Calibration of Dual-Channel Raman Spectrometer via Optical Frequency Comb
Abstract
:1. Introduction
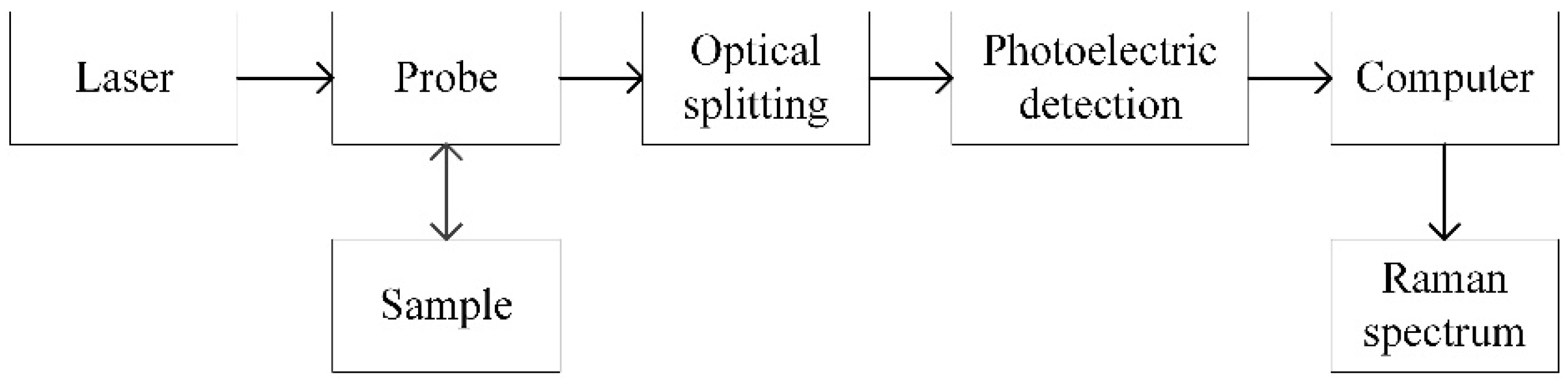
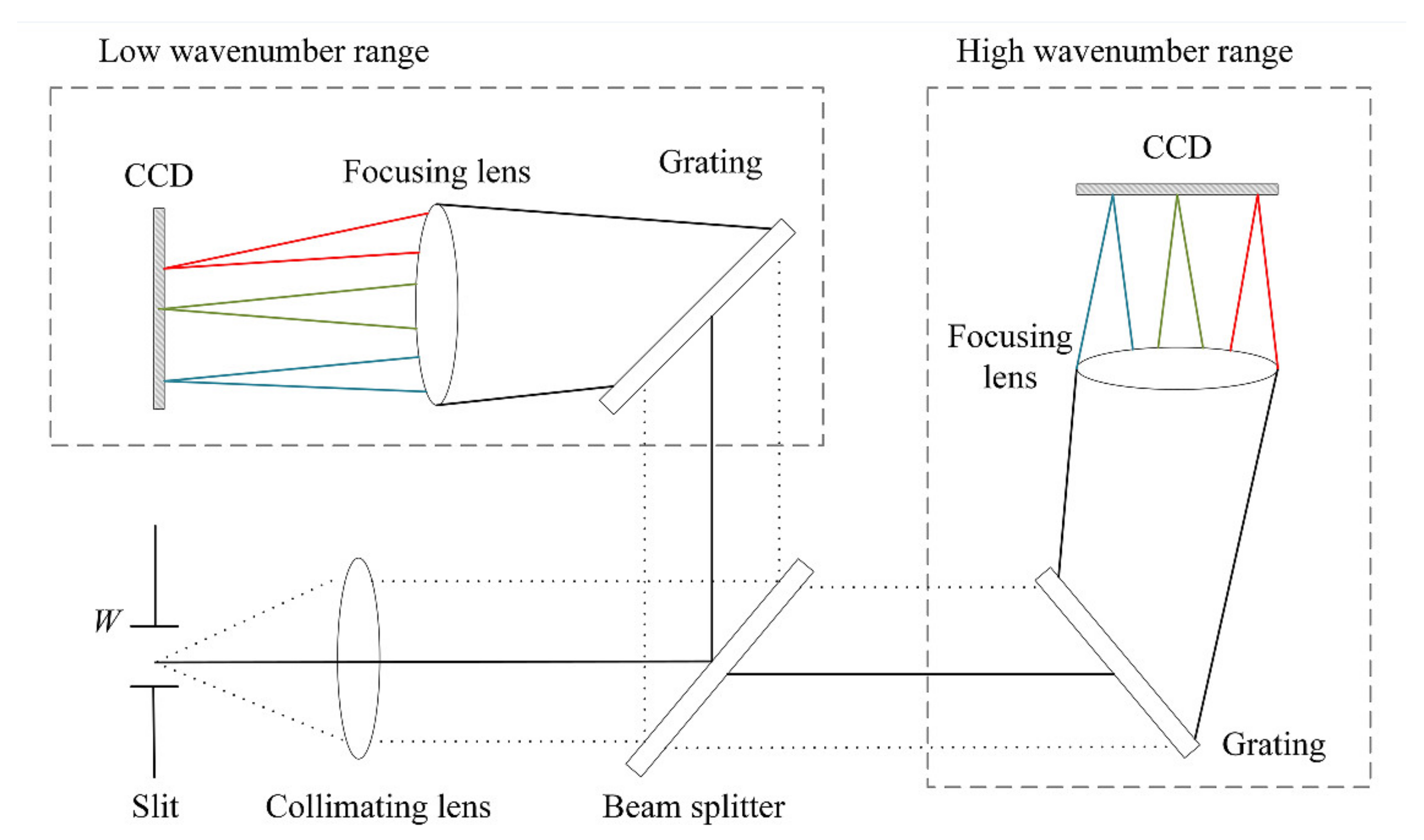
2. Structure of Dual-Channel Portable Raman Spectrometer
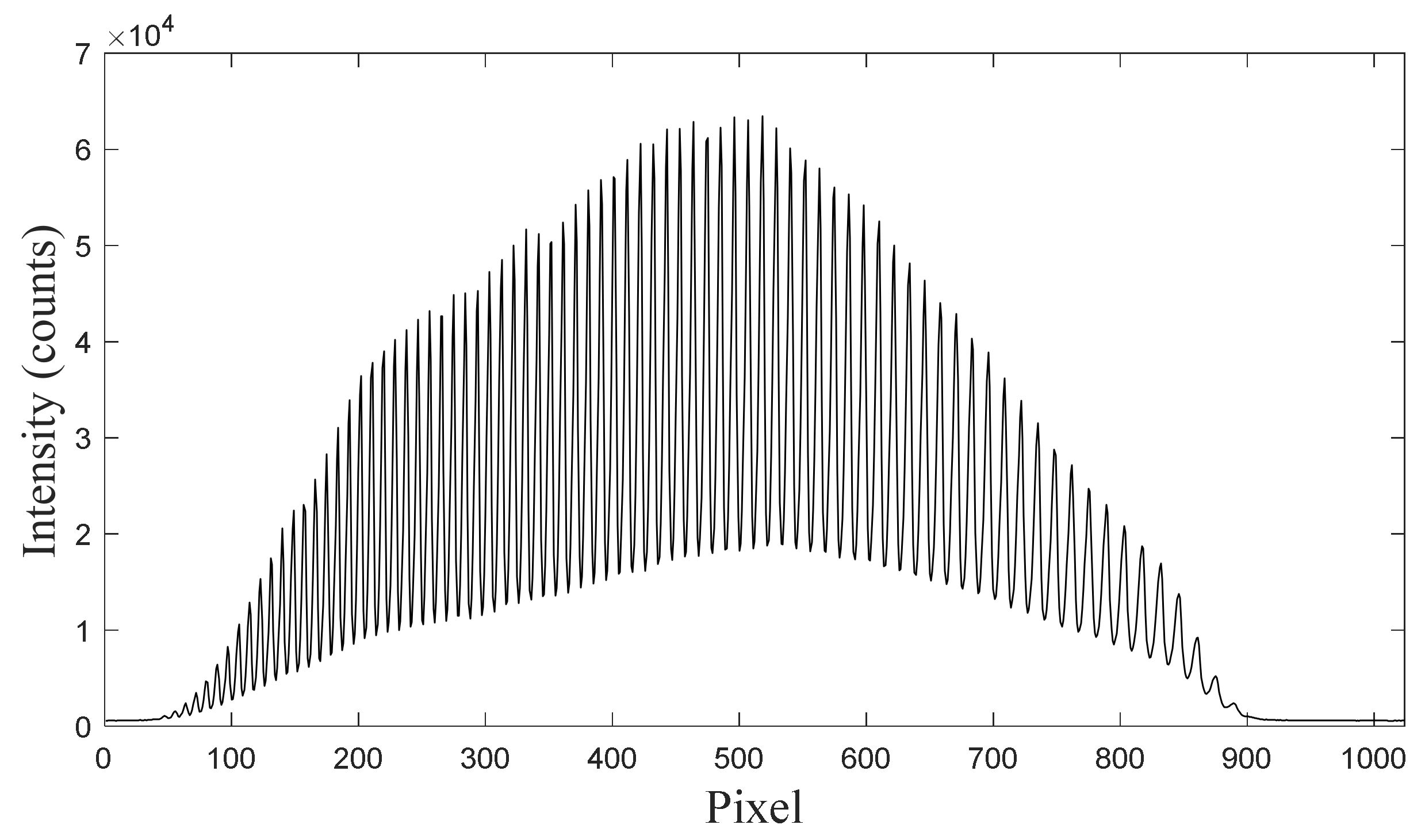
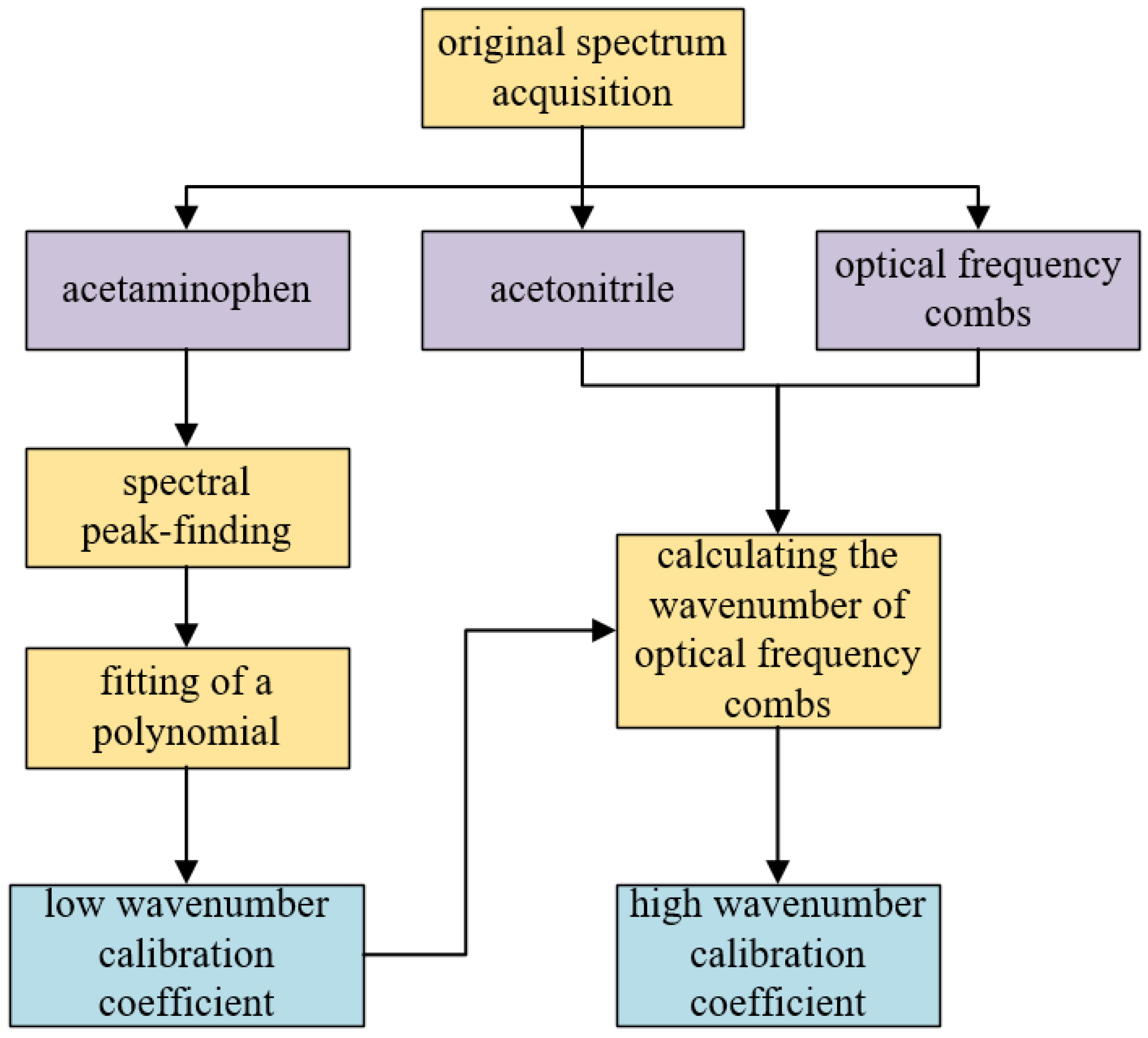
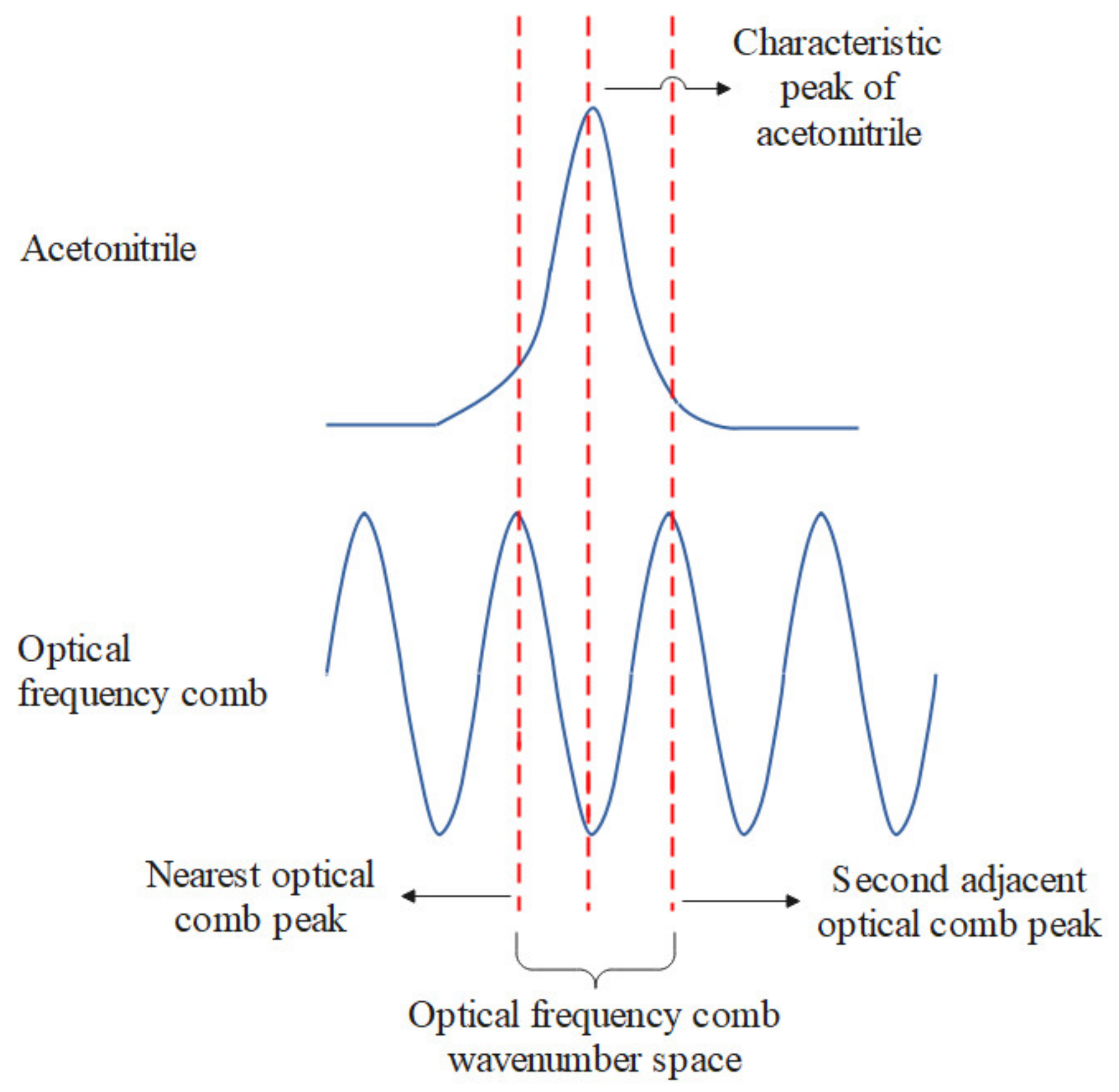
3. Dual-Band Wavelength Calibration Method Based on Optical Frequency Comb
4. Experimental Results and Analysis
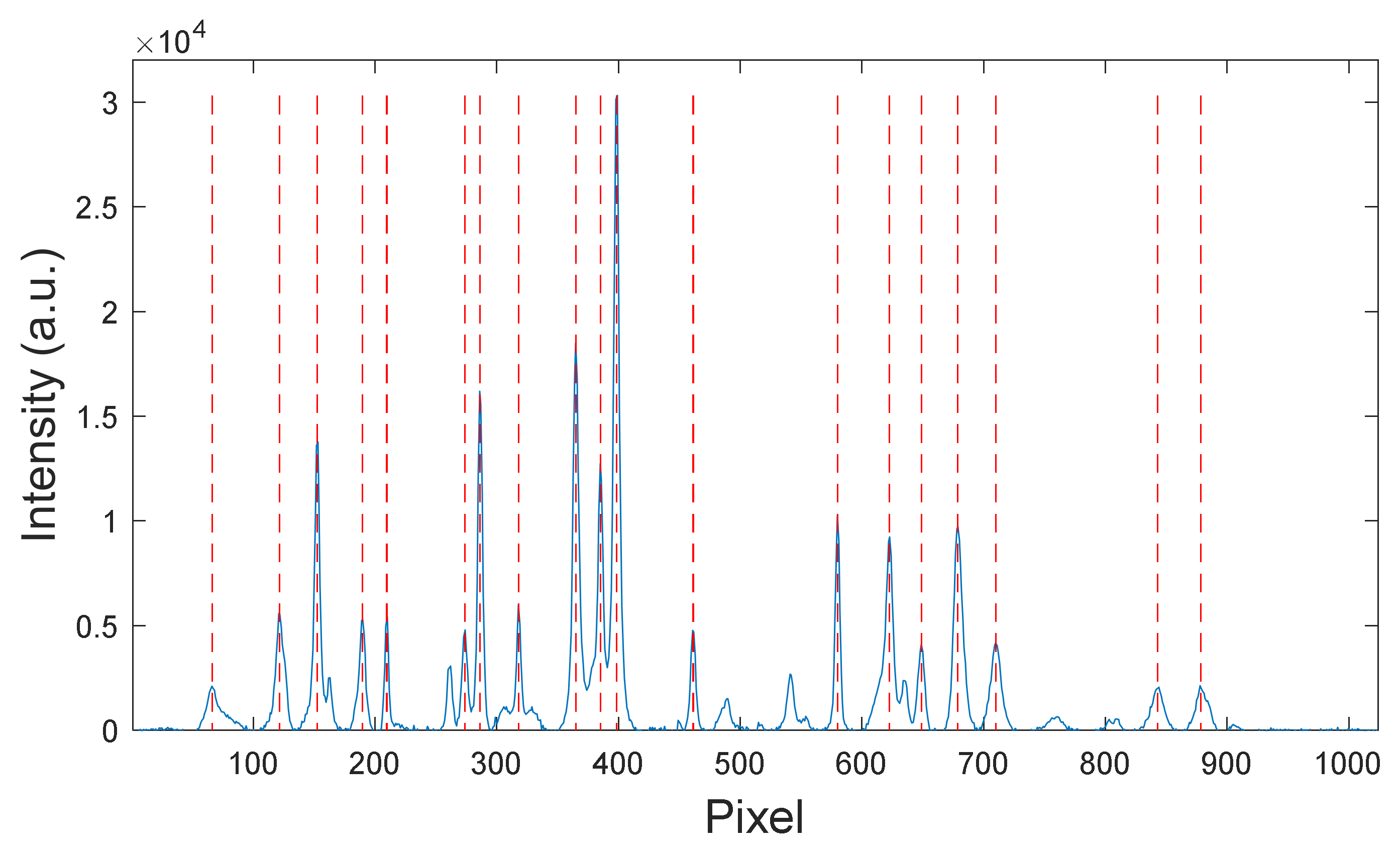
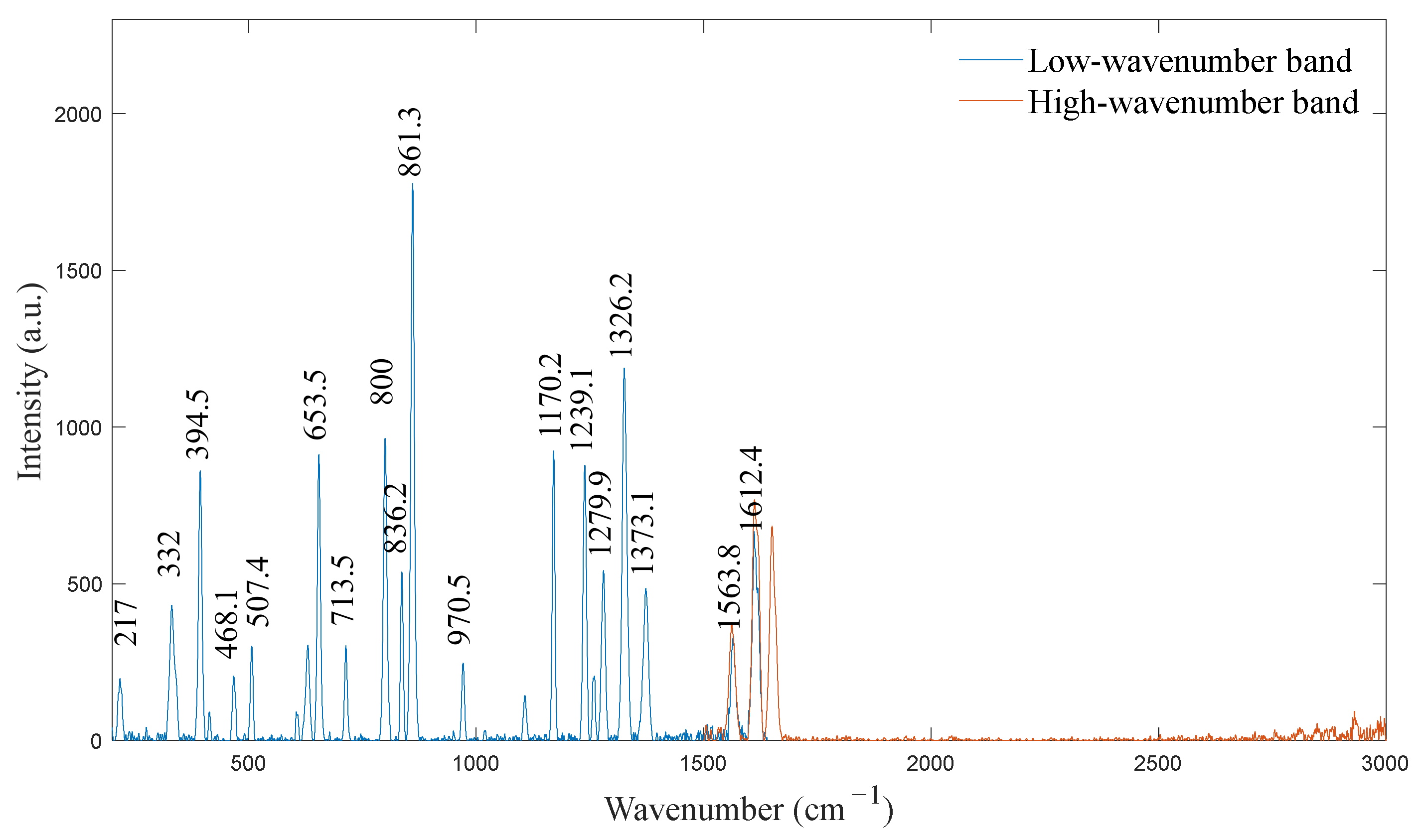
4.1. Spectrometer Calibration
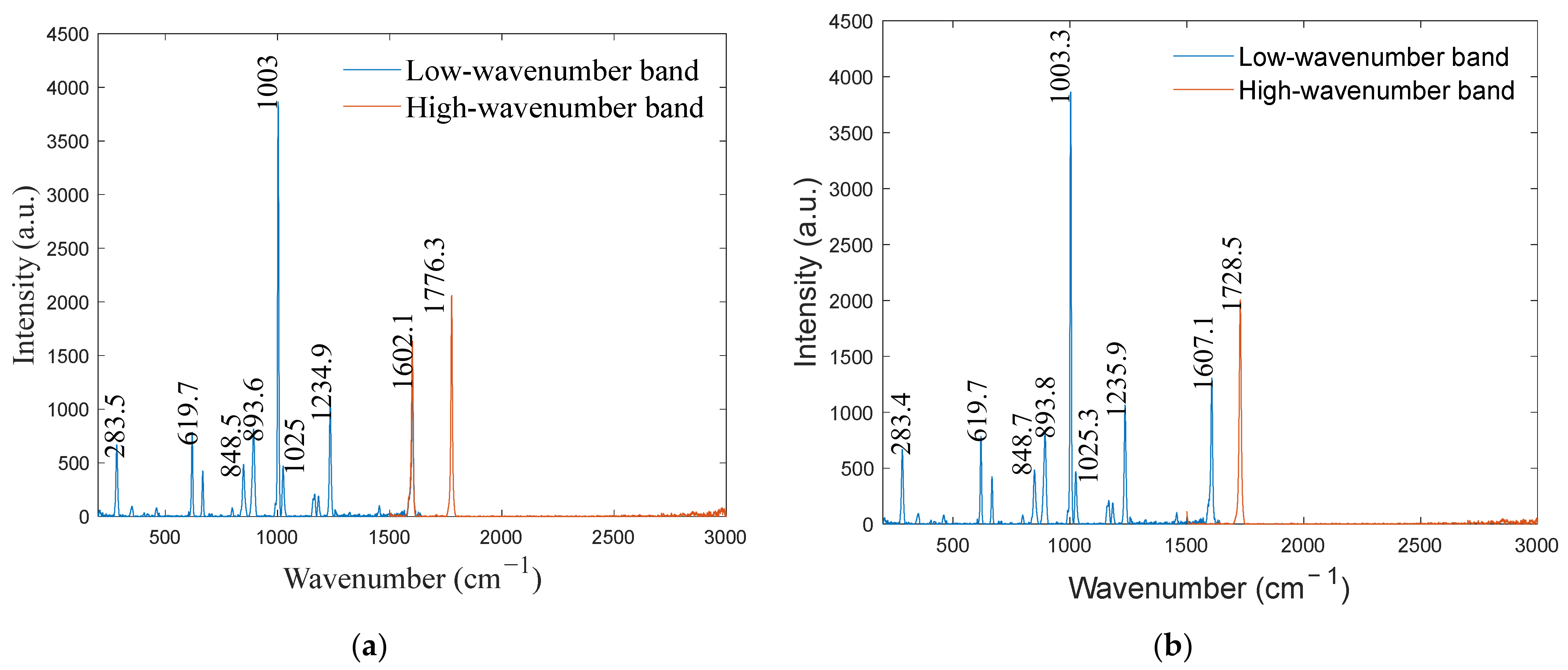
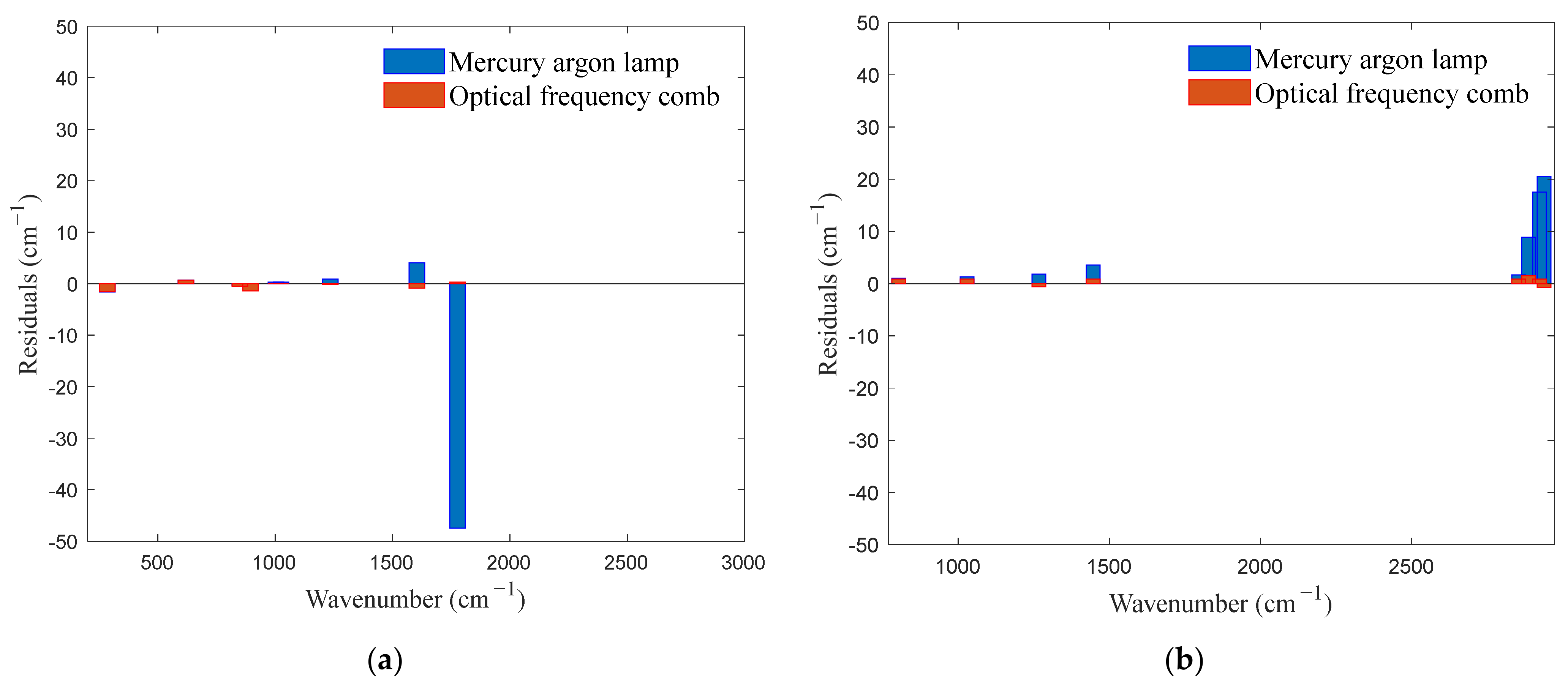
4.2. Experimental Verification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.G.; Jarvis, I.; Gillmore, G.; Stephenson, M. Raman spectroscopy as a tool to determine the thermal maturity of organic matter: Application to sedimentary, metamorphic and structural geology. Earth-Sci. Rev. 2019, 198, 102936. [Google Scholar] [CrossRef]
- Wang, K.; Li, Z.; Li, J.; Lin, H. Raman spectroscopic techniques for nondestructive analysis of agri-foods: A state-of-the-art review. Trends Food Sci. Technol. 2021, 118, 490–504. [Google Scholar] [CrossRef]
