Aluminum Nitride Thin Film Piezoelectric Pressure Sensor for Respiratory Rate Detection
Abstract
1. Introduction
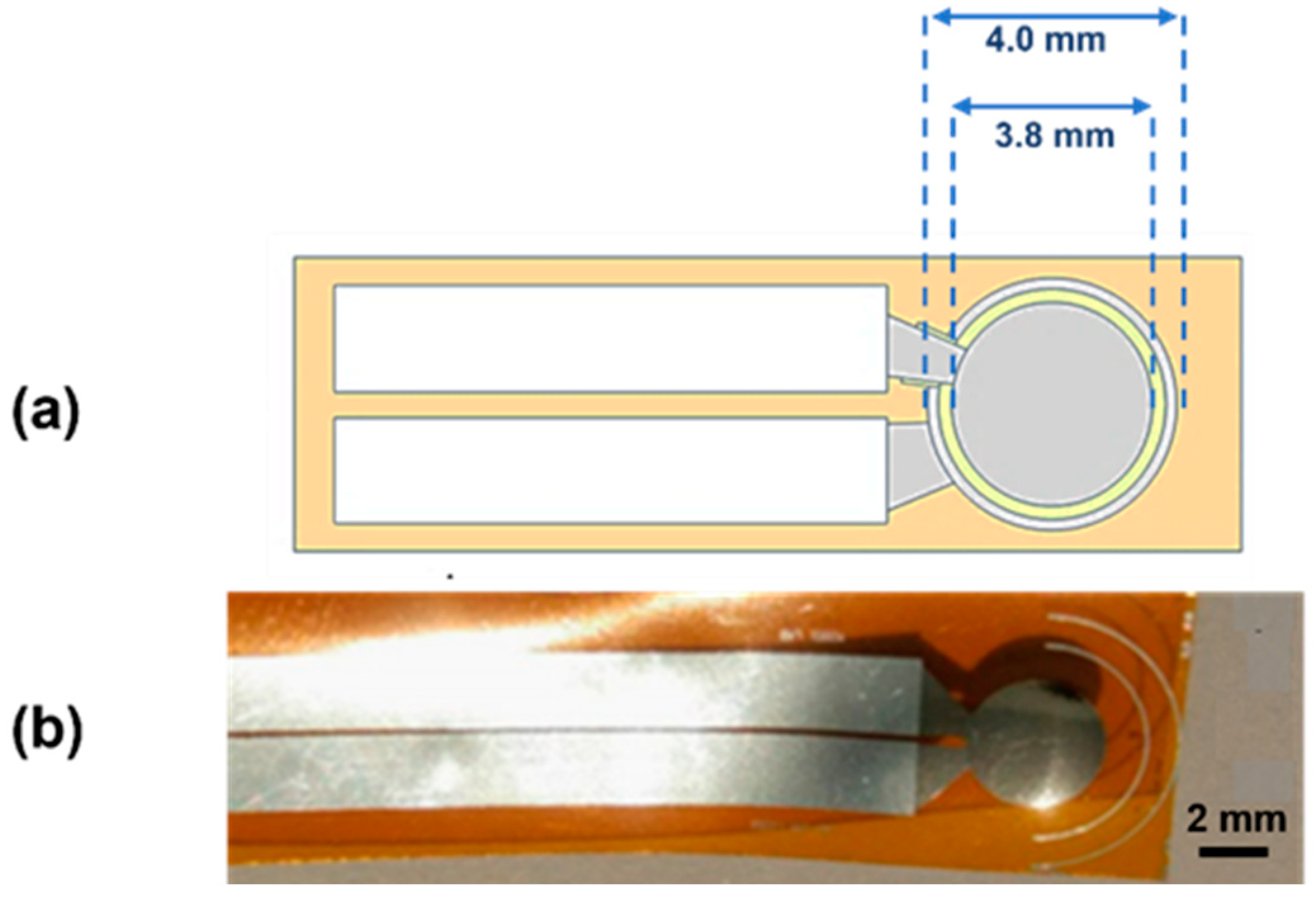
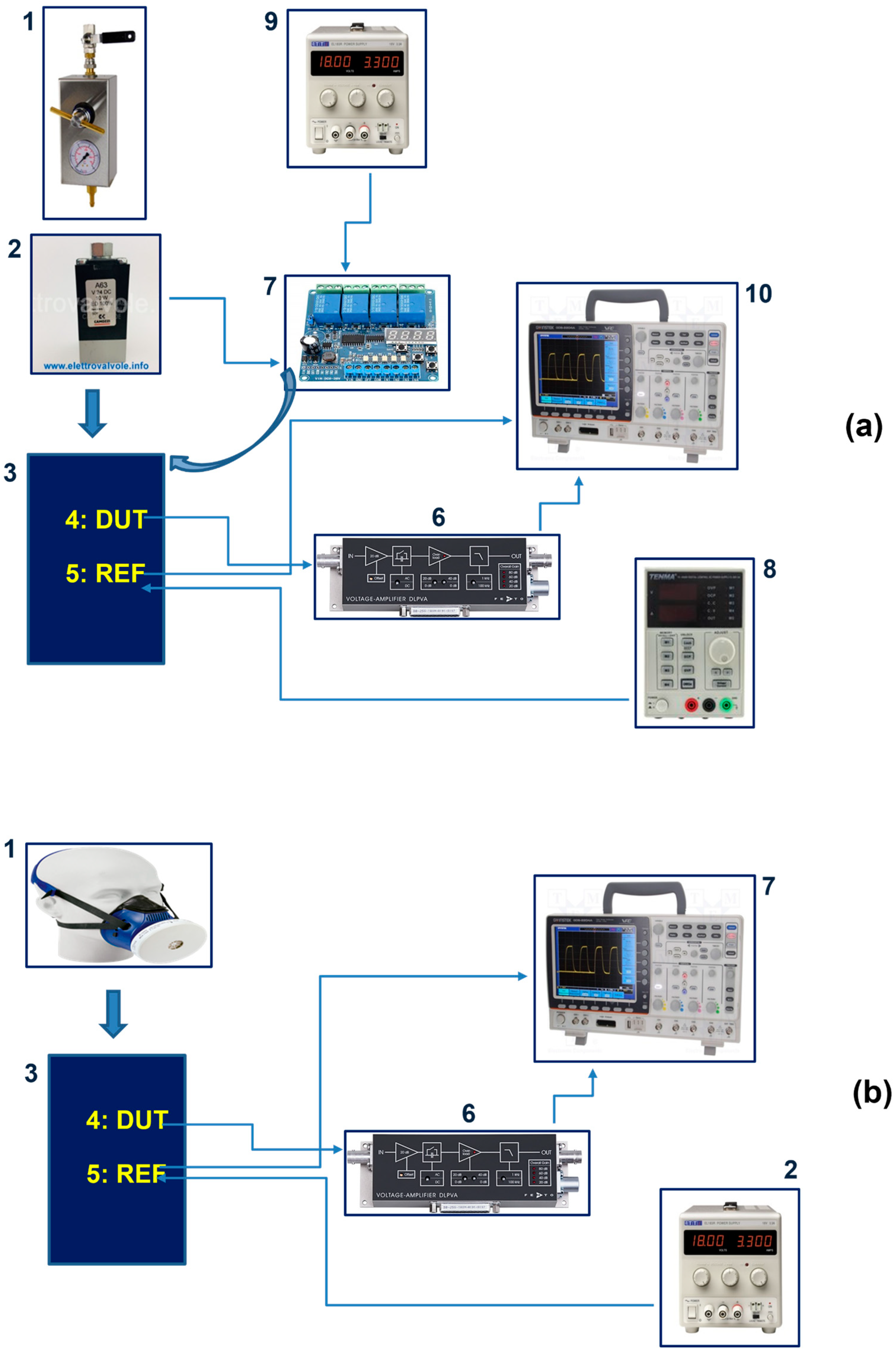
2. Materials and Methods
3. Results and Discussion
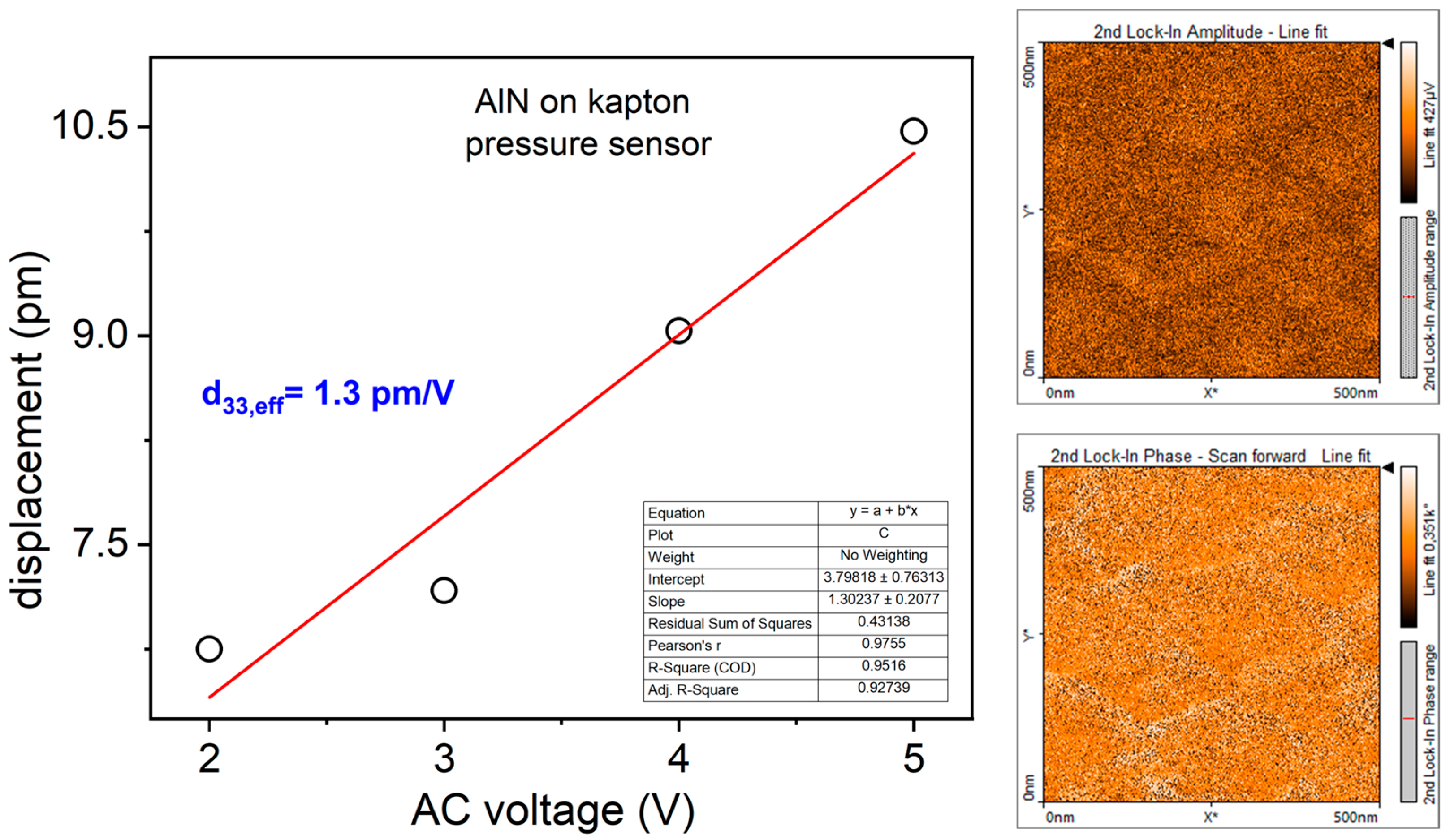
3.1. Morphological, Structural, and Piezoelectric Characterization of AlN Thin Film: AFM, XRD and PFM Analyses
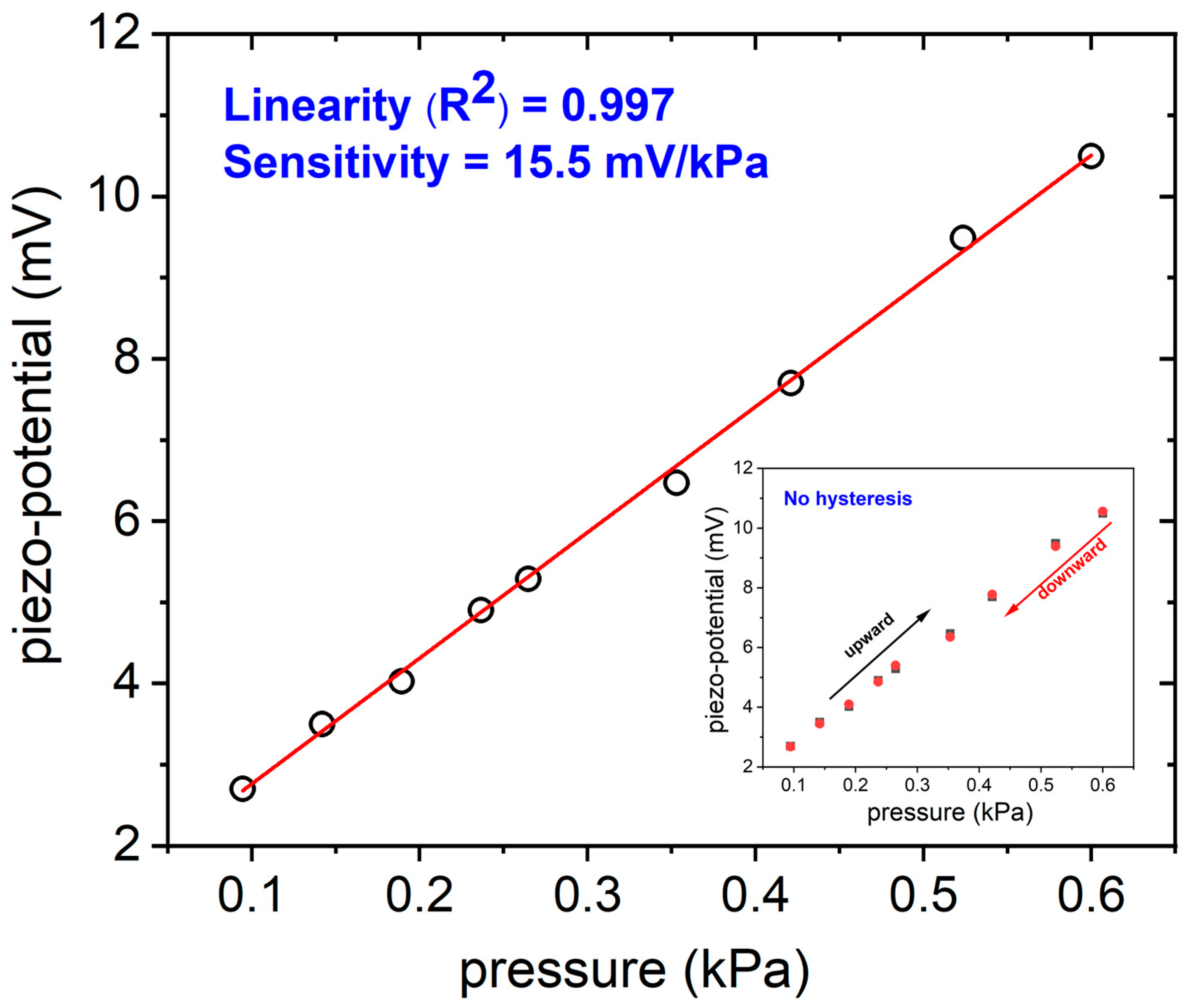
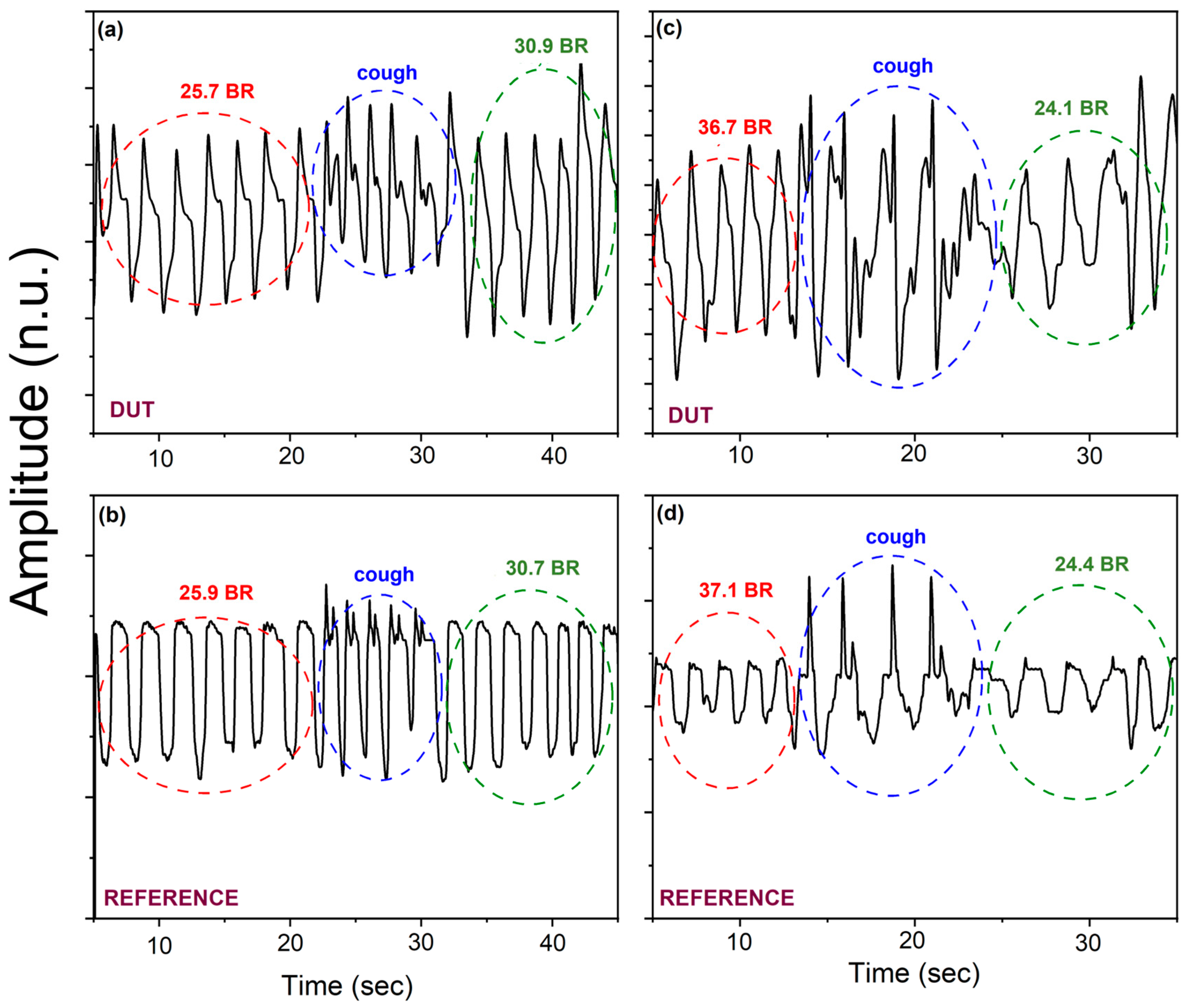
3.2. Piezoelectric Pressure Sensor Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seesaard, T.; Wongchoosuk, C. Flexible and Stretchable Pressure Sensors: From Basic Principles to State-of-the-Art Applications. Micromachines 2023, 14, 1638. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, L.; Wu, T.; Song, H.; Tang, C. Flexible piezoelectric pressure sensor with high sensitivity for electronic skin using near-field electrohydrodynamic direct-writing method. Extr. Mech. Lett. 2021, 48, 101279. [Google Scholar] [CrossRef]
