Detecting Psychological Interventions Using Bilateral Electromyographic Wearable Sensors
Abstract
1. Introduction
2. Methodology
2.1. Experimental Protocol
2.1.1. Study Design
2.1.2. Screening
2.1.3. Participants
2.1.4. Auditory Stimulation for Four Interventions
- Silence: Participants were instructed to wear noise-canceling headphones to eliminate external auditory stimuli during the walking sessions.
- Music: Participants were allowed to choose and listen to music of their preference via a music streaming application of their choice, such as Amazon Music, Pandora, Spotify, iHeartRadio, or Apple Music, during the walking sessions.
- Positive Reinforcement: Participants received auditory feedback in the form of positive reinforcement through the headphones every 30 s.
- Negative Reinforcement: Participants received negative reinforcement through the headphones every 30 s.
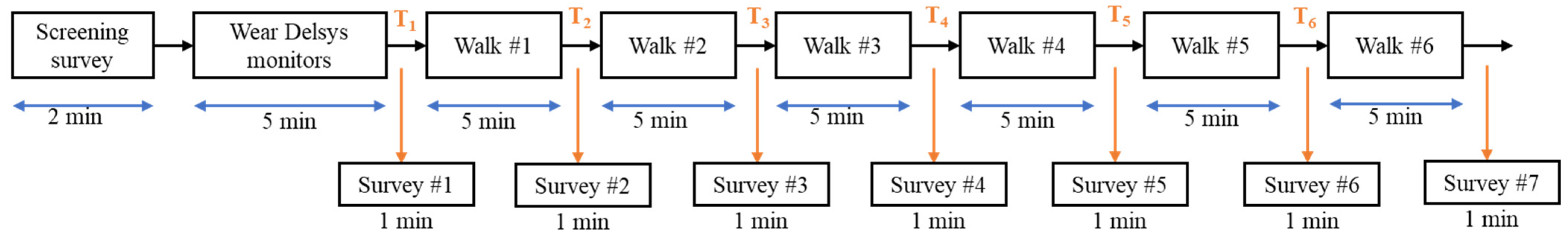
2.1.5. Experimental Procedure
2.2. Pre-Processing of Recorded EMG Signals
2.2.1. Filtering
2.2.2. Resampling
2.2.3. Segment Removal
2.2.4. Handling Short Segments with Spline Interpolation
2.3. Feature Extraction
2.4. Statistical Analysis and Classification
3. Results
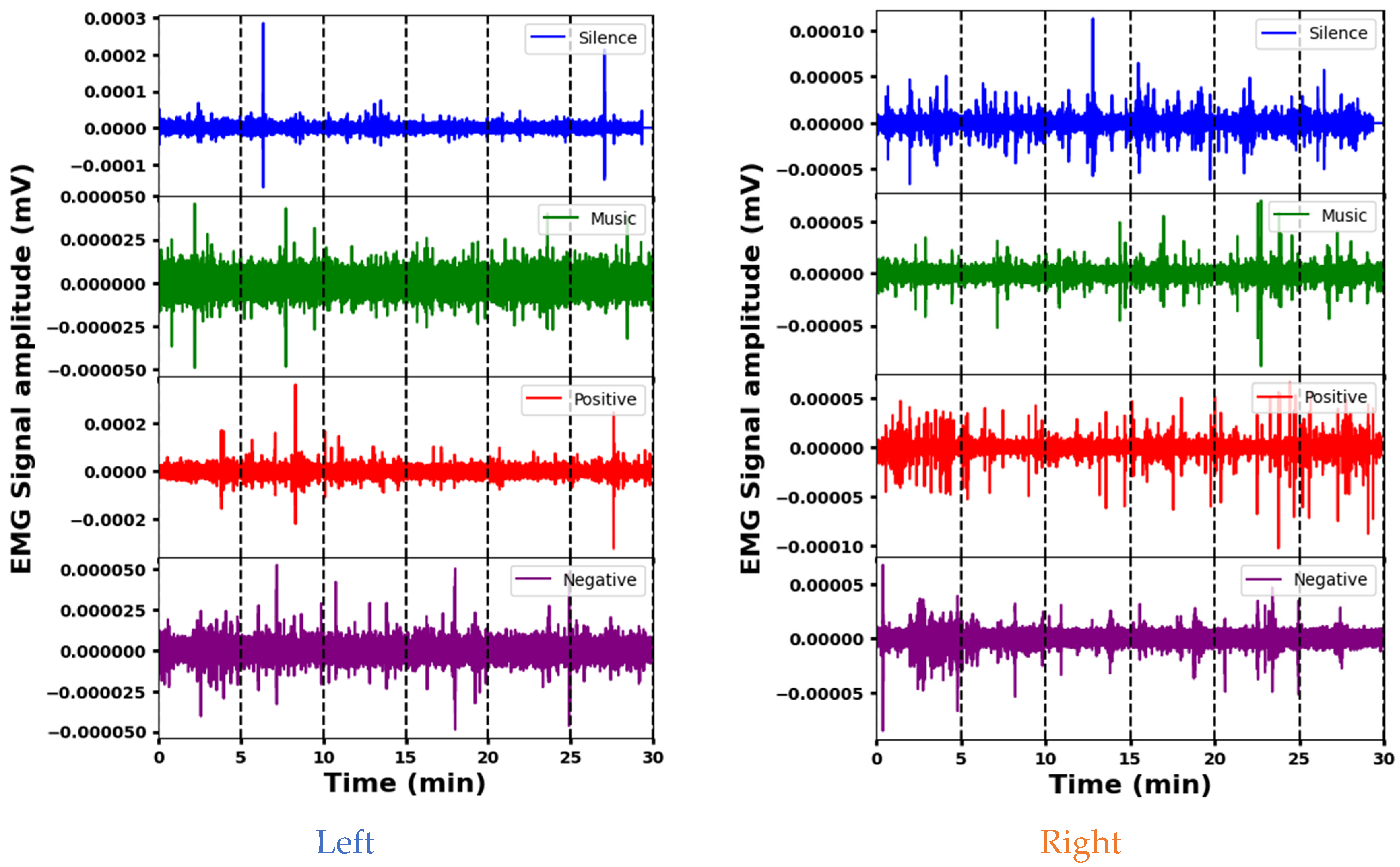
3.1. Representative EMG Signals during Four Psychological Interventions
3.2. Post Hoc Analysis of Extracted Features across Four Muscle Sites
3.2.1. Analysis of Sternocleidomastoid Muscle (SCM)
3.2.2. Analysis of Cervical Erector Muscle (CEM)
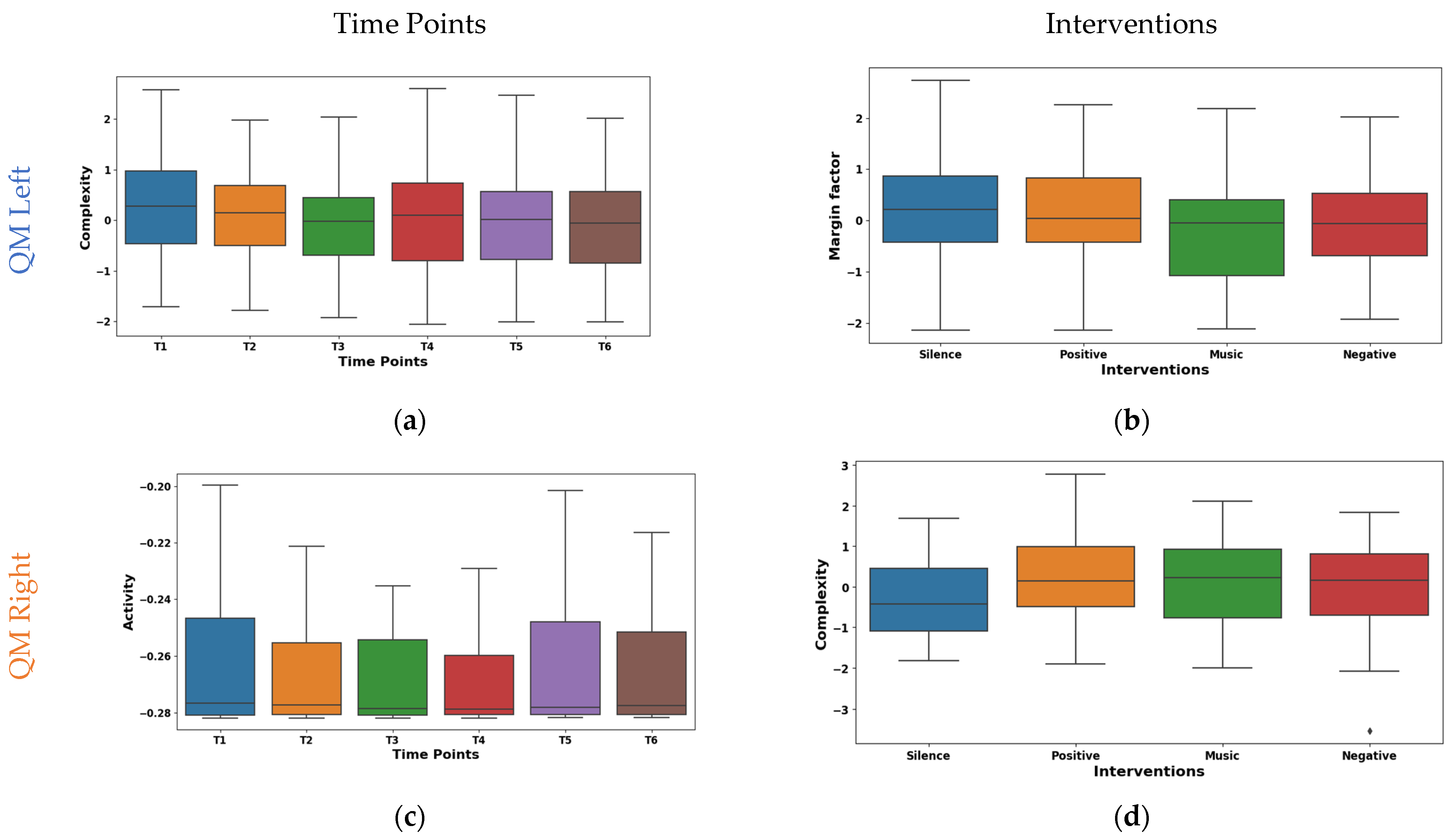
3.2.3. Analysis of Quad Muscle (QM)
3.2.4. Analysis of Tibialis Muscle (TM)
3.3. Classification of Psychological Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuna, J.M., Jr.; Tudor-Locke, C. Step by Step: Accumulated Knowledge and Future Directions of Step-Defined Ambulatory Activity. Res. Exerc. Epidemiol. 2012, 14, 107–116. [Google Scholar]
- Winter, D.A. Energy Generation and Absorption at the Ankle and Knee during Fast, Natural, and Slow Cadences. Clin. Orthop. Relat. Res. 1983, 14, 147–154. [Google Scholar] [CrossRef]
- Godde, B.; Voelcker-Rehage, C. Cognitive Resources Necessary for Motor Control in Older Adults Are Reduced by Walking and Coordination Training. Front. Hum. Neurosci. 2017, 11, 156. [Google Scholar] [CrossRef]
