A Comparative Study of Narrow/Ultra-Wideband Microwave Sensors for the Continuous Monitoring of Vital Signs and Lung Water Level
Abstract
:1. Introduction
2. Design of Microwave Sensors
2.1. Narrowband Antenna (NB)
2.2. Ultra-Wideband (UWB) Microwave Sensor
3. Microwave Sensors with Human Chest Phantom
3.1. Narrowband Microwave Sensors
3.2. Ultra-Wideband Microwave Sensors
4. Specific Absorption Rate Measurement
5. Proposed System for Vital Sign Detection
5.1. Principle of Operation
5.2. Sensor Assessment in Vital Sign Detection
5.2.1. Breathing Rate
Narrowband Sensor
UWB Sensor
5.2.2. Heartbeat Rate
Narrowband Sensor
UWB Sensor
6. Lung Water Level Monitoring
6.1. Lung Phantom Preparation: Mimicking Human Lung Tissue
6.1.1. Narrowband Sensor
6.1.2. UWB Sensor
7. Discussion and Comparisons
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Zhang, G.; Yu, B.; Qiao, Z.; Wang, J. Wearable human motion posture capture and medical health monitoring based on wireless sensor networks. Measurement 2020, 166, 108252. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, M.; Xia, B.; Wang, S.; Wang, M.; Chen, M.; Dai, S.; Wang, T.; Ye, T.T. E-textile battery-less displacement and strain sensor for human activities tracking. IEEE Internet Things J. 2021, 8, 16486–16497. [Google Scholar] [CrossRef]
- Vanegas, E.; Igual, R.; Plaza, I. Sensing systems for respiration monitoring: A technical systematic review. Sensors 2020, 20, 5446. [Google Scholar] [CrossRef]
- Celik, N.; Gagarin, R.; Huang, G.C.; Iskander, M.F.; Berg, B.W. Microwave stethoscope: Development and benchmarking of a vital signs sensor using computer-controlled phantoms and human studies. IEEE Trans. Biomed. Eng. 2013, 61, 2341–2349. [Google Scholar] [CrossRef]
- Perron, R.R.; Iskander, M.F.; Seto, T.B.; Huang, G.C.; Bibb, D.A. Electromagnetics in medical applications: The cardiopulmonary stethoscope journey. In The World of Applied Electromagnetics: In Appreciation of Magdy Fahmy Iskander; Springer: Cham, Switzerland, 2018; pp. 443–479. [Google Scholar]
- Massaroni, C.; Nicolò, A.; Lo Presti, D.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-based methods for measuring respiratory rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef]
- Massaroni, C.; Di Tocco, J.; Presti, D.L.; Longo, U.G.; Miccinilli, S.; Sterzi, S.; Formica, D.; Saccomandi, P.; Schena, E. Smart textile based on piezoresistive sensing elements for respiratory monitoring. IEEE Sens. J. 2019, 19, 7718–7725. [Google Scholar] [CrossRef]
- Dionisi, A.; Marioli, D.; Sardini, E.; Serpelloni, M. Autonomous wearable system for vital signs measurement with energy-harvesting module. IEEE Trans. Instrum. Meas. 2016, 65, 1423–1434. [Google Scholar] [CrossRef]
- Roudjane, M.; Messaddeq, Y. Innovative wearable sensors based on hybrid materials for real-time breath monitoring. In Wireless Sensor Networks-Design, Deployment and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Kaneko, H.; Horie, J.; Ishikawa, A. New scale to assess breathing movements of the chest and abdominal wall: Preliminary reliability testing. J. Phys. Ther. Sci. 2015, 27, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, N.; Zhong, H.; Zhang, W.; Zhang, Y.; Yin, X.; He, B. Textile-based mechanical sensors: A review. Materials 2021, 14, 6073. [Google Scholar] [CrossRef]
