Non-Invasive Alcohol Concentration Measurement Using a Spectroscopic Module: Outlook for the Development of a Drunk Driving Prevention System
Abstract
1. Introduction
2. Research Background
3. Materials and Methods
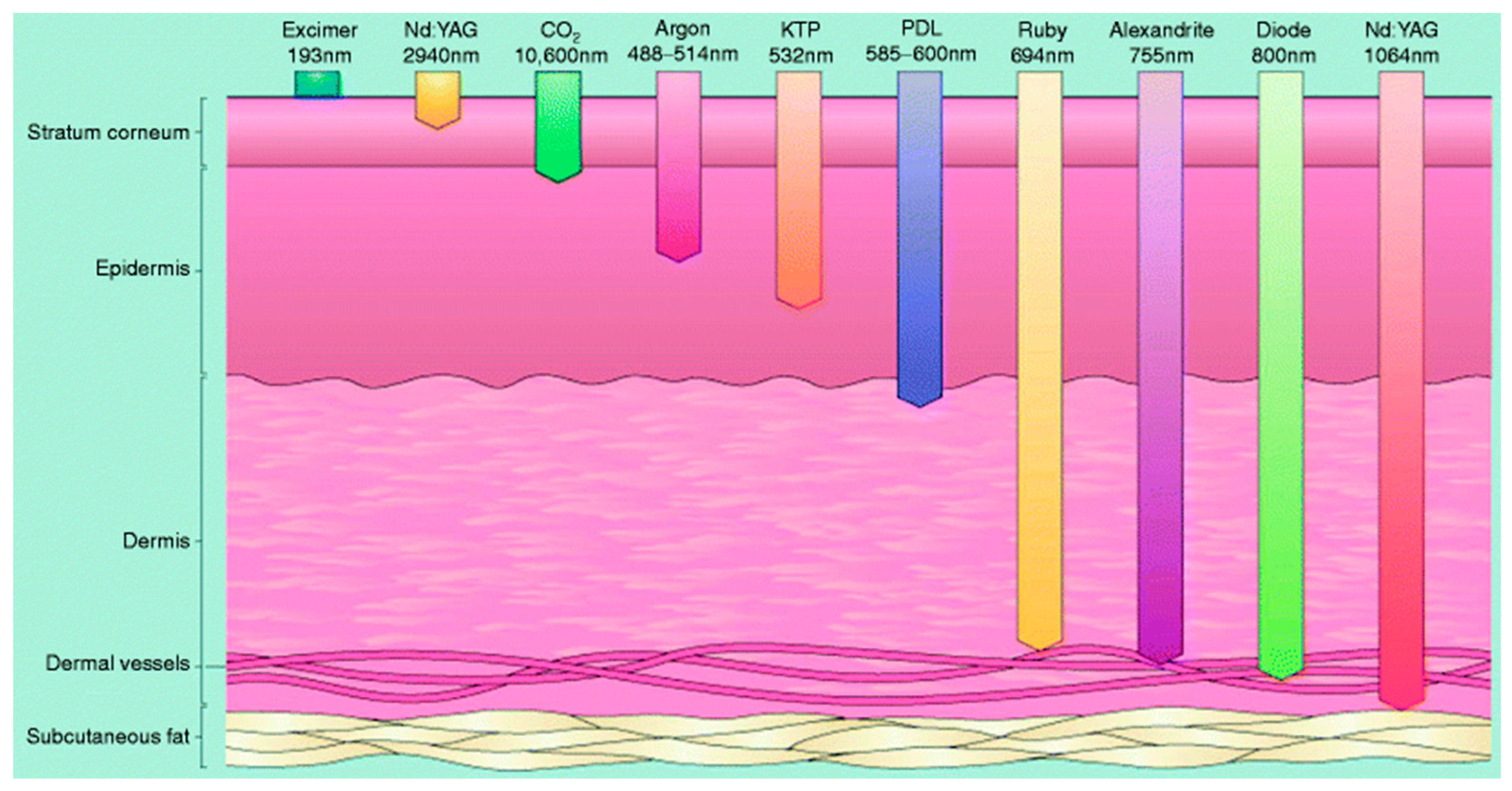
