Machine Learning for the Design and the Simulation of Radiofrequency Magnetic Resonance Coils: Literature Review, Challenges, and Perspectives
Abstract
:1. Introduction
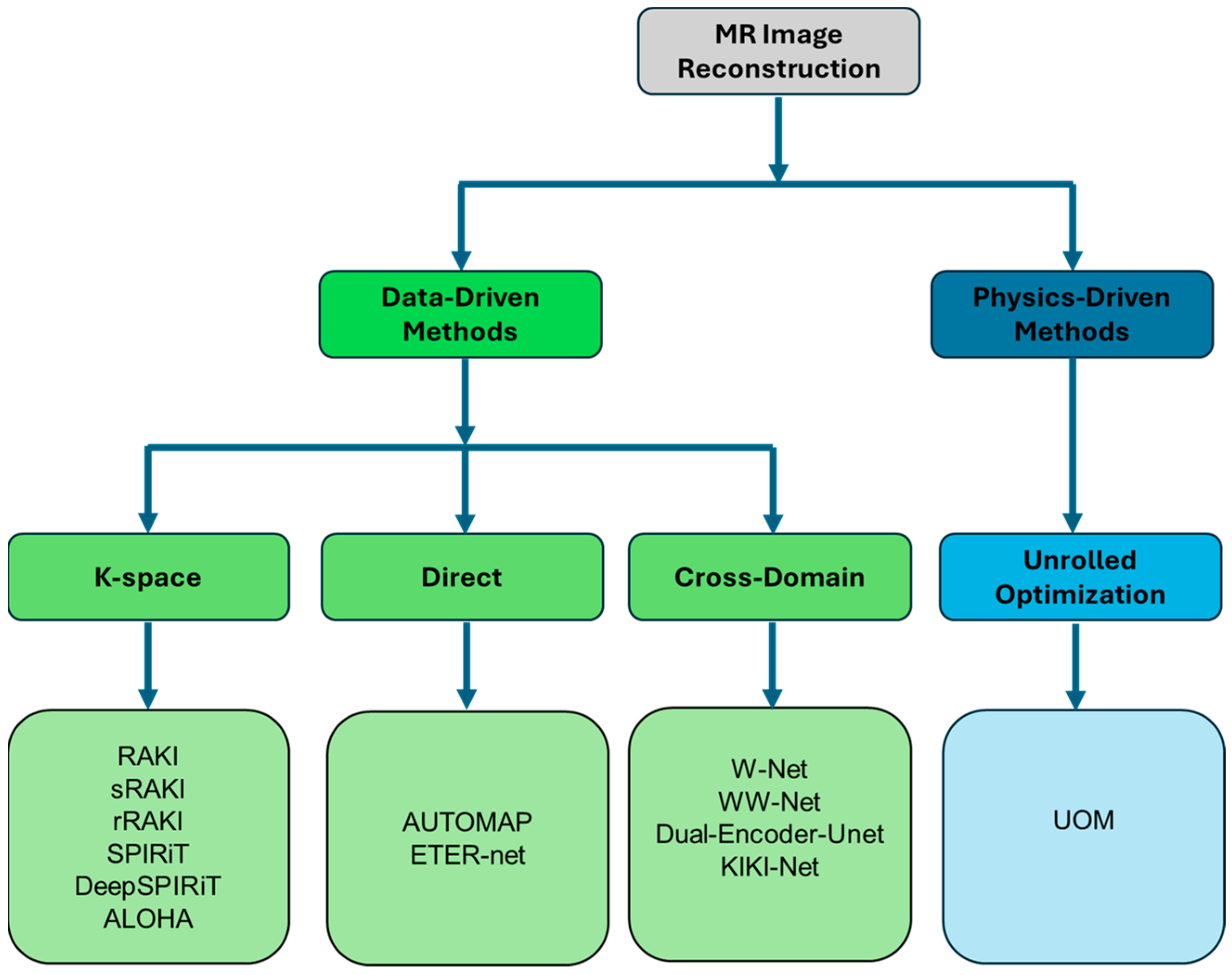
2. Machine Learning Applications in MR Reconstruction
2.1. K-Space Methods
2.2. Direct Methods
2.3. Cross-Domain Methods
2.4. Unrolled Optimization Methods (UOM)
3. Theory and Optimization of Coil Design
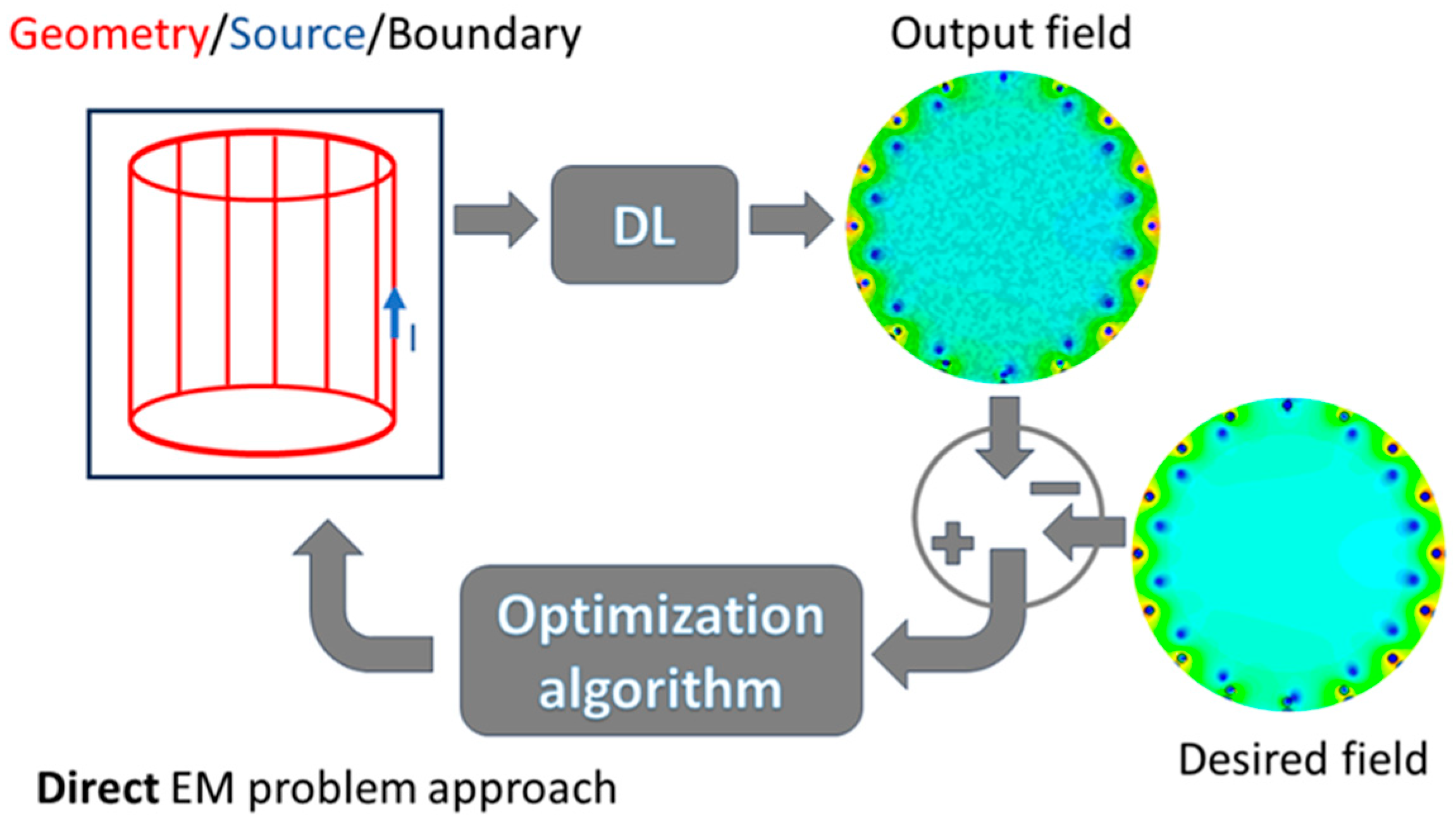
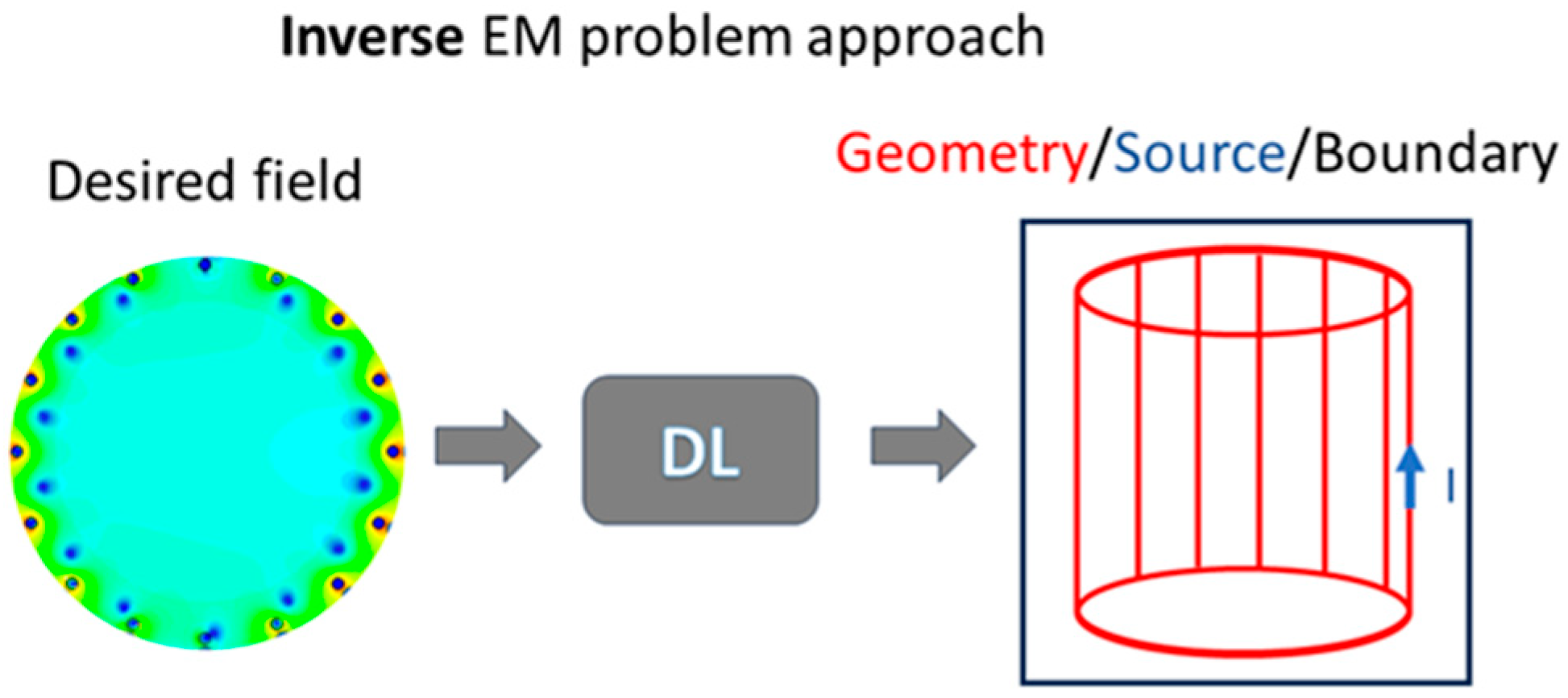
4. Deep Learning and Machine Learning Applied to Electromagnetic Problems
5. Machine Learning for RF Coil Design Procedure
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, J. Electromagnetic Analysis and Design in Magnetic Resonance Imaging; CRC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Gassenmaier, S.; Afat, S.; Nickel, D.; Mostapha, M.; Herrmann, J.; Othman, A.E. Deep learning-accelerated T2-weighted imaging of the prostate: Reduction of acquisition time and improvement of image quality. Eur. J. Radiol. 2021, 137, 109600. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, T.; Squarcina, L.; Giubergia, A.; Bertoldo, A.; Brambilla, P.; Peruzzo, D. Review on deep learning fetal brain segmentation from Magnetic Resonance images. Artif. Intell. Med. 2023, 143, 102608. [Google Scholar] [CrossRef] [PubMed]
- Krishnapriya, S.; Karuna, Y. Pre-trained deep learning models for brain MRI image classification. Front. Hum. Neurosci. 2023, 17, 1150120. [Google Scholar] [CrossRef] [PubMed]