- Bogey, R.A.; Barnes, L.A.; Perry, J. Computer Algorithms to Characterize Individual Subject EMG Profiles during Gait. Arch. Phys. Med. Rehabil. 1992, 73, 835–841. [Google Scholar] [PubMed]
- Ford, M.P.; Wagenaar, R.C.; Newell, K.M. Arm Constraint and Walking in Healthy Adults. Gait Posture 2007, 26, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, L.; Rivellini, G.; Figura, F. EMG Patterns during Running: Intra-and Inter-Individual Variability. J. Electromyogr. Kinesiol. 1996, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, E.B.; Alkjær, T. The Variability Problem of Normal Human Walking. Med. Eng. Phys. 2012, 34, 219–224. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Masiello, D.; Rossi, S. Factorization of EMG via Muscle Synergies in Walking Task: Evaluation of Intra-Subject and Inter-Subject Variability. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Turin, Italy, 22–25 May 2017; pp. 1–6. [Google Scholar]
- Chang, K.-M.; Liu, S.-H.; Wu, X.-H. A Wireless sEMG Recording System and Its Application to Muscle Fatigue Detection. Sensors 2012, 12, 489–499. [Google Scholar] [CrossRef]
- Kyeong, S.; Shin, W.; Kim, J. Predicting Walking Intentions Using sEMG and Mechanical Sensors for Various Environment. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4414–4417. [Google Scholar]
- Saraiva, M.; Castro, M.A.; Vilas-Boas, J.P. Influence of Cognitive and Motor Tasks Using Smartphone during Gait: EMG and Gait Performance Analysis–Dual-Task Study. Hum. Mov. Sci. 2023, 89, 103097. [Google Scholar] [CrossRef]
- Waldon, K.T.; Stout, A.; Manning, K.; Gray, L.; Wilson, D.G.; Kang, G.E. Dual-Task Interference Effects on Lower-Extremity Muscle Activities during Gait Initiation and Steady-State Gait among Healthy Young Individuals, Measured Using Wireless Electromyography Sensors. Sensors 2023, 23, 8842. [Google Scholar] [CrossRef]
- Pitts, J.; Singhal, K.; Apte, Y.; Patel, P.; Kannan, L.; Bhatt, T. The Effect of Cognitive Task, Gait Speed, and Age on Cognitive–Motor Interference during Walking. Sensors 2023, 23, 7368. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, R.; Del Din, S.; Donaghy, P.; Galna, B.; Thomas, A.J.; Rochester, L. The Impact of Environment on Gait Assessment: Considerations from Real-World Gait Analysis in Dementia Subtypes. Sensors 2021, 21, 813. [Google Scholar] [CrossRef] [PubMed]