- Long, Z.; Liu, X.; Xu, J.; Huang, Y.; Wang, Z. High-Sensitivity Flexible Piezoresistive Pressure Sensor Using PDMS/MWNTS Nanocomposite Membrane Reinforced with Isopropanol for Pulse Detection. Sensors 2022, 22, 4765. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Liu, Z.; Fu, J.; Shan, T.; Yang, X.; Lei, Q.; Yang, Y.; Li, D. Flexible capacitive pressure sensors for wearable electronics. J. Mater. Chem. C 2022, 10, 1594–1605. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Shi, Y.; Li, L.; Wang, W.; He, L.; Liu, R. Recent Developments for Flexible Pressure Sensors: A Review. Micromachines 2018, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.I.; Lee, J.M.; Moradnia, M.; Chen, J.; Pouladi, S.; Yarali, M.; Kim, J.Y.; Kwon, M.K.; Lee, T.R.; Ryou, J.H. Biocompatible composite thin-film wearable piezoelectric pressure sensor for monitoring of physiological and muscle motions. Soft. Sci. 2022, 2, 8. [Google Scholar] [CrossRef]
- Pang, Z.; Zhao, Y.; Luo, N.; Chen, D.; Chen, M. Flexible pressure and temperature dual-mode sensor based on buckling carbon nanofibers for respiration pattern recognition. Sci. Rep. 2022, 12, 17434. [Google Scholar] [CrossRef]
- MacKinnon, G.E.; Brittain, E.L. Mobile Health Technologies in Cardiopulmonary Disease. Chest 2020, 157, 654–664. [Google Scholar] [CrossRef]
- Miller, D.J.; Capodilupo, J.V.; Lastella, M.; Sargent, C.; Roach, G.D.; Lee, V.H.; Capodilupo, E.R. Analyzing changes in respiratory rate to predict the risk of COVID-19 infection. PLoS ONE 2020, 15, e0243693. [Google Scholar] [CrossRef]
- Kainan, P.; Sinchai, A.; Tuwanut, P.; Wardkein, P. New pulse oximetry detection based on the light absorbance ratio as determined from amplitude modulation indexes in the time and frequency domains. Biomed. Signal Process. Control 2022, 75, 524–525. [Google Scholar] [CrossRef]
