ZnO Decorated Graphene-Based NFC Tag for Personal NO2 Exposure Monitoring during a Workday
Abstract
1. Introduction
2. Experimental
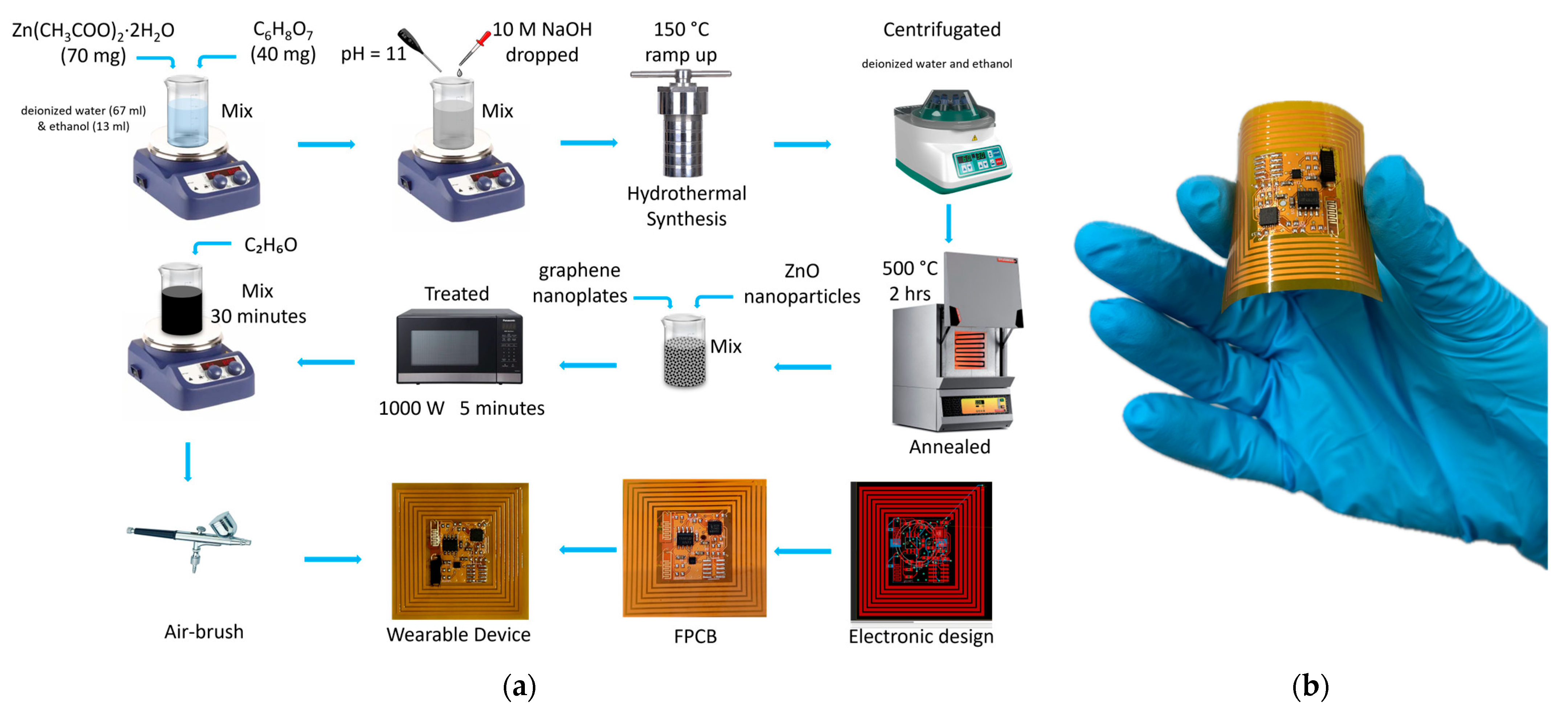
2.1. Wearable NFC Tag System Preparation
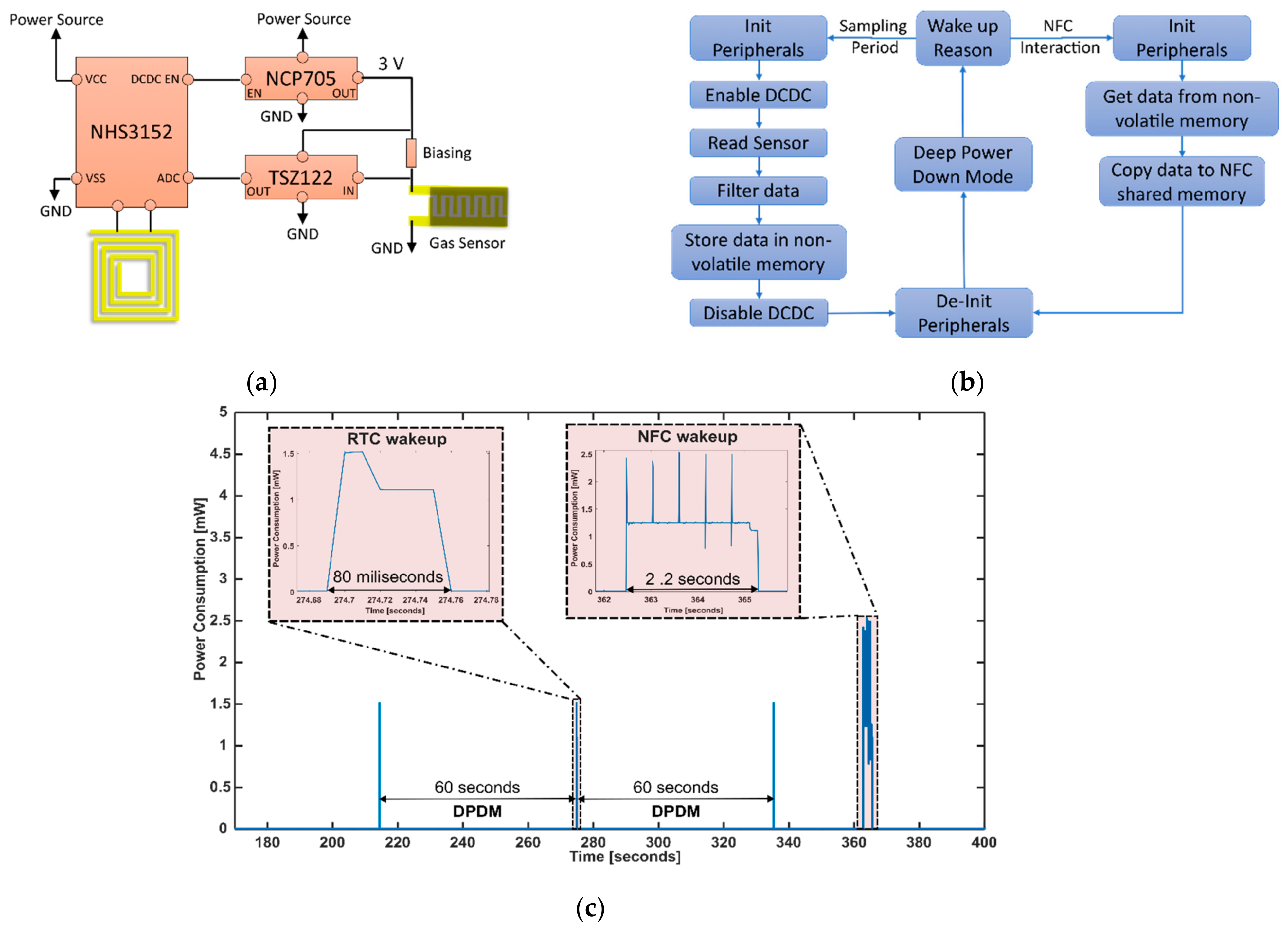
2.2. Electronic and Firmware Design
2.3. Active Layer Characterization
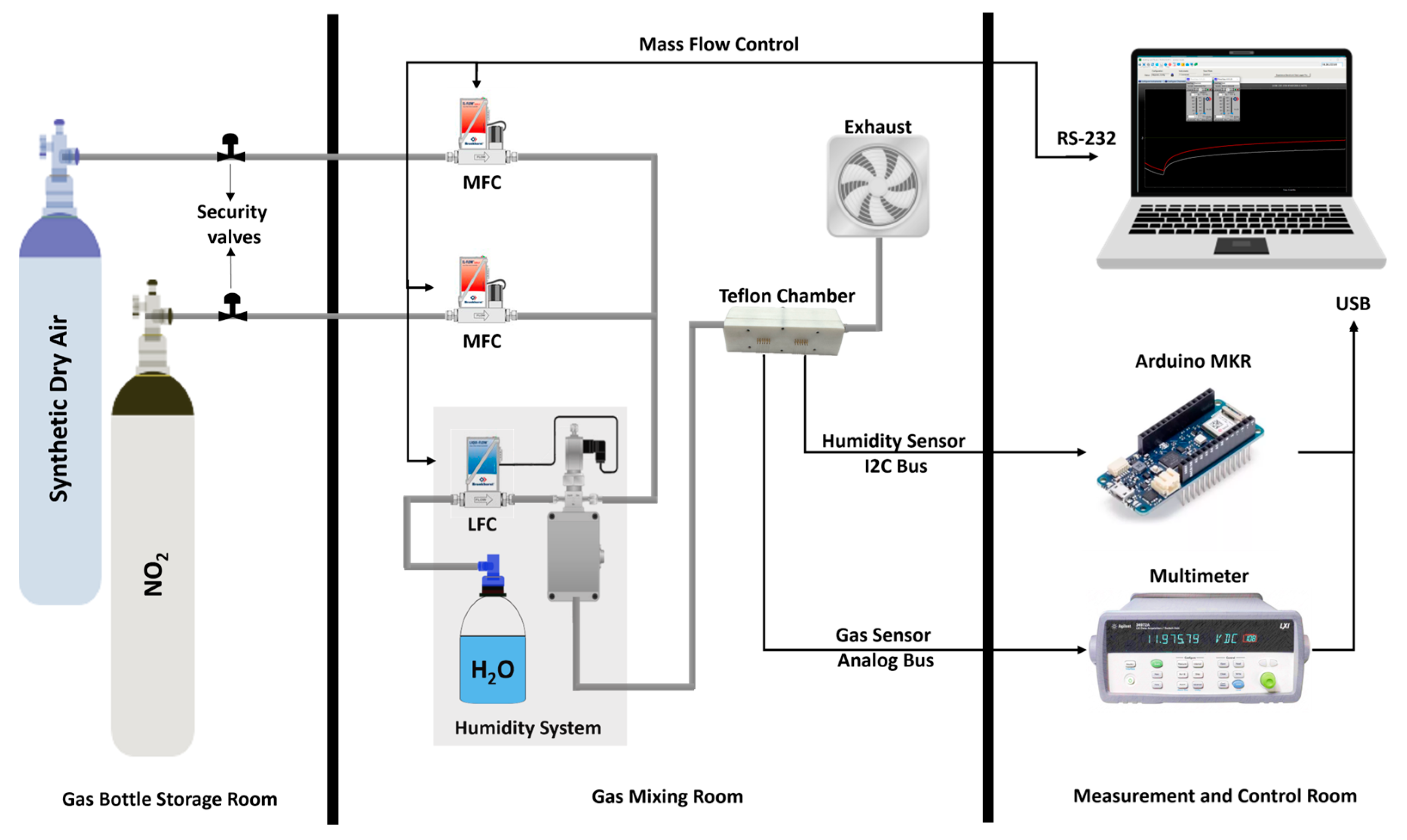
2.4. Gas Measurement System
3. Results and Discussion
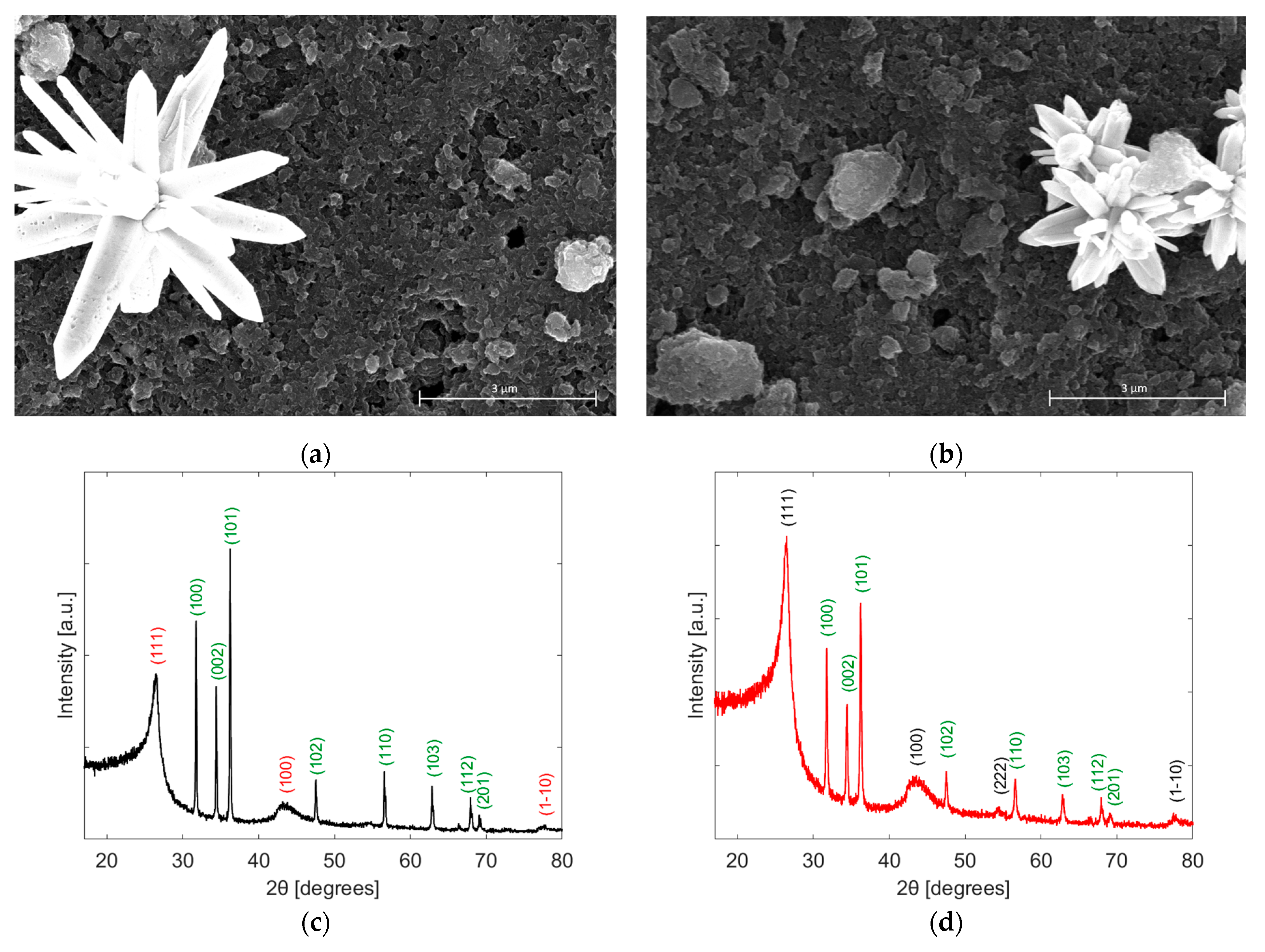