- Soomro, T.A.; Zheng, L.; Afifi, A.J.; Ali, A.; Soomro, S.; Yin, M.; Gao, J. Image Segmentation for MR Brain Tumor Detection Using Machine Learning: A Review. IEEE Rev. Biomed. Eng. 2023, 16, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Bäse, A.; Morra, L.; Meyer-Bäse, U.; Pinker, K. Current Status and Future Perspectives of Artificial Intelligence in Magnetic Resonance Breast Imaging. Contrast Media Mol. Imaging 2020, 2020, 6805710. [Google Scholar] [CrossRef] [PubMed]
- Twilt, J.J.; van Leeuwen, K.G.; Huisman, H.J.; Fütterer, J.J.; de Rooij, M. Artificial Intelligence Based Algorithms for Prostate Cancer Classification and Detection on Magnetic Resonance Imaging: A Narrative Review. Diagnostics 2021, 11, 959. [Google Scholar] [CrossRef]
- Miller, Z.; Johnson, K.M. Motion compensated self supervised deep learning for highly accelerated 3D ultrashort Echo time pulmonary MRI. Magn. Reson. Med. 2023, 89, 2361–2375. [Google Scholar] [CrossRef]
- Spieker, V.; Eichhorn, H.; Hammernik, K.; Rueckert, D.; Preibisch, C.; Karampinos, D.C.; Schnabel, J.A. Deep Learning for Retrospective Motion Correction in MRI: A Comprehensive Review. IEEE Trans. Med. Imaging 2024, 43, 846–859. [Google Scholar] [CrossRef]
- Shafieizargar, B.; Byanju, R.; Sijbers, J.; Klein, S.; den Dekker, A.J.; Poot, D.H.J. Systematic review of reconstruction techniques for accelerated quantitative MRI. Magn. Reson. Med. 2023, 90, 1172–1208. [Google Scholar] [CrossRef]
- Tao, Q.; Lelieveldt, B.P.F.; van der Geest, R.J. Deep Learning for Quantitative Cardiac MRI. AJR Am. J. Roentgenol. 2020, 214, 529–535. [Google Scholar] [CrossRef]