- Bao, X.; Abdala, A.K.; Kamavuako, E.N. Estimation of the Respiratory Rate from Localised ECG at Different Auscultation Sites. Sensors 2020, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hui, X.; Zhou, J.; Conroy, T.B.; Kan, E.C. Wearable radio-frequency sensing of respiratory rate, respiratory volume, and heart rate. NPJ Digit. Med. 2020, 3, 98. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, A.S.; Lenahan, J.L.; Izadnegahdar, R.; Ansermino, J.M. A Systematic Review of Tools to Measure Respiratory Rate in Order to Identify Childhood Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Kallioinen, N.; Hill, A.; Christofidis, M.J.; Horswill, M.S.; Watson, M.O. Quantitative systematic review: Sources of inaccuracy in manually measured adult respiratory rate data. J. Adv. Nurs. 2021, 77, 98–124. [Google Scholar] [CrossRef] [PubMed]
- Patout, M.; Arbane, G.; Cuvelier, A.; Muir, J.F.; Hart, N.; Murphy, P.B. Polysomnography versus limited respiratory monitoring and nurse-led titration to optimise non-invasive ventilation set-up: A pilot randomised clinical trial. Thorax 2019, 74, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Frase, L.; Nissen, C.; Spiegelhalder, K.; Feige, B. The importance and limitations of polysomnographyin insomnia disorder—A critical appraisal. J. Sleep Res. 2023, 32, 14036. [Google Scholar] [CrossRef] [PubMed]
- Bok Kim, K.; Jae Baek, H. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics 2023, 12, 2923. [Google Scholar] [CrossRef]
- Naik, G.R.; Breen, P.P.; Jayarathna, T.; Tong, B.K.; Eckert, D.J.; Gargiulo, G.D. Morphic Sensors for Respiratory Parameters Estimation: Validation against Overnight Polysomnography. Biosensors 2023, 13, 703. [Google Scholar] [CrossRef]
- Ming, D.K.; Sangkaew, S.; Chanh, H.Q.; Nhat, P.T.H.; Yacoub, S.; Georgiou, P.; Holmes, A.H. Continuous physiological monitoring using wearable technology to inform individual management of infectious diseases, public health and outbreak responses. J. Infect. Dis 2020, 96, 648–654. [Google Scholar] [CrossRef]
- Gordon, B.D.; Darius, F.; Margaret, L.; Andrew, B.; Janek, M.; Arvind, D.K. Classifying signals from a wearable accelerometer device to measure respiratory rate. ERJ Open Res. 2021, 7, 00681–02020. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H.; Xu, H.; Jin, Y.; Chen, G.; Zheng, W.; Wang, W.; Wang, Y.; Gao, L. Highly sensitive and wearable bionic piezoelectric sensor for human respiratory monitoring. Sens. Act. A Phys. 2022, 345, 113818. [Google Scholar] [CrossRef]
- Pandit, S.; Schneider, M.; Berger, C.; Schwarz, S.; Schmid, U. Impact of AlN seed layer on microstructure and piezoelectric properties of YxAl1-xN (x=15%) thin films. Adv. Electron. Mater. 2023, 9, 2200789. [Google Scholar] [CrossRef]
- Sapra, A.; Malik, A.; Bhandari, P. Vital Sign Assessment. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553213/ (accessed on 29 November 2023).
