1. Introduction
In the past decade, micro fuel cells have received lots of attention in the electronic engineering field due to not only the world’s growing energy crisis but also the urgent need for micro power sources for highly integrated microelectronic devices and systems [
1,
2,
3]. Among novel technologies, micro direct methanol fuel cells (μDMFCs) have shown great advantages of light weight, safety and reliability, environmental friendliness, high energy density, and ease of carrying and use [
4,
5]. However, two major factors still hinder the prospect of the commercialization of μDMFC technology. One is the low reaction kinetics of methanol and water in room temperature and the other is that the commonly used membrane, namely Nafion membrane, is not able to prevent methanol fuel from crossover. For both factors, extensive research shows that methanol concentration plays such an important role that increasing fuel concentration promotes the methanol oxidation reaction rate but inevitably increases methanol crossover. This is due to the relatively high methanol permeability of the Nafion membrane [
6]. Up till now, a clear majority of μDMFCs have still operated with low concentrations of methanol; therefore, raising methanol concentration in μDMFCs is of great interest but also faces great challenges [
7,
8,
9,
10]. In addition, μDMFCs have to be stacked to obtain a sufficiently high output voltage for electronic devices or systems. Multiple-cell packaging for micro fuel cells is highly difficult and there is a huge potential risk of fuel leakage. For those μDMFCs aiming to be supplied with high-concentration methanol, an unexpected leak or heavy crossover of methanol can cause more damage. Therefore, given the fact that the performance of a μDMFCs is so sensitive to methanol concentration, it is necessary to detect it in system monitoring, and therefore the online monitoring of methanol concentration should be a must-have function in future μDMFC devices.
Traditional chemical and physical methods are commonly used for solute concentration detection. Although the chemical titration method is easy to conduct for ion concentration detection, it is not suitable for methanol concentration detection in fuel cells. Alternatively, one of the typical physical methods is gas chromatography. It was used to measure methanol concentration in a study of preventing methanol crossover in DMFCs [
11]. Unfortunately, this method contains a step of obtaining aqueous solutions from the fuel cell, either by a micropipette or a syringe, which makes it an intrusive way. However, in well-assembled fuel cell stacks, it is almost impossible to obtain a solution sample when in operation. Therefore, the online detection of fuel concentration in DMFCs needs noncontact and nonintrusive methods. In recent years, nonintrusive and online ways of solute concentration measurements have been in great demand, not only in the fuel cell industry, but also in the medical and pharmaceutical industry. In addition, real-time monitoring of fuel cell operating status should also be mentioned and carried out. Methanol concentration, as the main index of the performance of a DMFC, needs to be monitored constantly, besides other parameters like temperature, output voltage, etc.
Nowadays, microwave sensors based on radio frequency (RF) resonance have shown the ability to accurately quantify aqueous solutions in noncontact ways and have received great attention in areas like chemistry, food, and biological medicine. These sensors can be introduced into fuel cell systems for fuel concentration monitoring [
12,
13,
14]. In common cases, it operates with advantages like multidimensional detection-based enhanced accuracy, real-time operation, reusability, contactless corrosive materials, and lab-on-a-chip compatibility. To take advantage of these attractive features, the sensing region for the interaction between the material under test and the electromagnetic wave should be carefully designed. Since the resonant mode design is critical to the sensitivity and stability of the microwave sensor, it is commonly seen that the sensitivity of an RF resonance sensor is approximated using the product of loaded quality and filling factors.
In this paper, we exploit a nonintrusive, online way of methanol concentration detection and operation status monitoring for μDMFCs. The proposed RF sensor is based on a square ring resonator. The integration of the sensor with the μDMFC guarantees a stable sensitivity of the RF resonance sensor and makes the device more compact. Besides the primary function of the sensor, i.e., the online detection of fuel concentration, it also makes it easy to access the real-time record of the operation status of the μDMFC in case of fuel leakage or abnormal fuel crossover. Fuel utilization, often regarded as the performance index for fuel cells, can also be calculated for any long-term discharging process.
2. Materials and Methods
Figure 1 shows the schematic of the proposed sensor-integrated μDMFC, the key components of the μDMFC, and the layout of the RF resonance sensor. As can be seen from the figures, the multilayer structure of the μDMFC consists of a cathode end plate, a cathode current collector, a membrane electrolyte assembly (MEA), an anode current collector, and an anode chamber. The MEA, with multiple porous layers, catalytic structures, and a proton exchange membrane in between, has an active area of 1 cm
2. The RF resonance sensor is attached to the bottom of the μDMFC; thus, the sensing area is directly below the anode chamber. Originally, the anode chamber had a volume of about 1 cm
3 for fuel, namely methanol aqueous solution. But in order to keep the fuel close to the sensor, we intentionally hollowed out a 6 × 6 mm
2 area at the bottom of the anode chamber so the fuel could be located closer to the sensor.