- Si, G.; Fang, Y.; Liu, J.; Huang, W.; Li, Z.; Yu, X.; Si, B.; Cheng, Z. A new eye-safe compact UV-Raman spectroscopy setup for the proximal detection of hazardous chemicals. In AOPC 2021: Optical Spectroscopy and Imaging; SPIE: Beijing, China, 2021; pp. 99–105. [Google Scholar]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A comprehensive review on Raman spectroscopy applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Bocklitz, T.W.; Dörfer, T.; Heinke, R.; Schmitt, M.; Popp, J. Spectrometer calibration protocol for Raman spectra recorded with different excitation wavelengths. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Workman, J.J. A review of calibration transfer practices and instrument differences in spectroscopy. Appl. Spectrosc. 2015, 72, 340–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Zheng, F.; Zhang, G.; Xu, Y. Accuracy control and influence analysis of wavelength calibration for compact CCD spectrometer. Chin. J. Sci. Instrum. 2019, 40, 19–27. [Google Scholar]
- Jakubek, R.S.; Fries, M.D. Calibration of Raman wavenumber in large Raman images using a mercury-argon lamp. J. Raman Spectrosc. 2020, 51, 1172–1185. [Google Scholar] [CrossRef]
- Travis, J.C.; Zwinkels, J.C.; Mercader, F.; Ruíz, A.; Early, E.A.; Smith, M.V.; Duewer, D.L. An international evaluation of holmium oxide solution reference materials for wavelength calibration in molecular absorption spectrophotometry. Anal. Chem. 2002, 74, 3408–3415. [Google Scholar] [CrossRef]
- Schinke, C.; Pollex, H.; Hinken, D.; Wolf, M.; Bothe, K.; Kröger, I.; Nevas, S.; Winter, S. Calibrating spectrometers for measurements of the spectral irradiance caused by solar radiation. Metrologia 2020, 57, 065027. [Google Scholar] [CrossRef]
- Youngquist, R.C.; Simmons, S.M.; Belanger, A.M. Spectrometer wavelength calibration using spectrally resolved white interferometry. Opt. Lett. 2010, 35, 2257–2259. [Google Scholar] [CrossRef] [PubMed]
- Perret, E.; Balmer, T.E.; Heuberger, M. Self-consistent algorithm for calibration spectrometers topicometer accuracy over the entire wavelength range. Appl. Spectrosc. 2010, 64, 1139–1144. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, M.; Wang, Y.; Zou, Y.; Sun, Z.; Cao, Z. Fast wavelength calibration method for spectrometers based on waveguide comb optical filter. Rev. Sci. Instrum. 2015, 86, 043103. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Sun, Y.; Li, B.; Chen, X.; Tan, Z. Fast spectral calibration method of spectral imager. Spectrosc. Spectr. Anal. 2022, 42, 2013–2017. [Google Scholar]