- Dayarathna, S.; Islam, K.T.; Uribe, S.; Yang, G.; Hayat, M.; Chen, Z. Deep learning based synthesis of MRI, CT and PET: Review and analysis. Med. Image Anal. 2024, 92, 103046. [Google Scholar] [CrossRef] [PubMed]
- Garcea, F.; Serra, A.; Lamberti, F.; Morra, L. Data augmentation for medical imaging: A systematic literature review. Comput. Biol. Med. 2023, 152, 106391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, J.Z.; Cauley, S.F.; Rosen, B.R.; Rosen, M.S. Image reconstruction by domain-transform manifold learning. Nature 2018, 555, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Montalt-Tordera, J.; Muthurangu, V.; Hauptmann, A.; Steeden, J.A. Machine learning in Magnetic Resonance Imaging: Image reconstruction. Phys. Medica 2021, 83, 79–87. [Google Scholar] [CrossRef]
- Zeng, G.; Guo, Y.; Zhan, J.; Wang, Z.; Lai, Z.; Du, X.; Qu, X.; Guo, D. A review on deep learning MRI reconstruction without fully sampled k-space. BMC Med. Imaging 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Knoll, F.; Hammernik, K.; Zhang, C.; Moeller, S.; Pock, T.; Sodickson, D.K.; Akcakaya, M. Deep-Learning Methods for Parallel Magnetic Resonance Imaging Reconstruction: A Survey of the Current Approaches, Trends, and Issues. IEEE Signal Process. Mag. 2020, 37, 128–140. [Google Scholar] [CrossRef]
- Ahishakiye, E.; Van Gijzen, M.B.; Tumwiine, J.; Wario, R.; Obungoloch, J. A survey on deep learning in medical image reconstruction. Intell. Med. 2021, 1, 118–127. [Google Scholar] [CrossRef]