3.1. Characteristics of Infrared Light Source
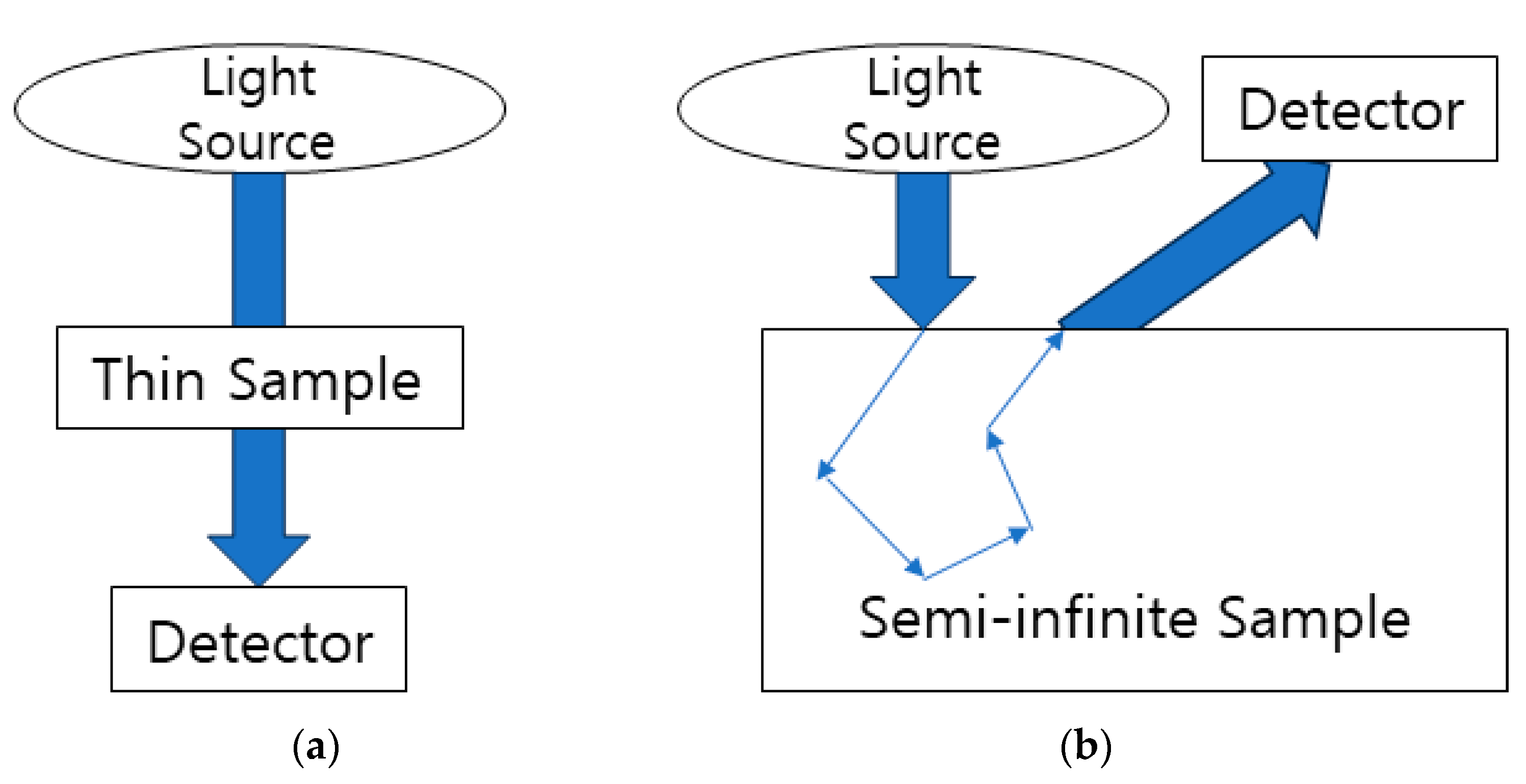
3.2. Reflectance and Transmittance Measurement Methods