- Camp, N.; Vagnetti, R.; Bisele, M.; Felton, P.; Hunter, K.; Magistro, D. The Effect of Cognitive Task Complexity on Healthy Gait in the Walking Corsi Test. Brain Sci. 2023, 13, 1019. [Google Scholar] [CrossRef]
- Feldman, R.; Schreiber, S.; Pick, C.G.; Been, E. Gait, Balance, Mobility and Muscle Strength in People with Anxiety Compared to Healthy Individuals. Hum. Mov. Sci. 2019, 67, 102513. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.; Huang, H.; Yu, L.-F.; Martin, R.; McCarthy, R.; Locke, E.; Yager, C.; Torad, A.A.; Kadry, A.M.; Elwan, M.A. Identifying Individuals Who Currently Report Feelings of Anxiety Using Walking Gait and Quiet Balance: An Exploratory Study Using Machine Learning. Sensors 2022, 22, 3163. [Google Scholar] [CrossRef] [PubMed]
- Belvederi Murri, M.; Triolo, F.; Coni, A.; Tacconi, C.; Nerozzi, E.; Escelsior, A.; Respino, M.; Neviani, F.; Bertolotti, M.; Bertakis, K.; et al. Instrumental Assessment of Balance and Gait in Depression: A Systematic Review. Psychiatry Res. 2020, 284, 112687. [Google Scholar] [CrossRef] [PubMed]
- Boolani, A.; Gruber, A.H.; Torad, A.A.; Stamatis, A. Identifying Current Feelings of Mild and Moderate to High Depression in Young, Healthy Individuals Using Gait and Balance: An Exploratory Study. Sensors 2023, 23, 6624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, Z.; Wang, Y.; Wang, J.; Li, B.; Zhu, T.; Xiang, Y. See Your Mental State from Your Walk: Recognizing Anxiety and Depression through Kinect-Recorded Gait Data. PLoS ONE 2019, 14, e0216591. [Google Scholar] [CrossRef]
- Vilmeau, S.R.; Boolani, A.; Papadakis, Z. Identifying Anger through Gait and Balance Analysis Using Machine Learning Models. Int. J. Exerc. Sci. Conf. Proc. 2023, 15, 5. [Google Scholar]
- Barbieri, F.A.; Dos Santos, P.C.R.; Lirani-Silva, E.; Vitório, R.; Gobbi, L.T.B.; Van Diëen, J.H. Systematic Review of the Effects of Fatigue on Spatiotemporal Gait Parameters. J. Back Musculoskelet. Rehabil. 2013, 26, 125–131. [Google Scholar] [CrossRef]