- Akçakaya, M.; Moeller, S.; Weingärtner, S.; Uğurbil, K. Scan-specific robust artificial-neural-networks for k-space interpolation (RAKI) reconstruction: Database-free deep learning for fast imaging. Magn. Reson. Med. 2019, 81, 439–453. [Google Scholar] [CrossRef]
- Hossein Hosseini, S.A.; Zhang, C.; Weingärtner, S.; Moeller, S.; Stuber, M.; Ugurbil, K.; Akçakaya, M. Accelerated coronary MRI with sRAKI: A database-free self-consistent neural network k-space reconstruction for arbitrary undersampling. PLoS ONE 2020, 15, e0229418. [Google Scholar] [CrossRef]
- Zhang, C.; Moeller, S.; Weingärtner, S.; Uǧurbil, K.; Akçakaya, M. (ISMRM 2019) Accelerated MRI Using Residual RAKI: Scan-specific Learning of Reconstruction Artifacts. In Proceedings of the ISMRM 2019, 27th Annual Meeting & Exhibition, Montreal, QC, Canada, 11–16 May 2019. [Google Scholar]
- Kim, T.H.; Garg, P.; Haldar, J.P. LORAKI: Autocalibrated Recurrent Neural Networks for Autoregressive MRI Reconstruction in k-Space. arXiv 2019, arXiv:1904.09390. [Google Scholar] [CrossRef]
- Hossein Hosseini, S.A.; Moeller, S.; Weingärtner, S.; Uǧurbil, K.; Akçakaya, M. Accelerated coronary MRI using 3D SPIRIT-RAKI with sparsity reularization. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 1692–1695. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Mardani, M.; Alley, M.T.; Pauly, J.M.; Vasanawala, S.S. Generalized Parallel Imaging using Deep Convolutional Neural Networks. In Proceedings of the ISMRM 2018, Joint Annual Meeting ISMRM-ESMRMB, Paris, France, 16–21 June 2018; Available online: https://archive.ismrm.org/2018/0570.html (accessed on 17 January 2024).