The RF sensor, based on a square ring resonator in which the two ports are orthogonal, is shown in
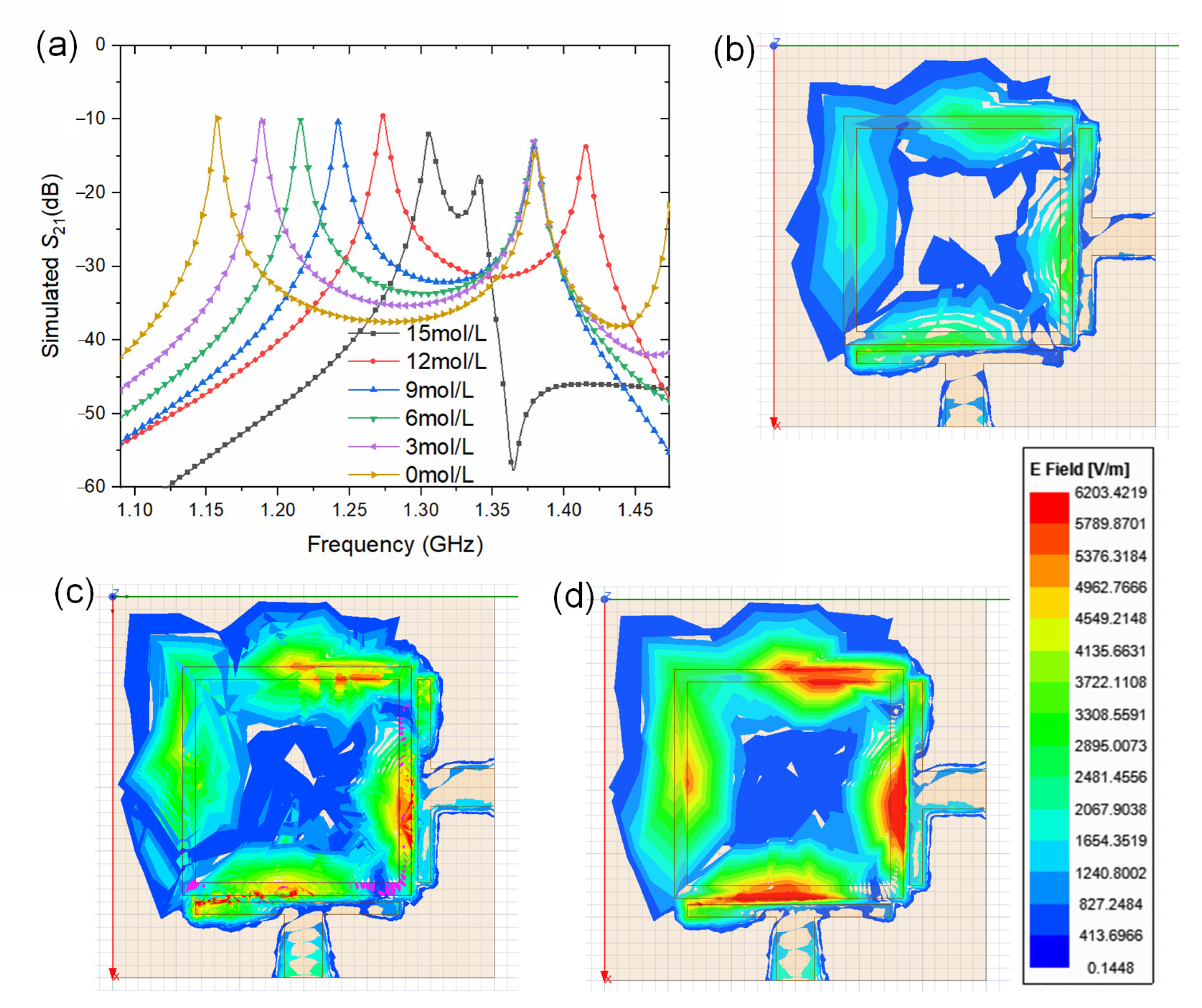
Figure 1c. The length difference between the two transmission paths from port 1 to port 2 equals π. Restrained by the placement of the ports, the resonator is not able to split the degenerate modes; therefore, a small chamber containing the methanol solution is placed in the 45° azimuth, which is defined as the sensing region. By forming a perturbation in the ring resonator, the balance of the resonator is destroyed. As a result, the coupling between the degenerate modes in the cavity of the square ring resonator becomes the key to generating dual-mode resonance. In order to implement the proposed RF resonance sensor with miniaturized size and enhanced filling factor, the side length and the line width of the square ring are set to 16 mm and 1 mm, and the line length and width of the interfacial part are 6.5 mm and 4 mm. It should be noted that the size of the resonator matches the size of a μDMFC, which is useful for further easy integration.