- Illahi, U.; Iqbal, J.; Sulaiman, M.I.; Alam, M.M.; Su’Ud, M.M.; Jamaluddin, M.H.; Yasin, M.N.M. Design of New Circularly Polarized Wearable Dielectric Resonator Antenna for Off-Body Communication in WBAN Applications. IEEE Access 2019, 7, 150573–150582. [Google Scholar] [CrossRef]
- Yun, Z.; Clemens, S.; Xiao, Y.; Perron, R.; Iskander, M.F. Mapping lung water signal distribution on human chest and predition of lung water content. In Proceedings of the 2019 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting, Atlanta, GA, USA, 7–12 July 2019; pp. 751–752. [Google Scholar]
- Leong, C.; Xiao, Y.; Yun, Z.; Iskander, M.F. Non-Invasive assessment of lung water content using chest patch RF sensors: A computer study using NIH patients CT scan database and AI classification algorithms. IEEE Access 2023, 11, 13058–13066. [Google Scholar] [CrossRef]
- Chen, P.; Wang, D.; Gan, Z. Flexible and small textile antenna for UWB wireless body area network. Micromachines 2023, 14, 718. [Google Scholar] [CrossRef] [PubMed]
- Wagih, M.; Malik, O.; Weddell, A.S.; Beeby, S. E-textile breathing sensor using fully textile wearable antennas. Eng. Proc. 2022, 15, 9. [Google Scholar]
- Abd El-Hameed, A.; Salem, D.; Abdallah, E.; Hashish, E. Crossbar fractal quasi self-complementary UWB antenna. In Proceedings of the 2014 IEEE Antennas and Propagation Society International Symposium (APSURSI), Memphis, TN, USA, 6–11 July 2014; pp. 219–220. [Google Scholar]
- Parameswari, S.; Chitra, C. Textile UWB antenna with metamaterial for healthcare monitoring. Int. J. Antennas Propag. 2021, 2021, 5855626. [Google Scholar] [CrossRef]
- Elashry, G.M.; Mohamed, H.A.; Abd-El-Hadi, A.A.; Abdallah, E.A. Cloverleaf filtenna with reconfigurable quintuple rejection bands using defected microstrip structure. AEU-Int. J. Electron. Commun. 2023, 168, 154708. [Google Scholar] [CrossRef]
- Yu, H.; Huang, W.; Du, B. SSA-VMD for UWB radar sensor vital sign extraction. Sensors 2023, 23, 756. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Kan, E.C. Monitoring vital signs over multiplexed radio by near-field coherent sensing. Nat. Electron. 2018, 1, 74–78. [Google Scholar] [CrossRef]
- Abd El-Hameed, A.S.; Wahab, M.G.; Elshafey, N.A.; Elpeltagy, M.S. Quad-port UWB MIMO antenna based on LPF with vast rejection band. AEU-Int. J. Electron. Commun. 2021, 134, 153712. [Google Scholar] [CrossRef]
- Abd El-Hameed, A.; Salem, D.; Abdallah, E.; Hashish, E. Fractal quasi-self complimentary miniaturized UWB antenna. In Proceedings of the IEEE Antennas and Propagation Society International Symposium (APSURSI), Orlando, FL, USA, 7–13 July 2013; pp. 15–16. [Google Scholar]
- Wahab, M.; Abd El-Hameed, A.; Swelam, W.; Abd ElAzeem, M. Design of miniaturized fractal quasi-self complimentary antenna for UWB applications. In Proceedings of the IEEE International Symposium on Antennas and Propagation (APSURSI), Fajardo, PR, USA, 26 June–1 July 2016; pp. 1809–1810. [Google Scholar]
- Elsheakh, D.N.; Elgendy, Y.K.; Elsayed, M.E.; Eldamak, A.R. Circularly polarized textile sensors for microwave-based smart bra monitoring system. Micromachines 2023, 14, 586. [Google Scholar] [CrossRef]
- Ejaz, A.; Jabeen, I.; Khan, Z.U.; Alomainy, A.; Aljaloud, K.; Alqahtani, A.H.; Hussain, N.; Hussain, R.; Amin, Y. A High performance all-textile wearable antenna for wristband application. Micromachines 2023, 14, 1169. [Google Scholar] [CrossRef]
- Kareem, F.R.; El Atrash, M.; Ibrahim, A.A.; Abdalla, M.A. All-textile inspired-folded dipole antennas for on/off-body communications medical applications. Alex. Eng. J. 2022, 61, 8751–8761. [Google Scholar] [CrossRef]