- Behrens, M.; Mau-Moeller, A.; Lischke, A.; Katlun, F.; Gube, M.; Zschorlich, V.; Skripitz, R.; Weippert, M. Mental Fatigue Increases Gait Variability during Dual-Task Walking in Old Adults. J. Gerontol. Ser. A 2017, 73, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Grobe, S.; Kakar, R.S.; Smith, M.L.; Mehta, R.; Baghurst, T.; Boolani, A. Impact of Cognitive Fatigue on Gait and Sway among Older Adults: A Literature Review. Prev. Med. Rep. 2017, 6, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Kadry, A.M.; Torad, A.; Elwan, M.A.; Kakar, R.S.; Bradley, D.; Chaudhry, S.; Boolani, A. Using Machine Learning to Identify Feelings of Energy and Fatigue in Single-Task Walking Gait: An Exploratory Study. Appl. Sci. 2022, 12, 3083. [Google Scholar] [CrossRef]
- Rumsey, H.E.; Aggarwal, S.; Hobson, E.M.; Park, J.; Pidcoe, P. Anxiety’s Effect on Muscle Activation and Fatigue in Trumpet Players: A Pilot Study. Med. Probl. Perform. Artist. 2015, 30, 203–210. [Google Scholar] [CrossRef] [PubMed]
- D’Attilio, M.; Rodolfino, D.; Saccucci, M.; Abate, M.; Romani, G.L.; Festa, F.; Merla, A. Effects of Viewing Affective Pictures on sEMG Activity of Masticatory and Postural Muscles. Neurosci. Lett. 2013, 544, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Boolani, A.; Lackman, J.; Baghurst, T.; LaRue, J.L.; Smith, M.L. Impact of Positive and Negative Motivation and Music on Jump Shot Efficiency among NAIA Division I College Basketball Players. Int. J. Exerc. Sci. 2019, 12, 100. [Google Scholar] [PubMed]
- Pilcher, J.J.; Huffcutt, A.I. Effects of Sleep Deprivation on Performance: A Meta-Analysis. Sleep 1996, 19, 318–326. [Google Scholar] [CrossRef]
- Boolani, A.; Martin, J.; Huang, H.; Yu, L.-F.; Stark, M.; Grin, Z.; Roy, M.; Yager, C.; Teymouri, S.; Bradley, D.; et al. Association between Self-Reported Prior Night’s Sleep and Single-Task Gait in Healthy, Young Adults: A Study Using Machine Learning. Sensors 2022, 22, 7406. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Ertuğrul, Ö.F. Estimation of Neurological Status from Non-Electroencephalography Bio-Signals by Motif Patterns. Appl. Soft Comput. 2019, 83, 105609. [Google Scholar] [CrossRef]
- Veeranki, Y.R.; Ganapathy, N.; Swaminathan, R. Analysis of Fluctuation Patterns in Emotional States Using Electrodermal Activity Signals and Improved Symbolic Aggregate Approximation. Fluct. Noise Lett. 2021, 21, 2250013. [Google Scholar] [CrossRef]
