pliuta, K.; Snigur, D. Simultaneous determination of Sunset Yellow and Tartrazine in soft drinks on carbon-paste electrode modified by silica impregnated with cetylpyridinium chloride. J. Food Meas. Charact. 2019, 13, 1964–1972. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Preparation and application of Sunset Yellow imprinted ionic liquid polymer − ionic liquid functionalized graphene composite film coated glassy carbon electrodes. Electrochim. Acta 2014, 115, 247–254. [Google Scholar] [CrossRef]
- Nguyen, Q.-T.; Le, T.-G.; Bergonzo, P.; Tran, Q.-T. One-step fabrication of nickel-electrochemically reduced graphene oxide nanocomposites modified electrodes and application to the detection of Sunset Yellow in drinks. Appl. Sci. 2022, 12, 2614. [Google Scholar] [CrossRef]
- Akkapinyo, C.; Subannajui, K.; Poo-arporn, Y.; Poo-arporn, R.P. Disposable electrochemical sensor for food colorants detection by reduced graphene oxide and methionine film modified screen printed carbon electrode. Molecules 2021, 26, 2312. [Google Scholar] [CrossRef]
- Măgeruşan, L.; Pogăcean, F.; Cozar, B.I.; Pruneanu, S. Analytical applicability of graphene-modified electrode in Sunset Yellow electrochemical assay. Sensors 2023, 23, 2160. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.G. Determination of standard rate constants for electrochemical irreversible processes from linear sweep voltammograms. Electroanalysis 1997, 9, 880–882. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Titova, M.; Davletshin, R. Electropolymerized 4-aminobenzoic acid based voltammetric sensor for the simultaneous determination of food azo dyes. Polymers 2022, 14, 5429. [Google Scholar] [CrossRef]
- Güneş, M.; Karakaya, S.; Dilgin, Y. Highly sensitive square wave voltammetric determination of Sunset Yellow at a functionalized-MWCNTs/poly(Alizarin Red-S) composite modified electrode. Electroanalysis 2022, 34, 1644–1655. [Google Scholar] [CrossRef]
- Setiyanto, H.; Hani, S.M.; Saraswaty, V.; Noviandri, I.; Rusli, H.; Rahayu, R.S.; Azis, M.Y.; Mufti, N. Sunset Yellow electrochemical sensor based on a molecularly imprinted poly-glycine film-decorated pencil graphite electrode. J. Electrochem. Soc. 2023, 170, 087503. [Google Scholar] [CrossRef]
- Jahani, P.M. Flower-like MoS2 screen-printed electrode based sensor for the sensitive detection of sunset yellow FCF in food samples. J. Electrochem. Sci. Eng. 2022, 12, 1099–1109. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Z.; Qin, S.; Huang, L.; Yang, J.; Wang, Y. A modified electrode based on a 3D reduced graphene oxide and MoS2 composite for simultaneous detection of sunset yellow and tartrazine. Anal. Methods 2023, 15, 4142–4148. [Google Scholar] [CrossRef]
- Moarefdoust, M.M.; Jahani, S.; Moradalizadeh, M.; Motaghi, M.M.; Foroughi, M.M. An electrochemical sensor based on hierarchical nickel oxide nanostructures doped with indium ions for voltammetric simultaneous determination of sunset yellow and tartrazine colorants in soft drink powders. Anal. Methods 2021, 13, 2396–2404. [Google Scholar] [CrossRef]