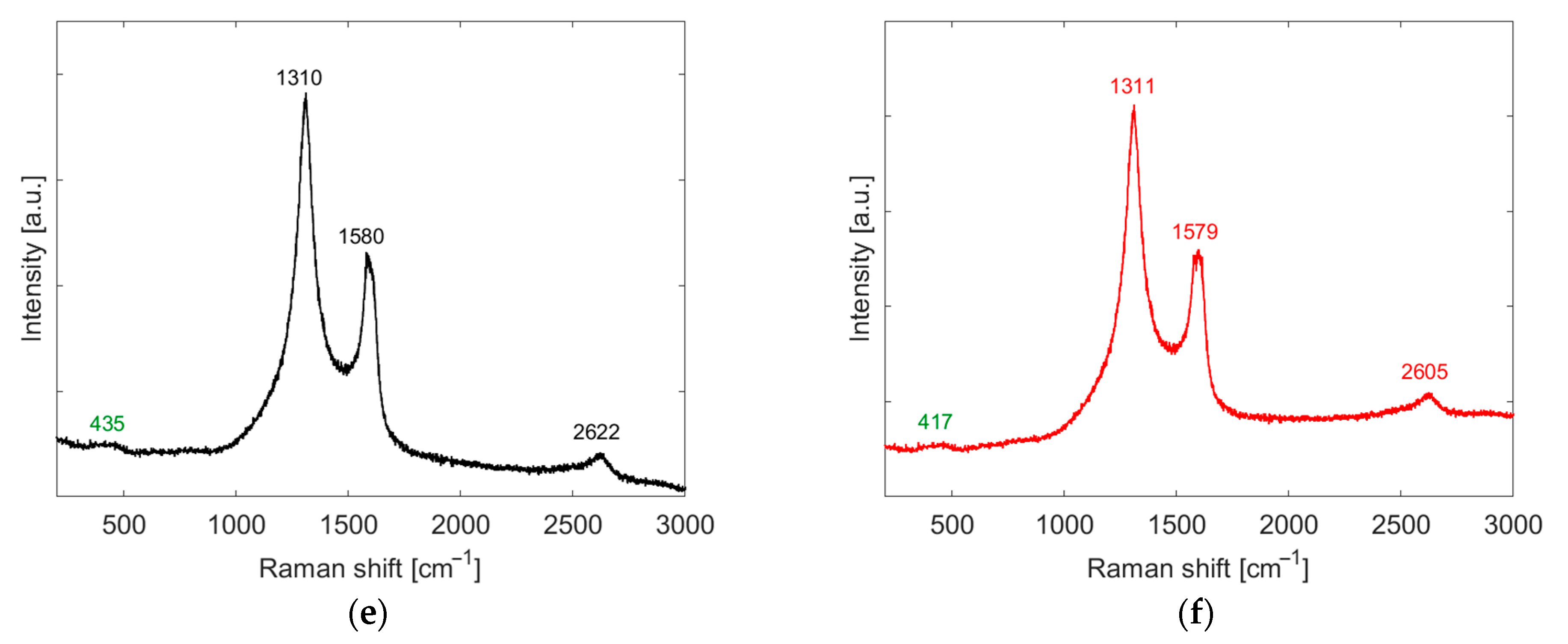
3.1. Active Layer Characterization
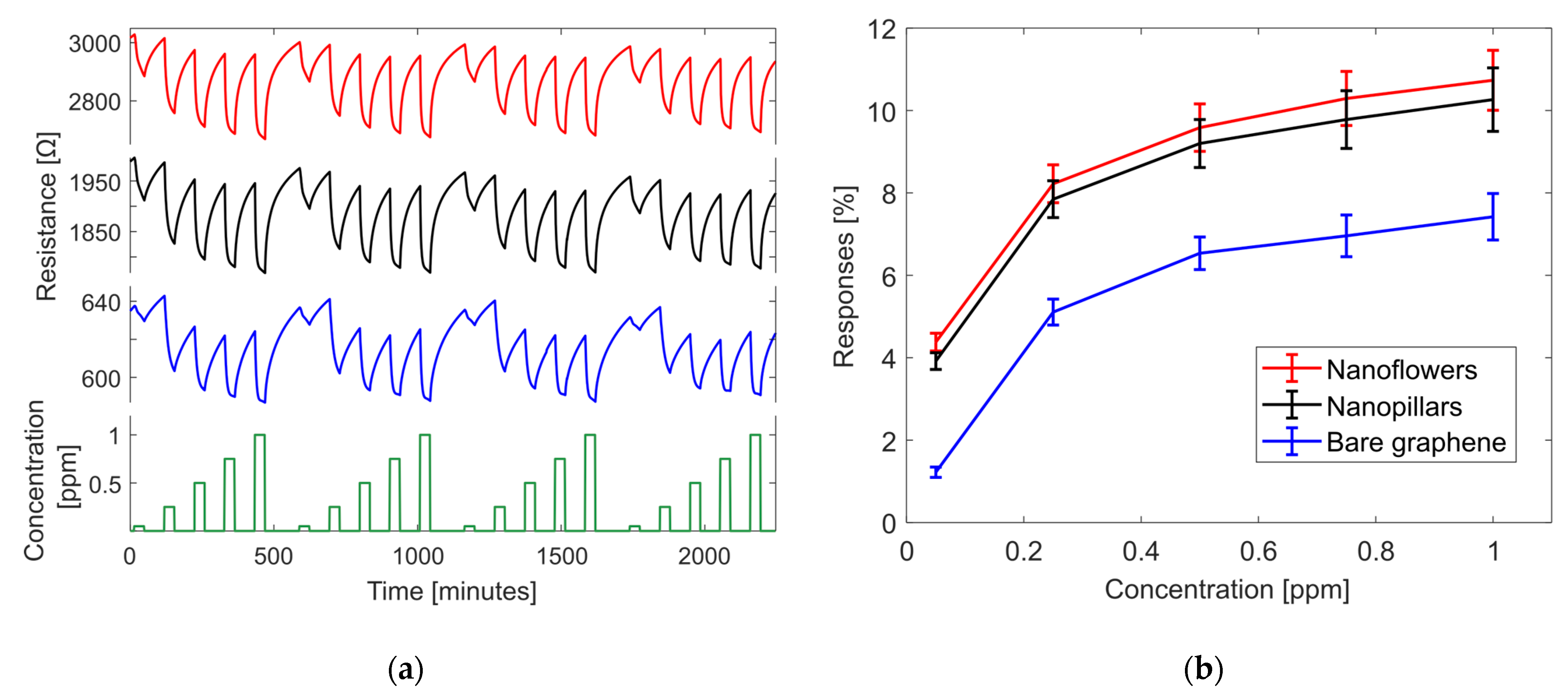
3.2. Gas Sensing Results
3.3. Sensing Mechanism to NO2
3.4. Gas Measurement Using NFC Tag System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitrogen Dioxide|American Lung Association. Available online: https://www.lung.org/clean-air/outdoors/what-makes-air-unhealthy/nitrogen-dioxide (accessed on 3 October 2023).