- Han, Y.; Sunwoo, L.; Ye, J.C. k -Space Deep Learning for Accelerated MRI. IEEE Trans. Med. Imaging 2020, 39, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yu, S.; Dong, H.; Slabaugh, G.; Dragotti, P.L.; Ye, X.; Liu, F.; Arridge, S.; Keegan, J.; Guo, Y.; et al. DAGAN: Deep De-Aliasing Generative Adversarial Networks for Fast Compressed Sensing MRI Reconstruction. IEEE Trans. Med. Imaging 2018, 37, 1310–1321. [Google Scholar] [CrossRef]
- Hyun, C.M.; Kim, H.P.; Lee, S.M.; Lee, S.; Seo, J.K. Deep learning for undersampled MRI reconstruction. Phys. Med. Biol. 2018, 63, 135007. [Google Scholar] [CrossRef] [PubMed]
- Kofler, A.; Dewey, M.; Schaeffter, T.; Wald, C.; Kolbitsch, C. Spatio-Temporal Deep Learning-Based Undersampling Artefact Reduction for 2D Radial Cine MRI With Limited Training Data. IEEE Trans. Med. Imaging 2020, 39, 703–717. [Google Scholar] [CrossRef]
- Pal, A.; Rathi, Y. A review and experimental evaluation of deep learning methods for MRI reconstruction. J. Mach. Learn. Biomed. Imaging 2022, 1, 001. [Google Scholar] [CrossRef]
- Oh, C.; Kim, D.; Chung, J.-Y.; Han, Y.; Park, H.W. ETER-net: End to End MR Image Reconstruction Using Recurrent Neural Network. In Proceedings of the Machine Learning for Medical Image Reconstruction: First International Workshop, MLMIR 2018, Held in Conjunction with MICCAI 2018, Granada, Spain, 16 September 2018; Part of the Lecture Notes in Computer Science Book Series (LNIP). Volume 11074. [Google Scholar] [CrossRef]
- Souza, R.; Frayne, R. A Hybrid Frequency-domain/Image-domain Deep Network for Magnetic Resonance Image Reconstruction. arXiv 2018, arXiv:1810.12473. [Google Scholar] [CrossRef]
- El-Rewaidy, H.; Fahmy, A.S.; Pashakhanloo, F.; Cai, X.; Kucukseymen, S.; Csecs, I.; Neisius, U.; Haji-Valizadeh, H.; Menze, B.; Nezafat, R. Multi-domain convolutional neural network (MD-CNN) for radial reconstruction of dynamic cardiac MRI. Magn. Reson. Med. 2021, 85, 1195–1208. [Google Scholar] [CrossRef]
- Jethi, A.K.; Murugesan, B.; Ram, K.; Sivaprakasam, M. Dual-Encoder-Unet For Fast Mri Reconstruction. In Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging Workshops (ISBI Workshops), Iowa City, IA, USA, 4 April 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Souza, R.; Bento, M.; Nogovitsyn, N.; Chung, K.J.; Loos, W.; Lebel, R.M.; Frayne, R. Dual-domain cascade of U-nets for multi-channel magnetic resonance image reconstruction. Magn. Reson. Imaging 2020, 71, 140–153. [Google Scholar] [CrossRef]
- Eo, T.; Jun, Y.; Kim, T.; Jang, J.; Lee, H.J.; Hwang, D. KIKI-net: Cross-domain convolutional neural networks for reconstructing undersampled magnetic resonance images. Magn. Reson. Med. 2018, 80, 2188–2201. [Google Scholar] [CrossRef]
- Hammernik, K.; Kustner, T.; Yaman, B.; Huang, Z.; Rueckert, D.; Knoll, F.; Akcakaya, M. Physics-Driven Deep Learning for Computational Magnetic Resonance Imaging: Combining physics and machine learning for improved medical imaging. IEEE Signal Process. Mag. 2023, 40, 98–114. [Google Scholar] [CrossRef]
- Mispelter, J.; Lupu, M.; Briguet, A. NMR Probeheads for Biophysical and Biomedical Experiments: Theoretical Principles & Practical Guidelines, 2nd ed.; Imperial College Press: London, UK, 2015. [Google Scholar]
- De Zanche, N.; Yahya, A.; Vermeulen, F.E.; Allen, P.S. Analytical approach to noncircular section birdcage coil design: Verification with a Cassinian oval coil. Magn. Reson. Med. 2005, 53, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Odoj, F.; Von Kienlin, M.; Warnking, J.; Fidler, F.; Weisser, A. NMR probeheads for in vivo applications. Concepts Magn. Reson. 2000, 12, 361–388. [Google Scholar] [CrossRef]