- Karami, M.; Shabani-Nooshabadi, M. Electrochemical analysis of Sunset Yellow, Brilliant Blue, and Tartrazine using sensor amplified with CuNiFe2O4 hollow spheres. J. Electroanal. Soc. 2023, 170, 087510. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Q.; Peng, X.Y.; Guo, Y. Facile synthesis of gold/graphene nanocomposites for simultaneous determination of Sunset Yellow and Tartrazine in soft drinks. Electroanalysis 2022, 34, 83–90. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Alharthi, S.S.; Al-Saidi, H.M.; Yadav, V.K.; Kumar, D.; Kumar, V. A novel ternary nanocomposite based electrochemical sensor coupled with regularized neural network for nanomolar detection of sunset yellow FCF. J. Alloys Compd. 2023, 968, 171934. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Zeng, W.; Zhou, S.; Zhang, W.; Ma, J. A new ratiometric molecularly imprinted electrochemical sensor for the detection of Sunset Yellow based on gold nanoparticles. Food Chem. 2023, 413, 135600. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, M. Applicability of a graphene oxide nanocomposite for fabrication of an electrochemical sensor for simultaneous detection of sunset yellow and rhodamine B in food samples. Anal. Methods 2023, 15, 5500–5509. [Google Scholar] [CrossRef] [PubMed]
- Tahtaisleyen, S.; Gorduk, O.; Sahin, Y. Electrochemical determination of Sunset Yellow using an electrochemically prepared graphene oxide modified—Pencil graphite electrode (EGO-PGE). Anal. Lett. 2021, 54, 394–416. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Khamzina, E.I.; Bukharinova, M.A.; Tarasov, A.V. An electrochemical sensor based on carbon paper modified with graphite powder for sensitive determination of Sunset Yellow and Tartrazine in drinks. Sensors 2022, 22, 4092. [Google Scholar] [CrossRef] [PubMed]
- Rozi, N.; Ahmad, A.; Yook Heng, L.; Shyuan, L.K.; Hanifah, S.A. Electrochemical Sunset Yellow biosensor based on photocured polyacrylamide membrane for food dye monitoring. Sensors 2018, 18, 101. [Google Scholar] [CrossRef]
- Bonan, S.; Fedrizzi, G.; Menotta, S.; Elisabetta, C. Simultaneous determination of synthetic dyes in foodstuffs and beverages by high-performance liquid chromatography coupled with diode-array detector. Dye. Pigm. 2013, 99, 36–40. [Google Scholar] [CrossRef]



| Sensor | Method | pH | Detection Limit, µM | Linear Dynamic Range, µM | Ref. |
|---|---|---|---|---|---|
| Nd2O3–CPE 1 | AdASWV 2 | 3.0 | 0.090 | 0.20–2.0 | [46] |
| MoO2–CPE | AdASWV | 4.1 | 0.060 | 0.16–2.0 | [47] |
| ZnO nanoflowers–CPE | AdASWV | 5.0 | 2.21 × 10−4 | 0.0011–0.022, 0.022–0.155 | [48] |
| La2O3–TiO2/CPE | AdASWV | 2.0 | 0.02 | 0.26–4.5 | [49] |
| Fe-Zr oxides/GCE 3 | DPV 4 | 2.0 | 5.7 | 19–270 | [50] |
| Hierarchical flower-like NiCo2O4 nanoplates/SPGE 5 | DPV | 7.0 | 0.008 | 0.02–145 | [51] |
| MnO2 nanorods–ERGO 6/GCE | AdASDLSV 7 | 4.5 | 0.002 | 0.01–2.0, 2.0–10, 10–100 | [52] |
| Dy2O3 NPs–MWCNTs(ox) 8/GCE | SWV 9 | 7.0 | 3.5 × 10−4 | 0.0010–0.14 | [53] |
| Pr6O11@Dy2O3−Carbon nanofibers/GCE | SWV | 7.0 | 3.12 × 10−4 | 0.0010–0.035 | [54] |
| Mesoporous TiO2 NPs–Graphene–CPE | AdASWV | 1.0 | 0.006 | 0.020–2.05 | [55] |
| Fe3O4@SiO2 NPs–MWCNTs-COOH 10–CPE | AdSSWV 11 | 6.0 | 0.050 | 0.50–100 | [56] |
| Cu@Cu2O-B and N codoped porous carbon/GCE | DPV | 6.5 | 0.0024 | 0.01–8 | [57] |
| Electrodeposited ZnO/Cysteinic acid/GCE | AdADPV 12 | 5.0 | 0.031 | 0.10–3.0 | [58] |
| Electrode | Epox, mV | Ipox, μA |
|---|---|---|
| Bare GCE | 906 | 0.18 ± 0.03 |
| GCE/SnO2–CeO2 NPs | 876 | 0.285 ± 0.005 |
| GCE/SnO2–CeO2 NPs–HDPB | 927 | 1.05 ± 0.06 |
| GCE/HDPB | 936 | 0.45 ± 0.01 |
| GCE/SnO2 NPs–HDPB | 927 | 0.67 ± 0.04 |
| GCE/CeO2 NPs–HDPB | 937 | 0.56 ± 0.05 |
| Electrode | A, mm2 | Rs, Ω | Ret, kΩ | Q, µΩ−1 | n | W, µΩ−1 | χ2 | ket, cm s−1 |
|---|---|---|---|---|---|---|---|---|