- Borio, V.G.; Vinha, R.; Nicolau, R.A.; de Oliveira, H.P.M.; de Lima, C.J.; Silveira, L. Quantitative evaluation of acetaminophen in oral solutions by dispersive Raman spectroscopy for quality control. Spectrosc. Int. J. 2012, 27, 215–228. [Google Scholar] [CrossRef]
- Dyer, S.A.; He, X. Least-squares fitting of data by polynomials. IEEE Instrum. Meas. Mag. 2001, 4, 46–51. [Google Scholar] [CrossRef]
- Jakubek, R.S. Effect of the spectrometer slit width and charge-coupled device detector on Raman intensities. J. Raman Spectrosc. 2021, 52, 1317–1333. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Shang, L.; Zhao, Y.; Yin, J.; Huang, B. Design and application of small NIR-Raman spectrometer based on dichroic and transmission collimating. Spectrosc. Spectr. Anal. 2018, 38, 1933–1937. [Google Scholar]
- Fortier, T.; Baumann, E. 20 years of developments in optical frequency comb technology and applications. Commun. Phys. 2019, 2, 153. [Google Scholar] [CrossRef]
- Kim, J.; Song, Y. Ultralow-noise mode-locked fiber lasers and frequency combs: Principles, status, and applications. Adv. Opt. Photonics 2016, 8, 465–540. [Google Scholar] [CrossRef]
- Kippenberg, T.J.; Holzwarth, R.; Diddams, S.A. Microresonator-based optical frequency combs. Science 2011, 332, 555–559. [Google Scholar] [CrossRef]