- Giovannetti, G.; Alecci, M.; Galante, A. Biot–Savart-Based Design and Workbench Validation at 100 MHz of Transverse Field Surface RF Coils. Electronics 2023, 12, 2578. [Google Scholar] [CrossRef]
- Roemer, P.B.; Edelstein, W.A.; Hayes, C.E.; Souza, S.P.; Mueller, O.M. The NMR phased array. Magn. Reson. Med. 1990, 16, 192–225. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.M.; Wald, L.L. Theory and application of array coils in MR spectroscopy. NMR Biomed. 1997, 10, 394–410. [Google Scholar] [CrossRef]
- Giovannetti, G.; Viti, V.; Positano, V.; Santarelli, M.F.; Landini, L.; Benassi, A. Coil sensitivity map-based filter for phased-array image reconstruction in Magnetic Resonance Imaging. Int. J. Biomed. Eng. Technol. 2007, 1, 4–17. [Google Scholar] [CrossRef]
- Ohliger, M.A.; Larson, P.E.; Bok, R.A.; Shin, P.; Hu, S.; Tropp, J.; Robb, F.; Carvajal, L.; Nelson, S.J.; Kurhanewicz, J.; et al. Combined parallel and partial fourier MR reconstruction for accelerated 8-channel hyperpolarized carbon-13 in vivo magnetic resonance Spectroscopic imaging (MRSI). J. Magn. Reson. Imaging 2013, 38, 701–713. [Google Scholar] [CrossRef]
- Mispelter, J.; Lupu, M. Homogeneous resonators for magnetic resonance: A review. Comptes Rendus Chim. 2008, 11, 340–355. [Google Scholar] [CrossRef]
- Giovannetti, G.; Frijia, F.; Attanasio, S.; Menichetti, L.; Hartwig, V.; Vanello, N.; Ardenkjaer-Larsen, J.H.; De Marchi, D.; Positano, V.; Schulte, R.; et al. Magnetic resonance butterfly coils: Design and application for hyperpolarized 13C studies. Measurement 2013, 46, 3282–3290. [Google Scholar] [CrossRef]
- Yeung, D.; Hutchison, J.M.; Lurie, D.J. An efficient birdcage resonator at 2.5 MHz using a novel multilayer self-capacitance construction technique. MAGMA 1995, 3, 163–168. Available online: https://link.springer.com/article/10.1007/BF01771702 (accessed on 10 January 2024). [CrossRef]
- 100C Series Porcelain Multilayer High RF Power Capacitors (MLCs). Available online: https://rfs.kyocera-avx.com/Product/25/100_C_Series_Porcelain_Multilayer_High_RF_Power_Capacitors_(MLCs) (accessed on 11 December 2023).
- Hoult, D.I.; Lauterbur, P.C. The sensitivity of the zeugmatographic experiment involving human samples. J. Magn. Reson. 1979, 34, 425–433. [Google Scholar] [CrossRef]
- Belevitch, V. Lateral skin effect in a flat conductor. Philips Tech. Rev. 1971, 32, 221–231. [Google Scholar]
- Giovannetti, G.; Hartwig, V.; Landini, L.; Santarelli, M.F. Classical and lateral skin effect contributions estimation in strip MR coils. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2012, 41, 57–61. [Google Scholar] [CrossRef]
- Grafendorfer, T.; Conolly, S.; Matter, N.; Pauly, J.; Scott, G. Optimized Litz Coil Design for Prepolarized Extremity MRI. Proc. Intl. Soc. Mag. Reson. Med. 2006, 14, 2613. [Google Scholar]
- Wright, A.C.; Song, H.K.; Wehrli, F.W. In vivo MR micro imaging with conventional radiofrequency coils cooled to 77 degrees K. Magn. Reson. Med. 2000, 43, 163–169. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Possible highTc superconductivity in the Ba−La−Cu−O system. Z. Für Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Giovannetti, G.; Hartwig, V.; Viti, V.; Zadaricchio, P.; Meini, L.; Landini, L.; Benassi, A. Low field elliptical MR coil array designed by FDTD. Conc. Magn. Reson. Part B Magn. Reson. Eng. 2008, 33, 32–38. [Google Scholar] [CrossRef]