- Elsheakh, D.N.; Mohamed, R.A.; Fahmy, O.M.; Ezzat, K.; Eldamak, A.R. Complete breast cancer Detection and monitoring system by using microwave textile based antenna sensors. Biosensors 2023, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, S. Printed compact asymmetric dual L-strip fed split-ring shaped EBG-based textile antenna for WBAN applications. Microw. Opt. Technol. Lett. 2020, 62, 3897–3904. [Google Scholar] [CrossRef]
- Pei, R.; Leach, M.P.; Lim, E.G.; Wang, Z.; Song, C.; Wang, J.; Zhang, W.; Jiang, Z.; Huang, Y. Wearable EBG-backed belt antenna for smart on-body applications. IEEE Trans. Ind. Inform. 2020, 16, 7177–7189. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.; Santos, J.; Takane, M. mHealth: New horizons for health through mobile technologies. World Health Organ. 2011, 64, 66–71. [Google Scholar]
- Dziuda, L.; Skibniewski, F.W.; Krej, M.; Lewandowski, J. Monitoring respiration and cardiac activity using fiber Bragg grating-based sensor. IEEE Trans. Biomed. Eng. 2012, 59, 1934–1942. [Google Scholar] [CrossRef]
- Guo, L.; Berglin, L.; Li, Y.; Mattila, H.; Mehrjerdi, A.K.; Skrifvars, M. ‘Disappearing sensor’-textile based sensor for monitoring breathing. In Proceedings of the 2011 International Conference on Control, Automation and Systems Engineering (CASE), Singapore, 30–31 July 2011; pp. 1–4. [Google Scholar]
- Irfansyah, A.; Harianto, B.; Pambudiyatno, N. Design of rectangular microstrip antenna 1×2 array for 5G communication. J. Phys. Conf. Ser. 2021, 2117, 012028. [Google Scholar] [CrossRef]
- Available online: https://www.solidworks.com/media/cst-studio-suite-electromagnetic-field-simulation-software (accessed on 1 June 2023).
- Balanis, C. Antenna Theory: Analysis and Design; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Durney, Y.C.; Iskander, M.F. Antennas for medical applications. In Antenna Handbook Theory, Applications, and Design, 1st ed.; Lo, Y.T., Lee, S.W., Eds.; Springer: New York, NY, USA, 1988; pp. 1729–1788. [Google Scholar]
- Staff, M.C. Pulmonary Edema Definition, Mayo Clinic. 2014. Available online: http://www.mayoclinic.org/diseases-conditions/pulmonary-edema/basics/definition/con-20022485 (accessed on 10 January 2017).
- Iskander, M.F.; Seto, T.B.; Perron, R.R.; Lim, E.; Qazi, F. Cardio-pulmonary stethoscope: Clinical validation with heart failure and hemodialysis patients. IEEE Trans. Biomed. Eng. 2017, 65, 1176–1180. [Google Scholar] [CrossRef]
- Perron, R.R.; Iskander, M.F.; Seto, T.B.; Qazi, F.A.; Bibb, D.A.; Lim, E. Multi-sensor Cardio-Pulmonary Stethoscope for quantitative lung water measurement. In Proceedings of the General Assembly and Scientific Symposium of the International Union of Radio Science (URSI GASS), Montreal, QC, Canada, 19–26 August 2017; pp. 1–2. [Google Scholar]
- Iskander, M.F.; Celik, N.; Gagarin, R.; Huang, G.C.; Bibb, D.A. Microwave Stethoscope for Measuring Cardio-Pulmonary Vital Signs and Lung Water Content. U.S. Patent US 9,526,438, 27 December 2016. [Google Scholar]
- National Electrical Manufacturers Association. NEMA Standards Publication MS 8-2016: Characterization of the Specific Absorption Rate (SAR) for Magnetic Resonance Imaging Systems; NEMA: Washington, DC, USA, 2016. [Google Scholar]
- Available online: https://www.suncoastsurgicalassociates.com/areas-of-expertise/thoracic-surgery/pleural-effusions (accessed on 25 June 2023).