- Plascak, M.E.; Ramirez, R.B.; Bagnell, K.; Delfyett, P.J. Tunable broadband electro-optic comb generation using an optically filtered optoelectronic oscillator. IEEE Photonics Technol. Lett. 2017, 30, 335–338. [Google Scholar] [CrossRef]
- Wu, Q.; Chan, H.P.; Chu, P.L.; Yu, C.; Hand, D. Compact tunable three-dimensional polymer optical waveguide comb filter. Opt. Commun. 2007, 277, 89–92. [Google Scholar] [CrossRef]
- Jing, W.; Wang, G.; Liu, K.; Zhang, Y.; Dong, H.; Jia, D.; Peng, G. Application of weighted wavelength algorithm on the demodulation of a fiber Bragg grating optical sensing system. J. Optoelectron. Laser 2007, 18, 1022–1025. [Google Scholar]
- Bi, Y.; Li, Y.; Zheng, R. The symmetric zero-area conversion adaptive peak-seeking method research for LIBS/Raman spectra. Spectrosc. Spectr. Anal. 2013, 33, 438–443. [Google Scholar]
- Yuan, J.; Zhao, J.; Li, H.; Liu, G. Research of peak-detection algorithm based on absolute wavelength calibration of excimer laser. Chin. J. Lasers 2018, 45, 19–25. [Google Scholar]