3.3. Sample Preparation
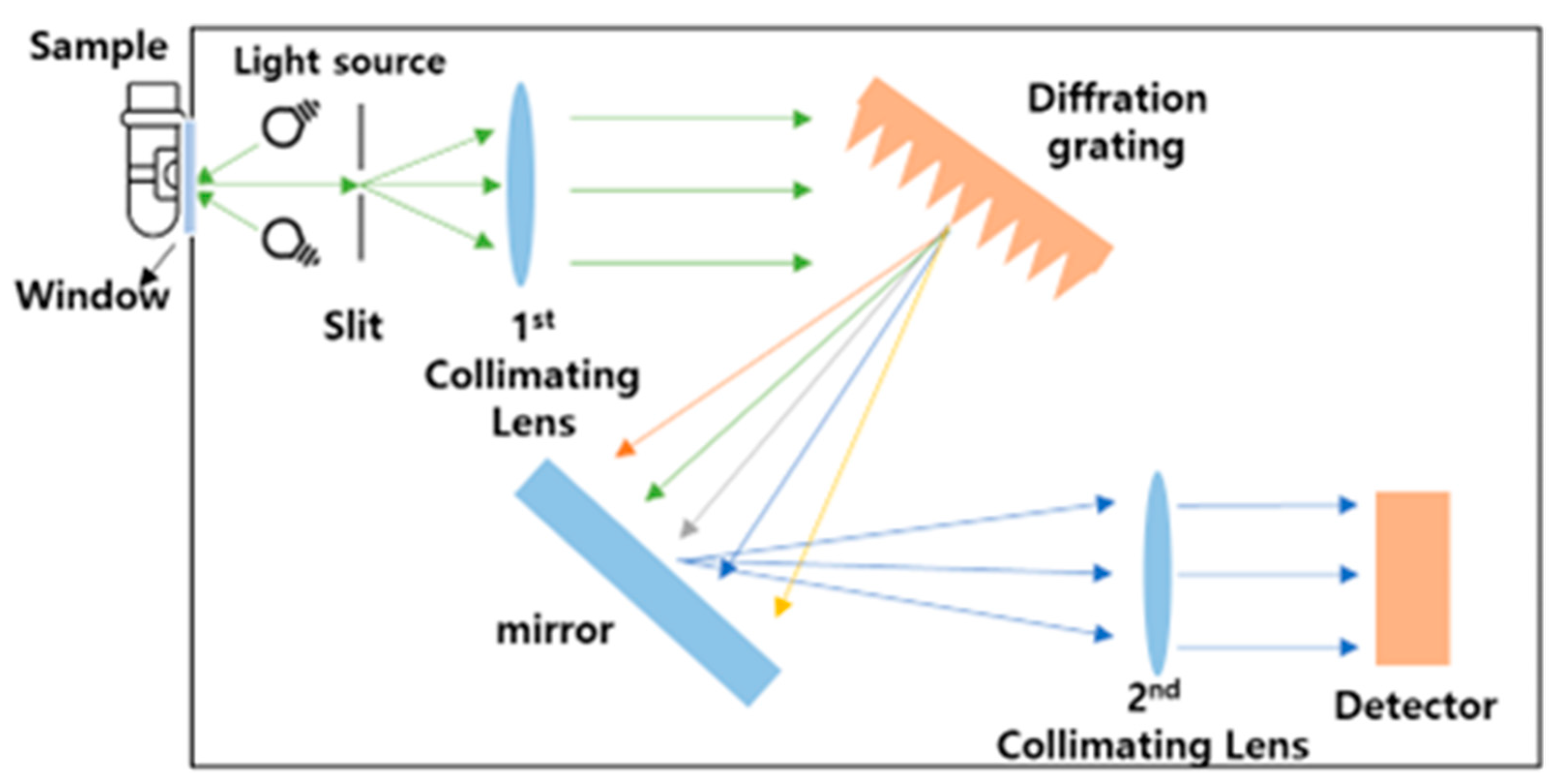
3.4. Sample Measurement Procedures