- Kramer, G.H.; Capello, K.; Bearrs, B.; Lauzon, A.; Normandeau, L. Linear dimensions and volumes of human lungs obtained from CT images. Health Phys. 2012, 102, 378–383. [Google Scholar] [CrossRef] [PubMed]
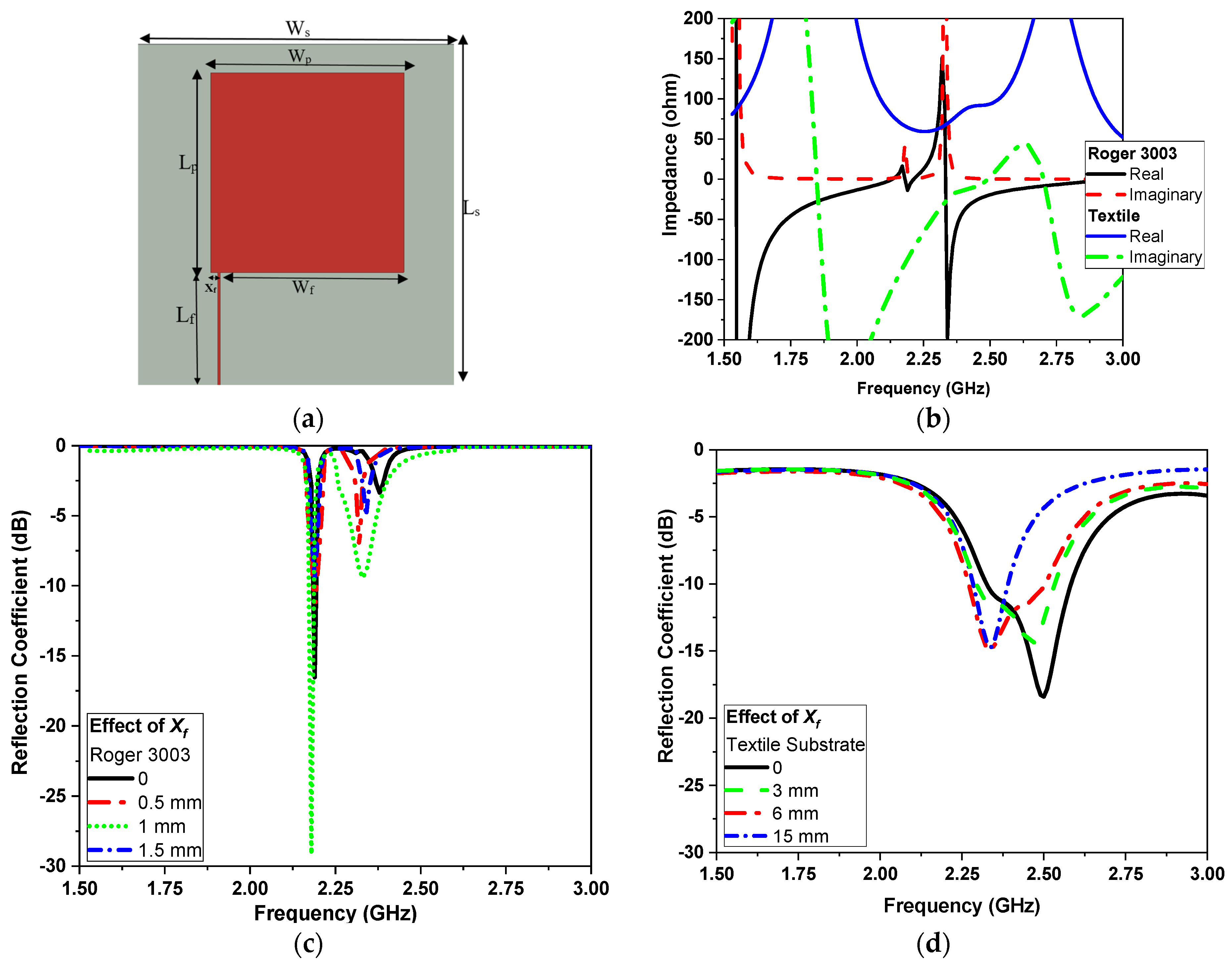

























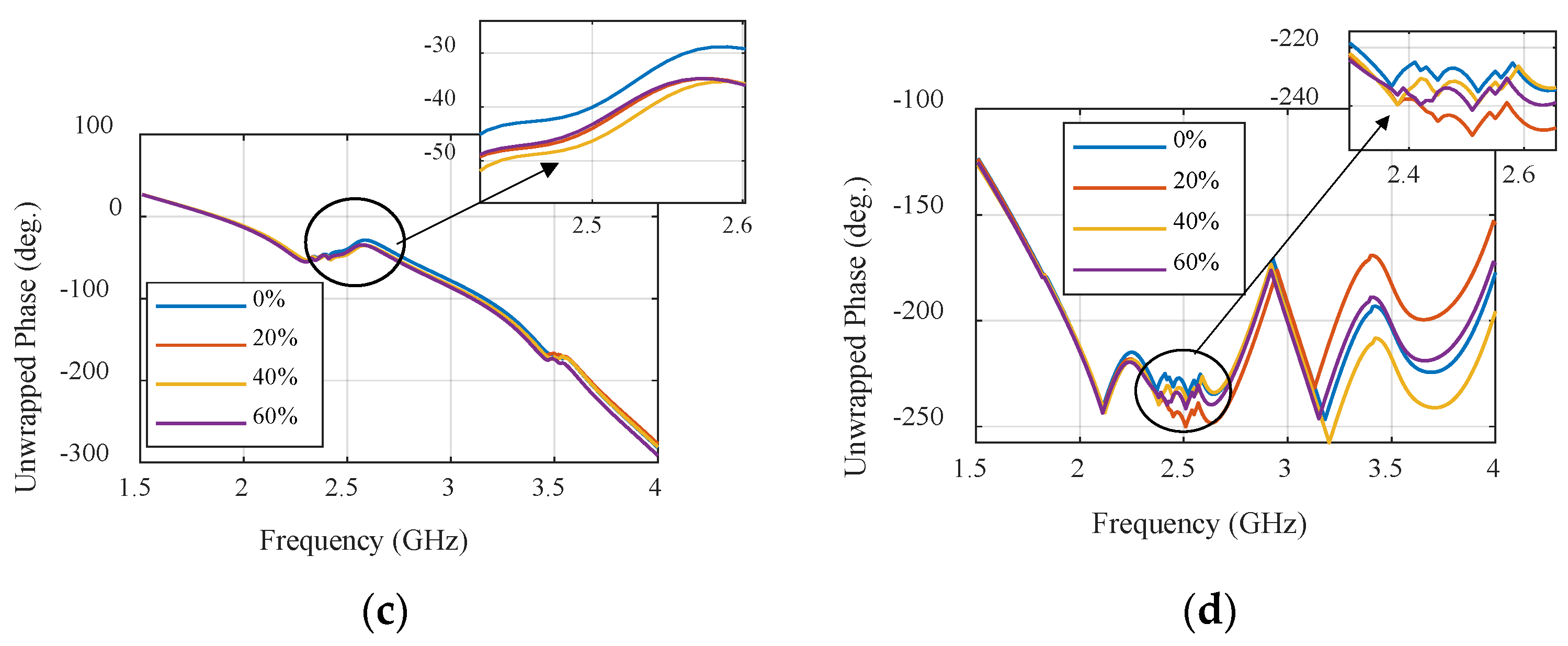
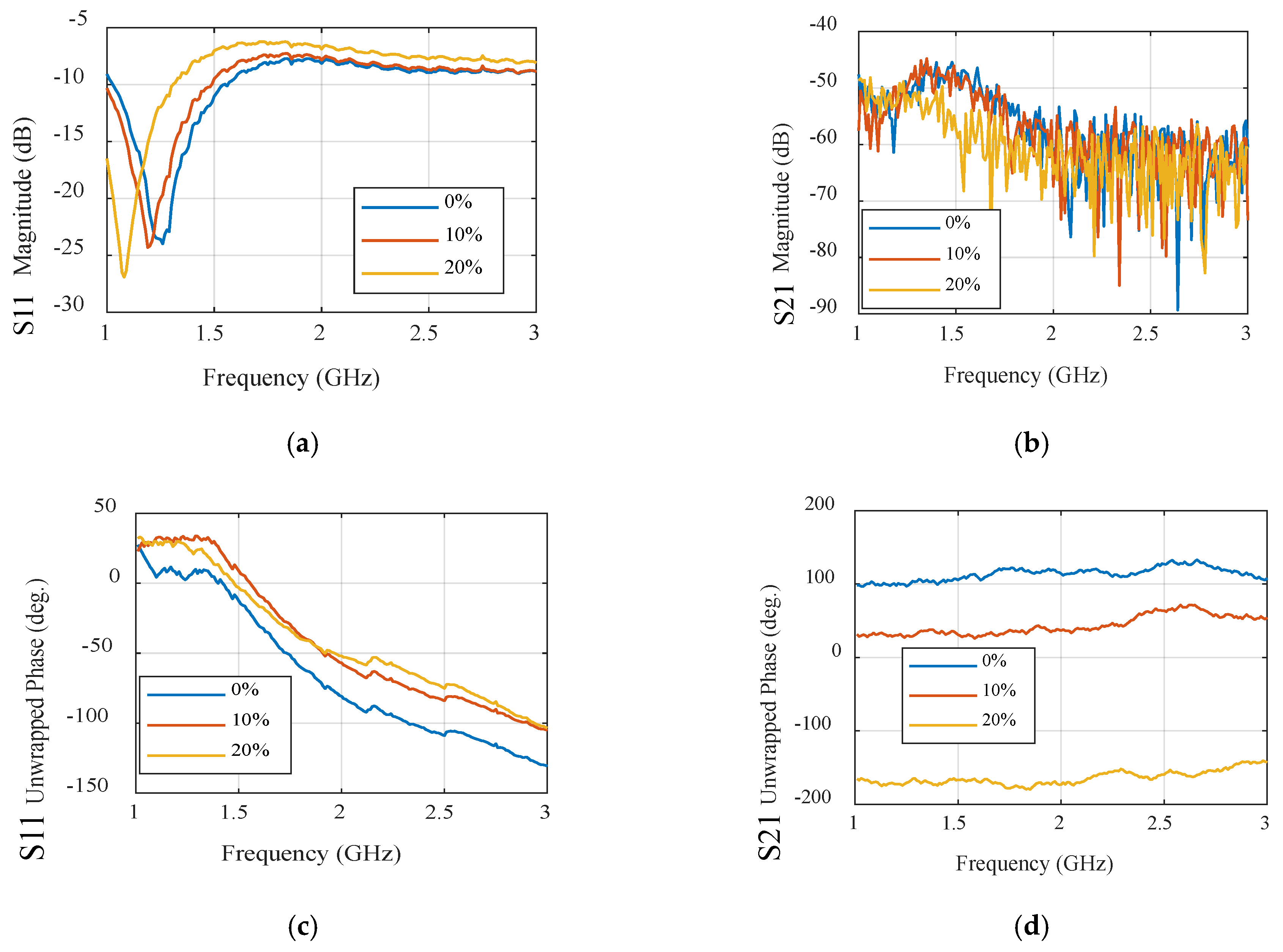



| Rogers Flexible Substrate | ||||||
|---|---|---|---|---|---|---|
| LS | Wf | Lp | Ws | Lf | Wp | Xf |
| 50 | 30 | 35 | 50 | 12 | 32 | 1.5 |
| Textile substrate | ||||||
| 60 | 24 | 35 | 60 | 20 | 32 | 6 |
| Ls | Lf | Lm | Lk | Lp | Lgg | Wp1 | S |
|---|---|---|---|---|---|---|---|
| 60 | 10 | 26 | 10 | 22 | 2 | 19.6 | 0.5 |
| Ws | Wf | Wm | Lg | Wp | Wgg | Wm1 | - |
| 60 | 6 | 12.7 | 10 | 44 | 2 | 6.7 | - |
| Narrowband Microwave Sensor | Ultra-Wideband Microwave Sensor | |||||
|---|---|---|---|---|---|---|
| 2.45 GHz | SAR (1 g) | SAR (10 g) | 2.45 GHz | SAR (1 g) | SAR (10 g) | |
 |  |  |  | |||
| Head | 10 dBm | 0.088 | 0.044 | 10 dBm | 0.25 | 0.124 |