- Giovannetti, G.; Fontana, N.; Monorchio, A.; Tosetti, M.; Tiberi, G. Estimation of losses in strip and circular wire conductors of radiofrequency planar surface coil by using the finite element method. Conc. Magn. Reson. Part B Magn. Reson. Eng. 2017, 47, e21358. [Google Scholar] [CrossRef]
- Kumar, A.; Bottomley, P.A. Optimized quadrature surface coil designs. Magn. Reson. Mater. Phys. Biol. Med. 2008, 21, 41. [Google Scholar] [CrossRef]
- Hartwig, V.; Vanello, N.; Giovannetti, G.; De Marchi, D.; Lombardi, M.; Landini, L.; Santarelli, M.F. B(1)(+)/actual flip angle and reception sensitivity mapping methods: Simulation and comparison. Magn. Reson. Imaging 2011, 29, 717–722. [Google Scholar] [CrossRef]
- Hartwig, V.; Giovannetti, G.; Vanello, N.; Landini, L.; Santarelli, M.F. Numerical Calculation of Peak-to-Average Specific Absorption Rate on Different Human Thorax Models for Magnetic Resonance Safety Considerations. Appl. Magn. Reson. 2010, 38, 337–348. [Google Scholar] [CrossRef]
- Chen, C.N.; Hoult, D.I. Biomedical Magnetic Resonance Technology; Adam Hilger: Bristol, UK, 1989. [Google Scholar]
- Giovannetti, G.; Hartwig, V.; Positano, V.; Vanello, N. Radiofrequency coils for magnetic resonance applications: Theory, design, and evaluation. Crit. Rev. Biomed. Eng. 2014, 42, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, G.; Landini, L.; Santarelli, M.F.; Positano, V. A fast and accurate simulator for the design of birdcage coils in MRI. Magn. Reson. Mater. Phys. Biol. Med. 2002, 15, 36–44. [Google Scholar] [CrossRef]
- Lucchini, F.; Torchio, R.; Cirimele, V.; Alotto, P.; Bettini, P. Topology optimization for electromagnetics: A survey. IEEE Access 2022, 10, 98593–98611. [Google Scholar] [CrossRef]
- Barmada, S.; Fontana, N.; Sani, L.; Thomopulos, D.; Tucci, M. Deep Learning and Reduced Models for Fast Optimization in Electromagnetics. IEEE Trans. Magn. 2020, 56, 7513604. [Google Scholar] [CrossRef]
- Barmada, S.; Fontana, N.; Formisano, A.; Thomopulos, D.; Tucci, M. A Deep Learning Surrogate Model for Topology Optimization. IEEE Trans. Magn. 2021, 57, 7200504. [Google Scholar] [CrossRef]
- Khan, A.; Ghorbanian, V.; Lowther, D. Deep Learning for Magnetic Field Estimation. IEEE Trans. Magn. 2019, 55, 7202304. [Google Scholar] [CrossRef]
- Sasaki, H.; Igarashi, H. Topology Optimization Accelerated by Deep Learning. IEEE Trans. Magn. 2019, 55, 7401305. [Google Scholar] [CrossRef]
- Li, Y.; Lei, G.; Bramerdorfer, G.; Peng, S.; Sun, X.; Zhu, J. Machine Learning for Design Optimization of Electromagnetic Devices: Recent Developments and Future Directions. Appl. Sci. 2021, 11, 1627. [Google Scholar] [CrossRef]
- Pollok, S.; Bjørk, R.; Jørgensen, P.S. Inverse Design of Magnetic Fields using Deep Learning. IEEE Trans. Magn. 2021, 57, 2101604. [Google Scholar] [CrossRef]
- Barmada, S.; Formisano, A.; Thomopulos, D.; Tucci, M. Deep learning as a tool for inverse problems resolution: A case study. COMPEL Int. J. Comput. Math. Electr. Electron. Eng. 2022, 41, 2120–2133. [Google Scholar] [CrossRef]
- Di Barba, P.; Mognaschi, M.E.; Sieni, E.; Ziolkowski, M. Convolutional neural networks for the shape design of a magnetic core for material testing: Forward and inverse approaches. Int. J. Appl. Electromagn. Mech. 2022, 69, 389–399. [Google Scholar] [CrossRef]
- Amjad, A.; Kamondetdacha, R.; Kildishev, A.V.; Park, S.M.; Nyenhuis, J.A. Power deposition inside a phantom for testing of MRI heating. IEEE Trans. Magn. 2005, 41, 4185–4187. [Google Scholar] [CrossRef]