- Basic Information about NO2|US EPA. Available online: https://www.epa.gov/no2-pollution/basic-information-about-no2 (accessed on 27 September 2023).
- Air Quality and Health. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts/types-of-pollutants (accessed on 3 October 2023).
- Carbone, U.; Montuori, P.; Novi, C.; Triassi, M. Respiratory Function in Power Plant Workers Exposed to Nitrogen Dioxide. Occup. Med. (Chic Ill) 2014, 64, 644–646. [Google Scholar] [CrossRef]
- Plato, N.; Bigert, C.; Larsson, B.-M.; Alderling, M.; Svartengren, M.; Gustavsson, P. Exposure to Particles and Nitrogen Dioxide Among Workers in the Stockholm Underground Train System. Saf. Health Work 2019, 10, 377–383. [Google Scholar] [CrossRef]
- Salonen, H.; Salthammer, T.; Morawska, L. Human Exposure to NO2 in School and Office Indoor Environments. Environ. Int. 2019, 130, 104887. [Google Scholar] [CrossRef]
- Dahmann, D.; Morfeld, P.; Monz, C.; Noll, B.; Gast, F. Exposure Assessment for Nitrogen Oxides and Carbon Monoxide in German Hard Coal Mining. Int. Arch Occup. Environ. Health 2009, 82, 1267–1279. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Sasmito, A.P.; Wong, W.Y.; Mujumdar, A.S. Prediction and Innovative Control Strategies for Oxygen and Hazardous Gases from Diesel Emission in Underground Mines. Sci. Total Environ. 2014, 481, 317–334. [Google Scholar] [CrossRef]
- Homepage—ECHA. Available online: https://echa.europa.eu/ (accessed on 3 October 2023).