| 15 dBm | 0.274 | 0.138 | 15 dBm | 0.794 | 0.393 | |
| 20 dBm | 0.887 | 0.447 | 20 dBm | 1.83 | 1.25 | |
| Body | 10 dBm | 0.093 | 0.042 | 10 dBm | 0.329 | 0.137 |
| 15 dBm | 0.304 | 0.137 | 15 dBm | 1.04 | 0.435 | |
| 20 dBm | 1.02 | 0.448 | 20 dBm | 1.91 | 1.39 | |
| Heartbeat Rate | CONTEC Machine Electrocardiograph ECG100G | Proposed System Using NB Sensors | Proposed System Using UWB Sensors |
|---|---|---|---|
| 37-year-old adult | 90 bpm | 93 bpm | 92.3 bpm |
| 8-year-old child | 87 bpm | 85.7 bpm | 86 bpm |
| Feature | NB Sensor | UWB Sensor |
|---|---|---|
| Power Consumption | Higher | Lower |
| Data Transmission Rates | Slower | Faster |
| Real-World Usability | Limited Range | Expanded Range |
| Cost | Lower | Higher |
| Applications | Specific | Diverse |
| Signal Processing | Specific | Diverse |
| Accuracy | Moderate | High |
| Ref | Antenna Material | Sensing Mechanism | Frequency (GHz) | Size (mm2) | Max. SAR (W/kg) | Data Post-Processing |
|---|---|---|---|---|---|---|