- Bujan, B.; Fischer, T.; Dietz-Terjung, S.; Bauerfeind, A.; Jedrysiak, P.; Sundrup, M.G.; Hamann, J.; Schöbel, C. Clinical validation of a contactless respiration rate monitor. Sci. Rep. 2023, 13, 3480. [Google Scholar] [CrossRef] [PubMed]
- Makowski, S.; Zawischa, M.; Schneider, D.; Barth, S.; Schettler, S.; Hoang, T.T.; Bartzsch, H.; Zimmermann, M. Surface acoustic wave spectroscopy for non-destructive coating and bulk characterization at temperatures up to 600 °C enabled by piezoelectric aluminum nitride coated sensor. Surf. Interface Anal. 2024, 1–14. [Google Scholar] [CrossRef]
- Kim, N.-I.; Yarali, M.; Moradnia, M.; Aqib, M.; Liao, C.-H.; AlQatari, F.; Nong, M.; Li, X.; Ryou, J.-H. Piezoelectric Sensors Operating at Very High Temperatures and in Extreme Environments Made of Flexible Ultrawide-Bandgap Single-Crystalline AlN Thin Films. Adv. Funct. Mater. 2023, 33, 2212538. [Google Scholar] [CrossRef]
- Telló, M.; Oliveira, L.; Parise, O.; Buzaid, A.C.; Oliveira, R.T.; Zanella, R.; Cardona, A. Electrochemical Therapy to Treat Cancer (In Vivo Treatment). In Proceedings of the 29th Annual International Conference of the IEEE EMBS Cité Internationale, Lyon, France, 23–26 August 2007. [Google Scholar]
- Mahmood Ali, S.; Mahmood, M.S.; Mahmood, N.S. Design of a Low-Cost Ventilator to Support Breathing for Patients with Respiratory Failure Arising from COVID-19. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 012143. [Google Scholar] [CrossRef]
- Constantin, C.P.; Aflori, M.; Damian, R.F.; Rusu, R.D. Biocompatibility of Polyimides: A Mini-Review. Materials 2019, 27, 3166. [Google Scholar] [CrossRef]
- Signore, M.A.; Velardi, L.; De Pascali, C.; Kuznetsova, I.; Blasi, L.; Biscaglia, F.; Quaranta, F.; Siciliano, P.; Francioso, L. Effect of silicon-based substrates and deposition type on sputtered AlN thin films: Physical & chemical properties and suitability for piezoelectric device integration. Appl. Surf. Sci. 2022, 599, 154017. [Google Scholar] [CrossRef]
- Hanawa, T. Biocompatibility of titanium from the viewpoint of its surface. Sci. Technol. Adv. Mater. 2022, 15, 457–472. [Google Scholar] [CrossRef]
- Signore, M.A.; Rescio, G.; De Pascali, C.; Iacovacci, V.; Dario, P.; Leone, A.; Quaranta, F.; Taurino, A.; Siciliano, P.; Francioso, L. Fabrication and characterization of AlN-based fexible piezoelectric pressure sensor integrated into an implantable artificial pancreas. Sci. Rep. 2019, 9, 17130. [Google Scholar] [CrossRef] [PubMed]
- Pleil, J.D.; Wallace, M.A.G.; Davis, M.D.; Matty, C.M. The Physics of human breathing: Flow, timing, volume, and pressure parameters for normal, on-demand, and ventilator respiration. J. Breath Res. 2021, 15, 042002. [Google Scholar] [CrossRef]
- Hu, J.; Duan, W.; Fan, S.; Xiao, H. A triangular wavy substrate-integrated wearable and flexible piezoelectric sensor for a linear pressure measurement and application in human health monitoring. Measurement 2022, 190, 110724. [Google Scholar] [CrossRef]
- Avalur, D.S. Human Breath Detection Using a Microphone. Master’s Thesis, University of Groningen, Groningen, The Netherlands, 30 August 2013. Available online: https://www.cs.rug.nl/~aiellom/tesi/avalur (accessed on 29 November 2023).