| Bare GCE | 8.9 ± 0.3 | 245 ± 5 | 72 ± 3 | 3.7 ± 0.2 | 0.789 | - | 0.03 | 4.15 × 10−5 |
| GCE/HDPB | 32 ± 1 | 35 ± 1 | 0.19 ± 0.03 | 96 ± 4 | 0.365 | 290 ± 7 | 0.01 | 4.38 × 10−3 |
| GCE/SnO2–CeO2 NPs | 36 ± 2 | 84 ± 2 | 9.0 ± 0.7 | 827 ± 9 | 0.887 | 264 ± 10 | 0.05 | 8.2 × 10−5 |
| GCE/SnO2–CeO2 NPs–HDPB | 40 ± 2 | 106 ± 3 | 0.42 ± 0.05 | 243 ± 2 | 0.466 | 138 ± 1 | 0.006 | 1.58 × 10−3 |
| Sensor | Method | Detection Limit, µM | Linear Dynamic Range, µM | Ref. |
|---|---|---|---|---|
| Poly(L-methionine)/Reduced graphene oxide/SPCE 1 | AdADPV | 0.048 | 1–10 and 10–50 | [70] |
| Exfoliate graphite/GCE | Amperometry | 0.0085 | 0.028–30 | [71] |
| Poly(4-aminobenzoic acid)/MWCNTs 2/GCE | DPV | 0.0023 | 0.010–0.75 and 0.75–5.0 | [73] |
| Functionalized MWCNTs/poly(Alizarin Red-S)/GCE | SWV | 0.16 | 0.50–256 | [74] |
| Molecularly imprinted polyglycine/Pencil graphite electrode | SWV | 0.753 | 2–100 | [75] |
| Flower-like MoS2/SPE 3 | DPV | 0.04 | 0.16–2.0 | [76] |
| 3D reduced graphene oxide loaded with cauliflower-like MoS2/GCE | DPV | 0.0176 | 0.05–10 and 10–60 | [77] |
| 3D raspberry-like In3+/NiO hierarchical nanostructures/GCE | DPV | 0.0027 | 0.01–700 | [78] |
| CuNiFe2O4 nanospheres–carbon paste electrode | DPV | 0.15 | 0.5–150 | [79] |
| Au NPs/PDDA 4–graphene/GCE | DPV | 0.002 | 0.006–5.0 | [80] |
| Ag NPs-Co3O4/Functionalized MWCNTs/SPCE | DPV | 0.0008 | 0.06–60 | [81] |
| Molecularly imprinted polymer-Au NPs/Indium-tin oxide | SWV | 0.0016 | 0.010–100 | [82] |
| Graphene oxide nanosheets–carbon paste electrode | DPV | 0.010 | 1–20 | [83] |
| Electrodeposited grapene oxide/Pencil graphite electrode | DPV | 0.057 | 0.5–10 | [84] |
| Fine-grained graphite powder/Carbon paper electrode | AdADPV | 0.00078 | 0.005–1.0 | [85] |
| Laccase/Poly(acrylamide-co-ethyl methacrylate) membrane/GCE | AdADPV | 0.02 | 0.08–10 | [86] |
| Added, μM | Found, μM | RSD, % | R, % |
|---|---|---|---|
| 0.010 | 0.0100 ± 0.0007 | 5.5 | 100 ± 7 |
| 0.25 | 0.249 ± 0.006 | 1.9 | 100 ± 2 |
| 1.0 | 1.00 ± 0.01 | 1.0 | 100 ± 1 |
| 7.5 | 7.51 ± 0.09 | 0.97 | 100 ± 1 |
| 100 | 100 ± 1 | 0.51 | 100 ± 1 |
| Interference | Epox, mV | Tolerance Limit, μM |
|---|---|---|
| K+ | ― | 500 |
| Mg2+ | ― | 500 |
| Ca2+ | ― | 500 |
| NO3− | ― | 500 |
| Cl− | ― | 500 |
| SO42− | ― | 500 |
| Glucose | ― | 50 |
| Rhamnose | ― | 50 |
| Sucrose | ― | 50 |
| Ascorbic acid | 391 | 25 |
| Sorbic acid | ― | 50 |
| Vanillin | 870 | 0.10 |
| Carminic acid | 557 | 2.5 |
| Riboflavin | ― | 50 |
| Tartrazine | 996 | 0.050 |
| Brilliant blue FCF | 965 | 0.10 |
| Sample | SY FCF Contents, mg L−1 | t-Test | F-Test | |||
|---|---|---|---|---|---|---|
| Voltammetry | RSD, % | HPLC | RSD, % | |||
| 1 | 2.6 ± 0.1 | 4.2 | 2.6 ± 0.3 | 5.5 | 0.341 | 1.63 |
| 2 | 8.0 ± 0.1 | 1.2 | 8.1 ± 0.6 | 2.7 | 1.18 | 4.98 |
| 3 | 1.87 ± 0.08 | 3.5 | 1.86 ± 0.06 | 1.4 | 0.748 | 6.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimadutdinova, L.; Ziyatdinova, G.; Davletshin, R. Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF. Sensors 2024, 24, 930. https://doi.org/10.3390/s24030930
Gimadutdinova L, Ziyatdinova G, Davletshin R. Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF. Sensors. 2024; 24(3):930. https://doi.org/10.3390/s24030930
Chicago/Turabian StyleGimadutdinova, Liliya, Guzel Ziyatdinova, and Rustam Davletshin. 2024. "Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF" Sensors 24, no. 3: 930. https://doi.org/10.3390/s24030930