| [26] | Textile (Denim) | Dielectric sensing | 2.45 | 35.4 × 82.4 | 0.295 | Peak detection |
| [27] | ShieldIt Super/Felt | Dielectric sensing | 2.4 | 80 × 20 | 0.0091 | N/A |
| [29] | Textile | Dielectric sensing | 2.45 | 40 × 20 | NA | N/A |
| [30] | Leather and Textile | Dielectric sensing | 2.45 | 120 × 120 | 0.04 | N/A |
| This Work | PCB and Textile | Dielectric sensing | 2.3 | 50 × 50 | 0.042 | Peak detection |
| Ref | Antenna Material | Sensing Mechanism | Bandwidth (GHz) | Size (mm2) | Sensitivity | Data Post-Processing |
|---|---|---|---|---|---|---|
| [16] | Textile | Dielectric sensing | 0.5-3 | 118 × 105 | 1.5–2.5 dB * | Peak detection |
| [21] | PCB | Dielectric sensing | N/A | N/A | 10% * | N/A |
| [2] | Stretchable Textile | Strain sensing | 0.4–1.4 | 120 × 30 | 1.5–7 dB &* | N/A |
| This Work | PCB and Textile | Dielectric sensing | 2–10 | 60 × 60 | 0.017 ** | Peak detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Hameed, A.S.; Elsheakh, D.M.; Elashry, G.M.; Abdallah, E.A. A