- Wang, A.; Hu, M.; Zhou, L.; Qiang, X. Self-Powered Wearable Pressure Sensors with Enhanced Piezoelectric Properties of Aligned P(VDF-TrFE)/MWCNT Composites for Monitoring Human Physiological and Muscle Motion Signs. Nanomaterials 2018, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, G.; Hong, Y.; He, W.; Wang, S.; Chen, Y.; Wang, C.; Tang, Y.; Sun, Y.; Zhu, Y. Highly sensitive, flexible and wearable piezoelectric motion sensor based on PT promoted β-phase PVDF. Sens. Act. A Phys. 2022, 337, 113415. [Google Scholar] [CrossRef]
- Panahi, A.; Hassanzadeh, A.; Moulavi, A. Design of a low cost, double triangle, piezoelectric sensor for respiratory monitoring applications. Sens. Bio-Sens. Res. 2020, 30, 100378. [Google Scholar] [CrossRef]

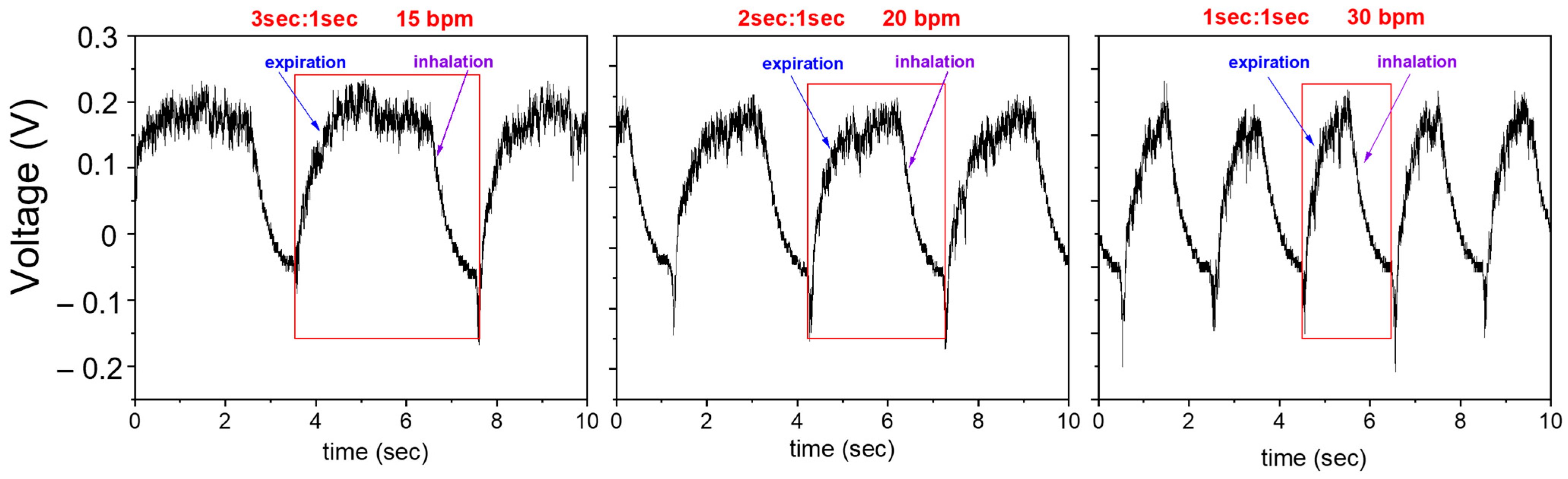


| Case of Study | E:I | bpm |
|---|---|---|
| A—moderate difficult breathing | 1:1 | 30 |
| B—borderline normal breathing | 2:1 | 20 |
| C—normal breathing | 3:1 | 15 |
| Material | Sensitivity | Working Range | Active Area Size (mm2) | References |
|---|---|---|---|---|
| PVDF | 19 mV kPa−1 | 0–500 Pa | 420 | [35] |
| P(VDF-TrFE)/MWCNT | 540 mV N−1 | 0.5–5 N | 225 | [37] |
| PVDF | 4.96 V N−1 940 mV N−1 | 0–0.3 N 0.3–3.8 N | 180 | [38] |
| AlN thin film | 15.5 mV kPa−1 | 0.08–0.6 kPa 0.001–0.006 N | 11.34 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signore, M.A.; Rescio, G.; Francioso, L.; Casino, F.; Leone, A. Aluminum Nitride Thin Film Piezoelectric Pressure Sensor for Respiratory Rate Detection. Sensors 2024, 24, 2071. https://doi.org/10.3390/s24072071
Signore MA, Rescio G, Francioso L, Casino F, Leone A. Aluminum Nitride Thin Film Piezoelectric Pressure Sensor for Respiratory Rate Detection. Sensors. 2024; 24(7):2071. https://doi.org/10.3390/s24072071
Chicago/Turabian StyleSignore, Maria Assunta, Gabriele Rescio, Luca Francioso, Flavio Casino, and Alessandro Leone. 2024. "Aluminum Nitride Thin Film Piezoelectric Pressure Sensor for Respiratory Rate Detection" Sensors 24, no. 7: 2071. https://doi.org/10.3390/s24072071
APA StyleSignore, M. A., Rescio, G., Francioso, L., Casino, F., & Leone, A. (2024). Aluminum Nitride Thin Film Piezoelectric Pressure Sensor for Respiratory Rate Detection. Sensors, 24(7), 2071. https://doi.org/10.3390/s24072071