- Park, N.-H.; Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Calorimetric Thermoelectric Gas Sensor for the Detection of Hydrogen, Methane and Mixed Gases. Sensors 2014, 14, 8350–8362. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent Advances in Carbon Material-Based NO2 Gas Sensors. Sens. Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide. Nanomicro Lett. 2021, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.; Rao, V.K.; Gupta, M.; Murthy, K.S.R.C.; Jain, R. Chemiresistive Gas Sensor for the Sensitive Detection of Nitrogen Dioxide Based on Nitrogen Doped Graphene Nanosheets. RSC Adv. 2016, 6, 1527–1534. [Google Scholar] [CrossRef]
- Ibañez, F.J.; Zamborini, F.P. Chemiresistive Sensing with Chemically Modified Metal and Alloy Nanoparticles. Small 2012, 8, 174–202. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.L. Metal Oxides. Metals 2020, 10, 820. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal Oxide Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting Metal Oxide Sensor Array for the Selective Detection of Combustion Gases. Sens. Actuators B Chem. 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Meixner, H.; Lampe, U. Metal Oxide Sensors. Sens. Actuators B Chem. 1996, 33, 198–202. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Xu, J.; Debliquy, M. Room Temperature Conductive Type Metal Oxide Semiconductor Gas Sensors for NO2 Detection. Sens. Actuators A Phys. 2019, 289, 118–133. [Google Scholar] [CrossRef]
- Drewniak, S.; Drewniak, Ł.; Pustelny, T. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review. Sensors 2022, 22, 5316. [Google Scholar] [CrossRef]
- Demon, S.Z.N.; Kamisan, A.I.; Abdullah, N.; Noor, S.A.M.; Khim, O.K.; Kasim, N.A.M.; Yahya, M.Z.A.; Manaf, N.A.A.; Azmi, A.F.M.; Halim, N.A. Graphene-Based Materials in Gas Sensor Applications: A Review. Sens. Mater. 2020, 32, 759. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Y.; Debliquy, M.; Zhang, C. Graphene-Enhanced Metal Oxide Gas Sensors at Room Temperature: A Review. Beilstein J. Nanotechnol. 2018, 9, 2832–2844. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, H.; Wang, C.; Pan, S.; He, J.; Liu, A.; Wang, J.; Sun, P.; Liu, F.; Lu, G. Room Temperature Wearable Gas Sensors for Fabrication and Applications. Adv. Sens. Res. 2023, 2300035. [Google Scholar] [CrossRef]
- Alouani, M.A.; Casanova-Cháfer, J.; Güell, F.; Peña-Martín, E.; Ruiz-Martínez-Alcocer, S.; de Bernardi-Martín, S.; García-Gómez, A.; Vilanova, X.; Llobet, E. ZnO-Loaded Graphene for NO2 Gas Sensing. Sensors 2023, 23, 6055. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Pumera, M. Wearable Sensors for Telehealth Based on Emerging Materials and Nanoarchitectonics. NPJ Flex. Electron. 2023, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shi, N.; Jiang, X.; Chen, M.; Jiang, J.; Zheng, Y.; Wu, W.; Cui, D.; Haick, H.; Tang, N. Techniques for Wearable Gas Sensors Fabrication. Sens. Actuators B Chem. 2022, 353, 131133. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A Review on Flexible Gas Sensors: From Materials to Devices. Sens. Actuators A Phys. 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping Healthcare with Wearable Biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, T.; Han, Y.; Lv, W.; Li, B.; Su, C.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; et al. Wearable NO2 Sensing and Wireless Application Based on ZnS Nanoparticles/Nitrogen-Doped Reduced Graphene Oxide. Sens. Actuators B Chem. 2021, 345, 130423. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, Q.; Han, F.; Wang, Z.; Tian, B.; Zhao, L.; Dong, T.; Jiang, Z. A Flexible and Wearable NO2 Gas Detection and Early Warning Device Based on a Spraying Process and an Interdigital Electrode at Room Temperature. Microsyst. Nanoeng. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Ha, G.; Wright, D.E.; Ma, Y.; Sen-Gupta, E.; Haubrich, N.R.; Branche, P.C.; Li, W.; Huppert, G.L.; Johnson, M.; et al. Highly Flexible, Wearable, and Disposable Cardiac Biosensors for Remote and Ambulatory Monitoring. NPJ Digit. Med. 2018, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Kim, H.-J.; Achavananthadith, S.; Kurt, S.A.; Tan, S.C.C.; Yao, H.; Tee, B.C.K.; Lee, J.K.W.; Ho, J.S. Wireless Battery-Free Body Sensor Networks Using near-Field-Enabled Clothing. Nat. Commun. 2020, 11, 444. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, P.; Ma, Z.; Li, S.; Gao, X.; Wu, R.; Pan, L.; Shi, Y. Near-Field Communication Sensors. Sensors 2019, 19, 3947. [Google Scholar] [CrossRef]