The sensor utilizes the parallel relationship of methanol concentration and dielectric properties of the methanol solution. When the concentration of the methanol solution changes, the dielectric constant changes accordingly. As a result, the resonant frequency of the RF sensor, which is based on near-field electromagnetic wave resonance, is affected by the change of the dielectric properties. With dual-mode resonance, the generated lower central frequency (
fL) of the square ring resonator will change accordingly with methanol concentration and can conveniently be measured as S-parameters. The penetration depth of the concentrated electromagnetic wave in the sensing region oscillating at the resonator’s
fL, which is a measure of how deeply RF radiation can penetrate the methanol chamber and sense methanol concentration-based permittivity variations, also depends on both complex permittivity and temperature. It is calculated according to the following equation:
where λ refers to the wavelength of the RF electromagnetic wave in free space.
The proposed device of a self-breathing transparent μDMFC integrated with an RF resonance sensor was fabricated in order to verify the simulation results. First and foremost, a piece of 5-layered MEA with 1 cm
2 active area was fabricated using the catalyst-coated-membrane (CCM) method. We employed a Pt-Ru/C catalyst of 4.0 mg cm
−2 loading on the anode and Pt/C catalyst of 2.0 mg cm
−2 on the cathode, with a Nafion117 membrane in between. Prior to the experimental tests, the following activation process was conducted at a temperature of 60 °C: Firstly, deionized water was fed into the anode chamber of the μDMFC to humidify the MEA for over 2 h. Secondly, the fuel cell was maintained at a discharging current density of 3 mA cm
−2 for 2 h with 2.0 M methanol solution. Thirdly, the fuel cell operated with a high current density of 30 mA cm
−2 until the output voltage reached its maximum value. The current collectors, made of 316 stainless steel, were fabricated by a computer numerical control (CNC) machine. The anode current collector was designed with a grid shape while the cathode current collector had a perforated structure. The anode chamber, made of organic glass, has a volume of 2 × 1.2 × 1.2 cm for methanol solution. In addition, silicone gaskets were fabricated by laser cutting to provide good sealing between the anode chamber, current collectors, and the MEA. Al-based endplates furthermore guaranteed good sealing and electrical contact of the fuel cell’s essential components.
Figure 2 illustrates the experimental setup for testing and monitoring. To conduct the electrical tests and measurements, the RF resonance sensor and the μDMFC were connected to an Agilent N9923A RF vector network analyzer (VNA) (Agilent Technologies, Inc., Santa Clara, CA, USA) and an Itech IT8511A+ DC electronic load (Itech Electronic co., Ltd., Nanjing, China), respectively. To automatically monitor the μDMFC in real time, both the VNA and the programmable DC load were connected to a personal computer (PC) through a network and RS232 protocol, respectively. Every 10 s, the status of the μDMFC was recorded and saved in the PC. An enlarged picture of the assembled μDMFC on top of the RF sensor is shown in
Figure 2b. The proposed RF resonance sensor with optimized dimensions was fabricated on a 0.508 mm thick substrate with a dielectric constant of 2.52 and a loss tangent of 0.002 using the wet etching printed circuit board (PCB) technique. The side length of the squared substrate is 32 mm. Both the top and bottom of the substrate was covered with a 0.018 mm thick copper layer with a conductivity of 5.8 × 10
7 S·m. The fabricated sensor was embedded under the anode chamber of the μDMFC and was fixed to the aluminum end plate.
4. Conclusions
In summary, in this work, a newly proposed technique of noncontact sensing and nonintrusive monitoring was demonstrated to be highly efficient in novel fuel cells by integrating a liquid-fed μDMFC with a high-sensitivity RF resonance sensor. Firstly, based on a dual-mode square ring resonator, the RF sensor was designed and numerically verified to be capable of accurately quantifying physiologically relevant concentrations of methanol in the anode chamber of a μDMFC. Secondly, a μDMFC with an active area of 1 × 1 cm2 and a maximum power density of 28.8 mW cm−2 at 30 °C was fabricated as well as the proposed RF sensor. Their integration was achieved by hollowing out the bottom of the anode chamber, which then acted as the sensing area for the sensor. The experimental results revealed a sensitivity of 9.5 MHz mol−1 L for the RF sensor, which was slightly smaller than the simulated data. Moreover, a linear and unidirectional negative correlation between fL and sample temperature enabled the proposed sensor to accurately quantify methanol concentration under a wide range of temperature variations. Finally, a long-term discharging operation was conducted through a demonstration of noncontact sensing and online monitoring for the integrated μDMFC device. Real-time fuel utilization was recorded, but to our disappointment, it was only about 28.5% in the end. The recorded data showed that there existed an enormous gap between the discharging loss and the actual loss of methanol, which was induced by methanol crossover. In the future, not only higher sensitivity of the sensor should be achieved, but also the compactness of the sensor-integrated fuel cell system should be improved.