- Jin, K.H.; McCann, M.T.; Froustey, E.; Unser, M. Deep Convolutional Neural Network for Inverse Problems in Imaging. IEEE Trans. Image Process. 2017, 26, 4509–4522. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Cheng, J.; Ke, Z.; Ying, L. Deep Magnetic Resonance Image Reconstruction: Inverse Problems Meet Neural Networks. IEEE Signal Process. Mag. 2020, 37, 141–151. [Google Scholar] [CrossRef]
- Ongie, G.; Jalal, A.; Metzler, C.A.; Baraniuk, R.G.; Dimakis, A.G.; Willett, R. Deep Learning Techniques for Inverse Problems in Imaging. arXiv 2020, arXiv:2005.06001. [Google Scholar] [CrossRef]
- Barmada, S.; Di Barba, P.; Formisano, A.; Mognaschi, M.E.; Tucci, M. Learning-Based Approaches to Current Identification from Magnetic Sensors. Sensors 2023, 23, 3832. [Google Scholar] [CrossRef] [PubMed]
- Puzyrev, V. Deep learning electromagnetic inversion with convolutional neural networks. Geophys. J. Int. 2019, 218, 817–832. [Google Scholar] [CrossRef]
- Barmada, S.; Barba, P.D.; Fontana, N.; Mognaschi, M.E.; Tucci, M. Electromagnetic Field Reconstruction and Source Identification Using Conditional Variational Autoencoder and CNN. IEEE J. Multiscale Multiphysics Comput. Tech. 2023, 8, 322–331. [Google Scholar] [CrossRef]
- Kanmaz, T.B.; Ozturk, E.; Demir, H.V.; Gunduz-Demir, C. Deep-learning-enabled electromagnetic near-field prediction and inverse design of metasurfaces. Optica 2023, 10, 1373–1382. [Google Scholar] [CrossRef]
- Yau, D.; Crozier, S. A Genetic Algorithm/Method of Moments Approach to the Optimization of an RF Coil for MRI Applications—Theoretical Considerations—Abstract. J. Electromagn. Waves Appl. 2003, 17, 753–754. [Google Scholar] [CrossRef]
- Rogovich, A.; Nepa, P.; Manara, G.; Monorchio, A. RF coils for MRI applications—A design procedure. In Proceedings of the 2005 IEEE Antennas and Propagation Society International Symposium, Washington, DC, USA, 3–8 July 2005; Volume 1, pp. 856–859. [Google Scholar] [CrossRef]
- Hadley, J.R.; Parker, D.; Furse, C.M. RF Coil Design for MRI Using a Genetic Algorithm. Appl. Comput. Electromagn. Soc. J. 2007, 22, 277–286. [Google Scholar]
- Nadig, K.; Potter, W.M.; Potter, W.D. Homogeneous RF Coil Design Using a GA. In Advanced Research in Applied Artificial Intelligence; Jiang, H., Ding, W., Ali, M., Wu, X., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; pp. 197–205. [Google Scholar] [CrossRef]
- Arshad, M.; Qureshi, M.; Inam, O.; Omer, H. Transfer learning in deep neural network-based receiver coil sensitivity map estimation. MAGMA 2021, 34, 717–728. [Google Scholar] [CrossRef]
- Hui, P.; Shen, G.X. Application of artificial neural network methods in HTS RF coil design for MRI. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2003, 18, 9–14. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannetti, G.; Fontana, N.; Flori, A.; Santarelli, M.F.; Tucci, M.; Positano, V.; Barmada, S.; Frijia, F. Machine Learning for the Design and the Simulation of Radiofrequency Magnetic Resonance Coils: Literature Review, Challenges, and Perspectives. Sensors 2024, 24, 1954. https://doi.org/10.3390/s24061954
Giovannetti G, Fontana N, Flori A, Santarelli MF, Tucci M, Positano V, Barmada S, Frijia F. Machine Learning for the Design and the Simulation of Radiofrequency Magnetic Resonance Coils: Literature Review, Challenges, and Perspectives. Sensors. 2024; 24(6):1954. https://doi.org/10.3390/s24061954
Chicago/Turabian StyleGiovannetti, Giulio, Nunzia Fontana, Alessandra Flori, Maria Filomena Santarelli, Mauro Tucci, Vincenzo Positano, Sami Barmada, and Francesca Frijia. 2024. "Machine Learning for the Design and the Simulation of Radiofrequency Magnetic Resonance Coils: Literature Review, Challenges, and Perspectives" Sensors 24, no. 6: 1954. https://doi.org/10.3390/s24061954