Comparative Study of Narrow/Ultra-Wideband Microwave Sensors for the Continuous Monitoring of Vital Signs and Lung Water Level. Sensors 2024, 24, 1658. https://doi.org/10.3390/s24051658
Abd El-Hameed AS, Elsheakh DM, Elashry GM, Abdallah EA. A Comparative Study of Narrow/Ultra-Wideband Microwave Sensors for the Continuous Monitoring of Vital Signs and Lung Water Level. Sensors. 2024; 24(5):1658. https://doi.org/10.3390/s24051658
Chicago/Turabian StyleAbd El-Hameed, Anwer S., Dalia M. Elsheakh, Gomaa M. Elashry, and Esmat A. Abdallah. 2024. "A Comparative Study of Narrow/Ultra-Wideband Microwave Sensors for the Continuous Monitoring of Vital Signs and Lung Water Level" Sensors 24, no. 5: 1658. https://doi.org/10.3390/s24051658
APA StyleAbd El-Hameed, A. S., Elsheakh, D. M., Elashry, G. M., & Abdallah, E. A. (2024). A Comparative Study of Narrow/Ultra-Wideband Microwave Sensors for the Continuous Monitoring of Vital Signs and Lung Water Level. Sensors, 24(5), 1658. https://doi.org/10.3390/s24051658