- Olenik, S.; Lee, H.S.; Güder, F. The Future of Near-Field Communication-Based Wireless Sensing. Nat. Rev. Mater 2021, 6, 286–288. [Google Scholar] [CrossRef]
- Escobedo, P.; Fernández-Ramos, M.D.; López-Ruiz, N.; Moyano-Rodríguez, O.; Martínez-Olmos, A.; Pérez de Vargas-Sansalvador, I.M.; Carvajal, M.A.; Capitán-Vallvey, L.F.; Palma, A.J. Smart Facemask for Wireless CO2 Monitoring. Nat. Commun. 2022, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, Q.; Kou, H.; Wu, D.; Zhang, W.; Xiong, J. Highly Sensitive NH3 Wireless Sensor Based on Ag-RGO Composite Operated at Room-Temperature. Sci. Rep. 2019, 9, 9942. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, P.; Erenas, M.M.; López-Ruiz, N.; Carvajal, M.A.; Gonzalez-Chocano, S.; de Orbe-Payá, I.; Capitán-Valley, L.F.; Palma, A.J.; Martínez-Olmos, A. Flexible Passive near Field Communication Tag for Multigas Sensing. Anal. Chem. 2017, 89, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Q.; Lu, Y.; Liu, L.; Ji, D.; Li, S.; Liu, Q. Passive and Wireless near Field Communication Tag Sensors for Biochemical Sensing with Smartphone. Sens. Actuators B Chem. 2017, 246, 748–755. [Google Scholar] [CrossRef]
- Salehnia, F.; Lazaro, A.; Villarino, R.; Lazaro, M.; Canyellas, N.; Vilanova, X.; Llobet, E.; Girbau, D. Battery-Free NFC Sub-ppm Gas Sensor for Distributed Gas Monitoring Applications at Room Temperature. IEEE J. Radio Freq. Identif. 2023, 7, 630–643. [Google Scholar] [CrossRef]
- NXP Semiconductors NFC Antenna Design Tool | Antenna Design Hub | NXP Semiconductors. Available online: https://www.nxp.com/products/rfid-nfc/nfc-hf/nfc-readers/nfc-antenna-design-hub:NFC-ANTENNA-DESIGN-TOOL (accessed on 30 November 2023).
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent Advances in Graphene-Based Humidity Sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Alfano, B.; Massera, E.; Polichetti, T.; Miglietta, M.L.; Di Francia, G. Effect of Humidity on the Hydrogen Sensing in Graphene Based Devices. In Lecture Notes in Electrical Engineering; Springer: Berlin, Germany, 2019; Volume 539, pp. 11–16. [Google Scholar] [CrossRef]
- Smith, A.D.; Elgammal, K.; Fan, X.; Lemme, M.C.; Delin, A.; Råsander, M.; Bergqvist, L.; Schröder, S.; Fischer, A.C.; Niklaus, F.; et al. Graphene-Based CO2 Sensing and Its Cross-Sensitivity with Humidity. RSC Adv. 2017, 7, 22329–22339. [Google Scholar] [CrossRef]
- Roso, S.; Bittencourt, C.; Umek, P.; González, O.; Güell, F.; Urakawa, A.; Llobet, E. Synthesis of Single Crystalline In2O3 Octahedra for the Selective Detection of NO2 and H2 at Trace Levels. J. Mater. Chem. C Mater. 2016, 4, 9418–9427. [Google Scholar] [CrossRef]
- Parra, M.; Elustondo, D.; Bermejo, R.; Santamaria, J. Ambient Air Levels of Volatile Organic Compounds (VOC) and Nitrogen Dioxide (NO2) in a Medium Size City in Northern Spain. Sci. Total Environ. 2008, 407, 999–1009. [Google Scholar] [CrossRef]
- Kountouriotis, A.; Aleiferis, P.G.; Charalambides, A.G. Numerical Investigation of VOC Levels in the Area of Petrol Stations. Sci. Total Environ. 2014, 470–471, 1205–1224. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic Oxidation of Volatile Organic Compounds (VOCs)—A Review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Liew, C.; Li, Y.; Wayne, S.; Clark, N. An Investigation on the Mechanism of the Increased NO2 Emissions from H2-Diesel Dual Fuel Engine. Int. J. Hydrog. Energy 2018, 43, 3837–3844. [Google Scholar] [CrossRef]
- Heck, R.M. Catalytic Abatement of Nitrogen Oxides–Stationary Applications. Catal. Today 1999, 53, 519–523. [Google Scholar] [CrossRef]
- ISO/IEC 14443-3:2018; Cards and Security Devices for Personal Identification—Contactless Proximity Objects—Part 3: Initialization and Anticollision. International Organization for Standardization: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/73598.html (accessed on 18 February 2024).