- Yuan, X.; Mayanovic, R.A. An empirical study on Raman peak fitting and its application to Raman quantitative research. Appl. Spectrosc. 2017, 71, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, F. Accurate wavelength calibration method using system parameters for grating spectrometers. Opt. Eng. 2013, 52, 013603. [Google Scholar] [CrossRef]
- Xie, Y.; Zhuang, S.; Zhang, B. Research on calibration of spectrometers based on linear CCD. Chin. J. Sci. Instrum. 2011, 32, 546–550. [Google Scholar]
- Quinet, O.; Champagne, B.; Rodriguez, V. Experimental and theoretical investigation of the Raman and hyper-Raman spectra of acetonitrile and its derivatives. J. Chem. Phys. 2006, 124, 244312. [Google Scholar] [CrossRef]
- Baek, S.J.; Park, A.; Ahn, Y.J.; Choo, J. Baseline correction using asymmetrically reweighted penalized least squares smoothing. Analyst 2015, 140, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Huang, C.; Xia, G.; Jin, S.Q.; Lu, H.B. Accurate wavelength calibration method for compact CCD spectrometer. JOSA A 2017, 34, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Ojanen, M.; Kärhä, P.; Ikonen, E. Spectral irradiance model for tungsten halogen lamps in 340–850 nm wavelength range. Appl. Opt. 2010, 49, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Y.; Min, Q.; Cao, S.; Zhang, Z.; Su, M.; Sun, D.; Dong, C. Relative Spectral Intensity Response Calibration of Spectrometers Using Ar Plasma Emission Branching Ratio Method. Spectrosc. Spectr. Anal. 2018, 38, 1375–1379. [Google Scholar]