- Wannawijit, I.; Kaiwansil, S.; Ruthaisujaritkul, S.; Yingthawornsuk, T. ECG Classification with Modification of Higher-Order Hjorth Descriptors. In Proceedings of the 2019 15th International Conference on Signal-Image Technology & Internet-Based Systems (SITIS), Sorrento, Italy, 26–29 November 2019; pp. 564–571. [Google Scholar]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 6th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007; ISBN 978-0-13-187715-3. [Google Scholar]
- Reinhart, A. Statistics Done Wrong: The Woefully Complete Guide, 1st ed.; No Starch Press: San Francisco, CA, USA, 2015; ISBN 978-1-59327-620-1. [Google Scholar]
- Keren, G.; Lewis, C. (Eds.) A Handbook for Data Analysis in the Behavioral Sciences: Statistical Issues; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1993; ISBN 978-0-8058-1093-6. [Google Scholar]
- ThriftBooks Experimental Design: Procedures for Book by Roger E. Kirk. Available online: https://www.thriftbooks.com/w/experimental-design-procedures-for-behavioral-sciences-psychology_roger-e-kirk/633045/ (accessed on 22 January 2024).
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Veeranki, Y.R.; McNaboe, R.; Posada-Quintero, H.F. EEG-Based Seizure Detection Using Variable-Frequency Complex Demodulation and Convolutional Neural Networks. Signals 2023, 4, 816–835. [Google Scholar] [CrossRef]
- Frigo, C.; Crenna, P. Multichannel SEMG in Clinical Gait Analysis: A Review and State-of-the-Art. Clin. Biomech. 2009, 24, 236–245. [Google Scholar] [CrossRef]
- Hase, K.; Stein, R.B. Turning Strategies During Human Walking. J. Neurophysiol. 1999, 81, 2914–2922. [Google Scholar] [CrossRef] [PubMed]






| Characteristic | N |
|---|---|
| Total subjects | 36 |
| Excluded subjects | 10 |
| Gender | 16 females |
| Height | 167.32 ± 20.20 cm |
| Weight | 69.93 ± 9.76 kg |
| Age | 23.04 ± 5.64 years |
| Positive Reinforcement | Negative Reinforcement |
|---|---|
| Good job, you’re doing awesome! | You’ve got to walk faster than that. |
| Keep up the good work! | You’re so slow! |
| You’ve got this! | Why do you walk like that? |
| You’re almost done, just a few more minutes! | Did you learn how to walk yesterday? |
| That’s a great pace! | You’re doing terrible. |
| You’re going strong! Keep it up! | Who walks like that? |
| Nice work. | You have potential but you don’t use it. |
| Great job! | You’ll never amount to anything. |
| Good stuff. Keep it up. | You’re not putting very much effort into this. |