- Zhang, B.; Cheng, M.; Liu, G.; Gao, Y.; Zhao, L.; Li, S.; Wang, Y.; Liu, F.; Liang, X.; Zhang, T.; et al. Room Temperature NO2 Gas Sensor Based on Porous Co3O4 Slices/Reduced Graphene Oxide Hybrid. Sens. Actuators B Chem. 2018, 263, 387–399. [Google Scholar] [CrossRef]
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.; Xiang, L.; Xie, D.; et al. Reduced Graphene Oxide/Mesoporous ZnO NSs Hybrid Fibers for Flexible, Stretchable, Twisted, and Wearable NO2 E-Textile Gas Sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, S.J.; Kim, Y.-J.; Shim, Y.-S.; Kim, S.Y.; Hong, B.H.; Jang, H.W. Self-Activated Transparent All-Graphene Gas Sensor with Endurance to Humidity and Mechanical Bending. ACS Nano 2015, 9, 10453–10460. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, S.; Nguyen, T.H.; Yang, W.; Noh, J.S. Polyurethane Sponges Decorated with Reduced Graphene Oxide and Silver Nanowires for Highly Stretchable Gas Sensors. Sens. Actuators B Chem. 2018, 265, 609–616. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.; Zhang, J.; Pu, J.; Lin, Y.; Xu, S.; Shen, L.; Chen, Q.; Shi, W. Fully Printed, Rapid-Response Sensors Based on Chemically Modified Graphene for Detecting NO2 at Room Temperature. ACS Appl. Mater. Interfaces 2014, 6, 7426–7433. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, S.; Fei, T.; Liu, S.; Zhang, T. Construction of ZnO/SnO2 Heterostructure on Reduced Graphene Oxide for Enhanced Nitrogen Dioxide Sensitive Performances at Room Temperature. ACS Sens. 2019, 4, 2048–2057. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, X. Novel 3D Graphene Aerogel–ZnO Composites as Efficient Detection for NO2 at Room Temperature. Sens. Actuators B Chem. 2015, 211, 220–226. [Google Scholar] [CrossRef]
- Ugale, A.D.; Umarji, G.G.; Jung, S.H.; Deshpande, N.G.; Lee, W.; Cho, H.K.; Yoo, J.B. ZnO Decorated Flexible and Strong Graphene Fibers for Sensing NO2 and H2S at Room Temperature. Sens. Actuators B Chem. 2020, 308, 127690. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsieh, H.F.; Ding, C.F.; Li, K.M.; Young, H.T.; Hsiao, W.T. Laser Surface Modification on RGO/ZnO Composite Materials for NO2 Gas Sensing. Mater. Chem. Phys. 2022, 290, 126551. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, J. One Step Solvothermal Synthesis of Urchin-like ZnO Nanorods/Graphene Hollow Spheres and Their NO2 Gas Sensing Properties. Ceram. Int. 2016, 42, 2085–2090. [Google Scholar] [CrossRef]
- Zhu, R.; Azzarelli, J.M.; Swager, T.M. Wireless Hazard Badges to Detect Nerve-Agent Simulants. Angew. Chem. Int. Ed. 2016, 55, 9662–9666. [Google Scholar] [CrossRef] [PubMed]
- Barandun, G.; Soprani, M.; Naficy, S.; Grell, M.; Kasimatis, M.; Chiu, K.L.; Ponzoni, A.; Güder, F. Cellulose Fibers Enable Near-Zero-Cost Electrical Sensing of Water-Soluble Gases. ACS Sens. 2019, 4, 1662–1669. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.; Shi, Y.; Yu, G. Highly Sensitive, Printable Nanostructured Conductive Polymer Wireless Sensor for Food Spoilage Detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Desroches, M.; Yoon, B.; Swager, T.M. Wireless Oxygen Sensors Enabled by Fe(II)-Polymer Wrapped Carbon Nanotubes. ACS Sens. 2017, 2, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Visagie, I.; Chen, Y.; Abbel, R.; Parker, K. NFC-Enabled Dual-Channel Flexible Printed Sensor Tag. Sensors 2023, 23, 6765. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Betancourt, A.; Santos-Ceballos, J.C.; Alouani, M.A.; Malik, S.B.; Romero, A.; Ramírez, J.L.; Vilanova, X.; Llobet, E. ZnO Decorated Graphene-Based NFC Tag for Personal NO2 Exposure Monitoring during a Workday. Sensors 2024, 24, 1431. https://doi.org/10.3390/s24051431
Santos-Betancourt A, Santos-Ceballos JC, Alouani MA, Malik SB, Romero A, Ramírez JL, Vilanova X, Llobet E. ZnO Decorated Graphene-Based NFC Tag for Personal NO2 Exposure Monitoring during a Workday. Sensors. 2024; 24(5):1431. https://doi.org/10.3390/s24051431
Chicago/Turabian StyleSantos-Betancourt, Alejandro, José Carlos Santos-Ceballos, Mohamed Ayoub Alouani, Shuja Bashir Malik, Alfonso Romero, José Luis Ramírez, Xavier Vilanova, and Eduard Llobet. 2024. "ZnO Decorated Graphene-Based NFC Tag for Personal NO2 Exposure Monitoring during a Workday" Sensors 24, no. 5: 1431. https://doi.org/10.3390/s24051431
APA StyleSantos-Betancourt, A., Santos-Ceballos, J. C., Alouani, M. A., Malik, S. B., Romero, A., Ramírez, J. L., Vilanova, X., & Llobet, E. (2024). ZnO Decorated Graphene-Based NFC Tag for Personal NO2 Exposure Monitoring during a Workday. Sensors, 24(5), 1431. https://doi.org/10.3390/s24051431