| Parameter | Value |
|---|---|
| Laser wavelength | 784.5 nm |
| Spectral range | 200~3000 cm−1 |
| Resolution ratio | 2.6 cm−1 |
| Pixel size | 14 × 14 μm |
| CCD pixel | 1024 × 64 pixels |
| Standard Wavenumber ) | Pixel Position (pixel) | Calibration Wavenumber ) | Deviation ) |
|---|---|---|---|
| 217 | 66.1 | 217.0 | 0 |
| 332 | 121.6 | 332.0 | 0 |
| 394 | 152.6 | 394.5 | 0.5 |
| 468 | 189.8 | 468.1 | 0.1 |
| 507 | 209.7 | 507.4 | 0.4 |
| 654 | 286.4 | 653.5 | −0.5 |
| 713 | 318.1 | 713.5 | 0.5 |
| 800 | 365.1 | 800.0 | 0 |
| 837 | 385.3 | 836.2 | −0.8 |
| 861 | 398.5 | 861.3 | 0.3 |
| 971 | 461.4 | 970.5 | −0.5 |
| 1170 | 580.1 | 1170.2 | 0.2 |
| 1239 | 622.7 | 1239.1 | 0.1 |
| 1280 | 648.9 | 1279.9 | −0.1 |
| 1326 | 678.8 | 1326.2 | 0.2 |
| 1373 | 710.2 | 1373.1 | 0.1 |
| 1564 | 843.1 | 1563.8 | −0.2 |
| 1612 | 878.4 | 1612.4 | 0.4 |
| Standard Wavenumber ) | Mercury–Argon Lamp Calibration ) | Deviation ) | Optical Frequency Comb Calibration ) | Deviation ) |
|---|---|---|---|---|
| 285 | 283.4 | −1.6 | 283.5 | −1.5 |
| 619 | 619.7 | 0.7 | 619.7 | 0.7 |
| 849 | 848.7 | −0.3 | 848.5 | −0.5 |
| 895 | 893.8 | −1.2 | 893.6 | −1.4 |