| You’re doing an amazing job. | This is the worst pace you’ve had yet. |
| Feature | Expression | Description |
|---|---|---|
| Coefficient of variance (CV) [31] | Measures relative variability, indicating how spread out values are compared to the mean. | |
| Shape factor (SF) [31] | Captures signal shape characteristics, often reflecting muscle contraction patterns. | |
| Margin factor (MF) [31] | Quantifies signal margin, representing the proportion of time a signal is active. | |
| Dynamic range (DR) [32] | Reflects the signal’s amplitude range, capturing its highest and lowest values. | |
| Max amplitude (MA) [32] | Represents the signal’s maximum amplitude, indicating its peak intensity. | |
| Activity (AC) [33] | Quantifies muscle activation intensity. | |
| Mobility (MO) [33] | Indicates muscle activation changes and movement. | |
| Complexity (CO) [33] | Combines activity and mobility indicators. | |
| Chaos (CH) [33] | Assesses irregular muscle activation patterns. | |
| Hazard (HZ) [33] | Measures sudden muscle activation shifts. |
| Metric | Measure |
|---|---|
| Acc | Overall performance of the model in correctly classifying both positive and negative cases. |
| Pre | The model’s ability to avoid false positives, ensuring that positive predictions are highly likely to be correct. |
| Rec | The model’s ability to detect true positives, ensuring that most actual positive cases are captured. |
| F1 | A balanced assessment of both precision and recall, capturing both the model’s ability to avoid false positives and its ability to detect true positives. |
| Spe | The model’s ability to avoid false negatives, ensuring that negative predictions are highly likely to be correct. |
| AUC | The model’s ability to distinguish between classes across all possible thresholds, representing its overall discriminative power. |
| Timepoint Pairs | Intervention Pairs | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pairs | T1 & T2 | T1 & T3 | T1 & T4 | T1 & T5 | T1 & T6 | T2 & T3 | T2 & T4 | T2 & T5 | T2 & T6 | T3 & T4 | T3 & T5 | T3 & T6 | T4 & T5 | T4 & T6 | T5 & T6 | M & N | M & P | M & S | N & P | N & S | P & S | |
| Features | ||||||||||||||||||||||
| CV | ||||||||||||||||||||||
| SF | L | L | ||||||||||||||||||||
| MF | ||||||||||||||||||||||
| DR | ||||||||||||||||||||||
| MA | ||||||||||||||||||||||
| AC | ||||||||||||||||||||||
| MO | R | R | R | R | R | R | R | R | R | R | R | |||||||||||
| CO | R | R | R | R | R | R | R | R | R | R | R | |||||||||||
| CH | R | R | R | R | R | R | R | R | R | R | R | |||||||||||
| HZ | R | R | R | R | R | R | R | R | R | |||||||||||||
| Timepoint Pairs | Intervention Pairs | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pairs | T1 & T2 | T1 & T3 | T1 & T4 | T1 & T5 | T1 & T6 | T2 & T3 | T2 & T4 | T2 & T5 | T2 & T6 | T3 & T4 | T3 & T5 | T3 & T6 | T4 & T5 | T4 & T6 | T5 & T6 | M & N | M & P | M & S | N & P | N & S | P & S | |
| Features | ||||||||||||||||||||||
| CV | ||||||||||||||||||||||
| SF | ||||||||||||||||||||||
| MF | ||||||||||||||||||||||
| DR | L | L | ||||||||||||||||||||
| MA | L | L | ||||||||||||||||||||
| AC | ||||||||||||||||||||||
| MO | L | |||||||||||||||||||||
| CO | R | R | R | |||||||||||||||||||
| CH | ||||||||||||||||||||||
| HZ | ||||||||||||||||||||||
| Timepoint Pairs | Intervention Pairs | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pairs | T1 & T2 | T1 & T3 | T1 & T4 | T1 & T5 | T1 & T6 | T2 & T3 | T2 & T4 | T2 & T5 | T2 & T6 | T3 & T4 | T3 & T5 | T3 & T6 | T4 & T5 | T4 & T6 | T5 & T6 | M & N | M & P | M & S | N & P | N & S | P & S | |
| Features | ||||||||||||||||||||||
| CV | ||||||||||||||||||||||
| SF | ||||||||||||||||||||||
| MF | L | |||||||||||||||||||||
| DR | ||||||||||||||||||||||
| MA | ||||||||||||||||||||||
| AC | ||||||||||||||||||||||
| MO | ||||||||||||||||||||||
| CO | L | L | L | L | L | L | R | |||||||||||||||
| CH | L | L | L | |||||||||||||||||||
| HZ | ||||||||||||||||||||||
| Timepoint Pairs | Intervention Pairs | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pairs | T1 & T2 | T1 & T3 | T1 & T4 | T1 & T5 | T1 & T6 | T2 & T3 | T2 & T4 | T2 & T5 | T2 & T6 | T3 & T4 | T3 & T5 | T3 & T6 | T4 & T5 | T4 & T6 | T5 & T6 | M & N | M & P | M & S | N & P | N & S | P & S | |
Features | ||||||||||||||||||||||
| CV | L | L R | L | L R | R | L R | L | L R | R | |||||||||||||
| SF | R | R | R | R | R | R | ||||||||||||||||
| MF | ||||||||||||||||||||||
| DR | R | R | R | R | ||||||||||||||||||
| MA | R | R | ||||||||||||||||||||
| AC | R | R | R | R | R | |||||||||||||||||