| 1003 | 1003.3 | 0.3 | 1003.0 | 0 |
| 1025 | 1025.3 | 0.3 | 1025.0 | 0 |
| 1235 | 1235.9 | 0.9 | 1234.9 | −0.1 |
| 1603 | 1607.1 | 4.1 | 1602.1 | −0.9 |
| 1776 | 1728.5 | −47.5 | 1776.3 | 0.3 |
| Standard Wavenumber ) | Mercury–Argon Lamp Calibration ) | Deviation ) | Optical Frequency Comb Calibration ) | Deviation ) |
|---|---|---|---|---|
| 803 | 804.0 | 1.0 | 803.9 | 0.9 |
| 1029 | 1030.3 | 1.3 | 1029.9 | 0.9 |
| 1267 | 1268.8 | 1.8 | 1267.6 | 0.6 |
| 1446 | 1449.6 | 3.6 | 1446.9 | 0.9 |
| 2853 | 2854.7 | 1.7 | 2853.9 | 0.9 |
| 2886 | 2894.9 | 8.9 | 2887.5 | 1.5 |
| 2923 | 2940.5 | 17.5 | 2923.9 | 0.9 |
| 2938 | 2958.5 | 20.5 | 2937.3 | −0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Lou, X.; Gai, Q.; Mu, T. Calibration of Dual-Channel Raman Spectrometer via Optical Frequency Comb. Sensors 2024, 24, 1217. https://doi.org/10.3390/s24041217
Lv S, Lou X, Gai Q, Mu T. Calibration of Dual-Channel Raman Spectrometer via Optical Frequency Comb. Sensors. 2024; 24(4):1217. https://doi.org/10.3390/s24041217
Chicago/Turabian StyleLv, Shengyujie, Xiaoping Lou, Qiaona Gai, and Taotao Mu. 2024. "Calibration of Dual-Channel Raman Spectrometer via Optical Frequency Comb" Sensors 24, no. 4: 1217. https://doi.org/10.3390/s24041217