| MO | L R | L R | L R | L R | L R | R | R | L R | L R | R | R | R | R | L R | ||||||||
| CO | L R | L R | L R | L R | L R | R | R | L R | L R | R | R | R | R | R | ||||||||
| CH | L R | L R | L R | L R | L R | L R | L R | L R | L R | R | L R | L R | R | L R | ||||||||
| HZ | L R | L R | L R | L R | L R | R | R | L R | L R | R | R | R | R | R | ||||||||
| Accuracy | Precision | Recall | F1-Score | Specificity | AUC | ||
|---|---|---|---|---|---|---|---|
| SCM | Left | 39.20 | 44.09 | 39.20 | 40.09 | 53.33 | 65.66 |
| Right | 41.60 | 46.67 | 41.60 | 42.38 | 57.14 | 65.23 | |
| CEM | Left | 56.80 | 59.55 | 56.80 | 57.26 | 82.35 | 77.40 |
| Right | 37.60 | 39.41 | 37.60 | 37.70 | 75.00 | 63.10 | |
| QM | Left | 46.40 | 47.82 | 46.40 | 46.77 | 70.59 | 74.98 |
| Right | 32.00 | 35.04 | 32.00 | 32.55 | 69.23 | 64.66 | |
| TM | Left | 63.20 | 64.42 | 63.20 | 63.34 | 75.00 | 84.61 |
| Right | 60.80 | 66.36 | 60.80 | 61.30 | 73.68 | 84.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeranki, Y.R.; Garcia-Retortillo, S.; Papadakis, Z.; Stamatis, A.; Appiah-Kubi, K.O.; Locke, E.; McCarthy, R.; Torad, A.A.; Kadry, A.M.; Elwan, M.A.; et al. Detecting Psychological Interventions Using Bilateral Electromyographic Wearable Sensors. Sensors 2024, 24, 1425. https://doi.org/10.3390/s24051425
Veeranki YR, Garcia-Retortillo S, Papadakis Z, Stamatis A, Appiah-Kubi KO, Locke E, McCarthy R, Torad AA, Kadry AM, Elwan MA, et al. Detecting Psychological Interventions Using Bilateral Electromyographic Wearable Sensors. Sensors. 2024; 24(5):1425. https://doi.org/10.3390/s24051425
Chicago/Turabian StyleVeeranki, Yedukondala Rao, Sergi Garcia-Retortillo, Zacharias Papadakis, Andreas Stamatis, Kwadwo Osei Appiah-Kubi, Emily Locke, Ryan McCarthy, Ahmed Ali Torad, Ahmed Mahmoud Kadry, Mostafa Ali Elwan, and et al. 2024. "Detecting Psychological Interventions Using Bilateral Electromyographic Wearable Sensors" Sensors 24, no. 5: 1425. https://doi.org/10.3390/s24051425
APA StyleVeeranki, Y. R., Garcia-Retortillo, S., Papadakis, Z., Stamatis, A., Appiah-Kubi, K. O., Locke, E., McCarthy, R., Torad, A. A., Kadry, A. M., Elwan, M. A., Boolani, A., & Posada-Quintero, H. F. (2024). Detecting Psychological Interventions Using Bilateral Electromyographic Wearable Sensors. Sensors, 24(5), 1425. https://doi.org/10.3390/s24051425