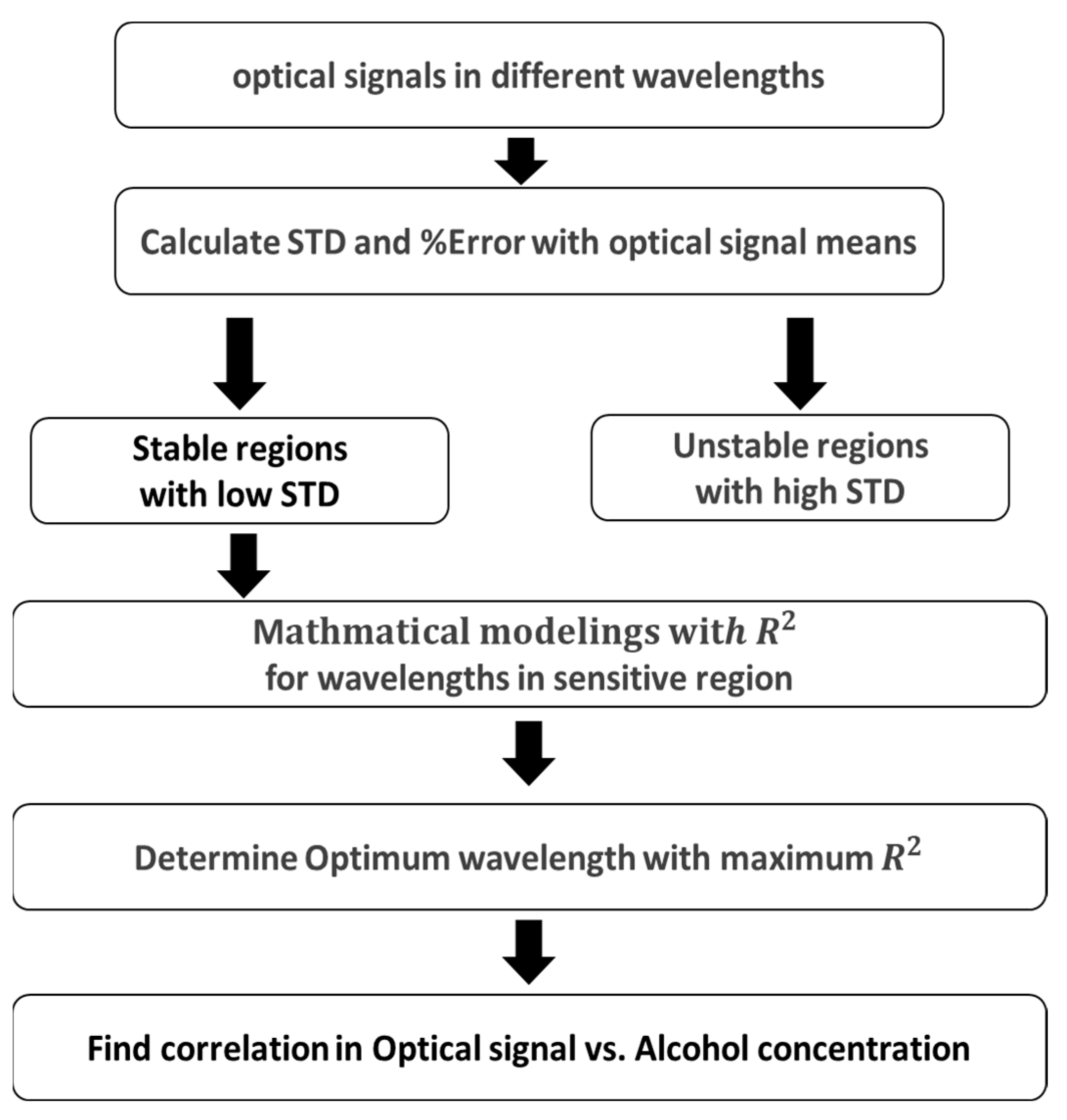
3.5. Data Analysis and Error Minimization
4. Results and Discussion
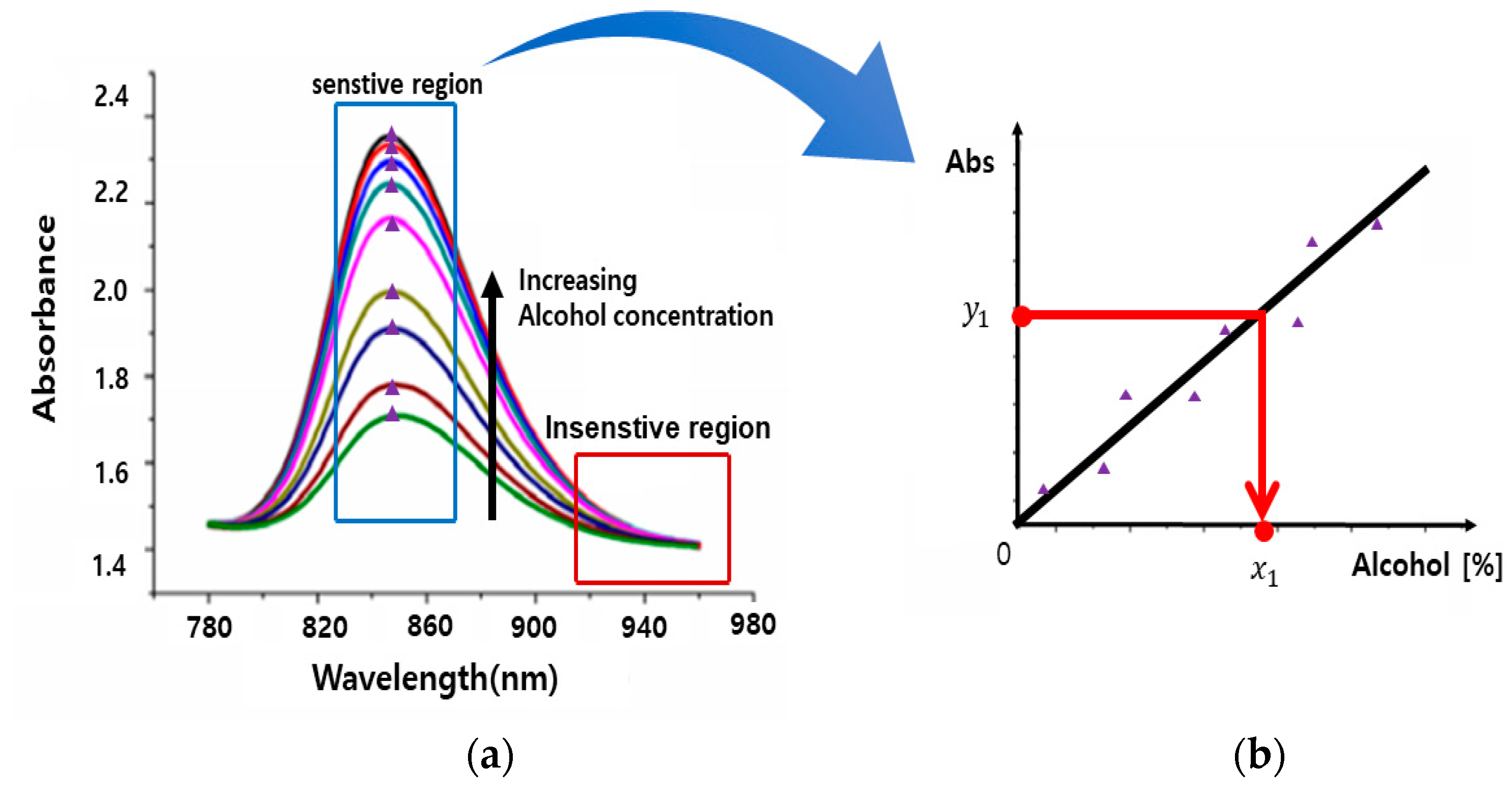
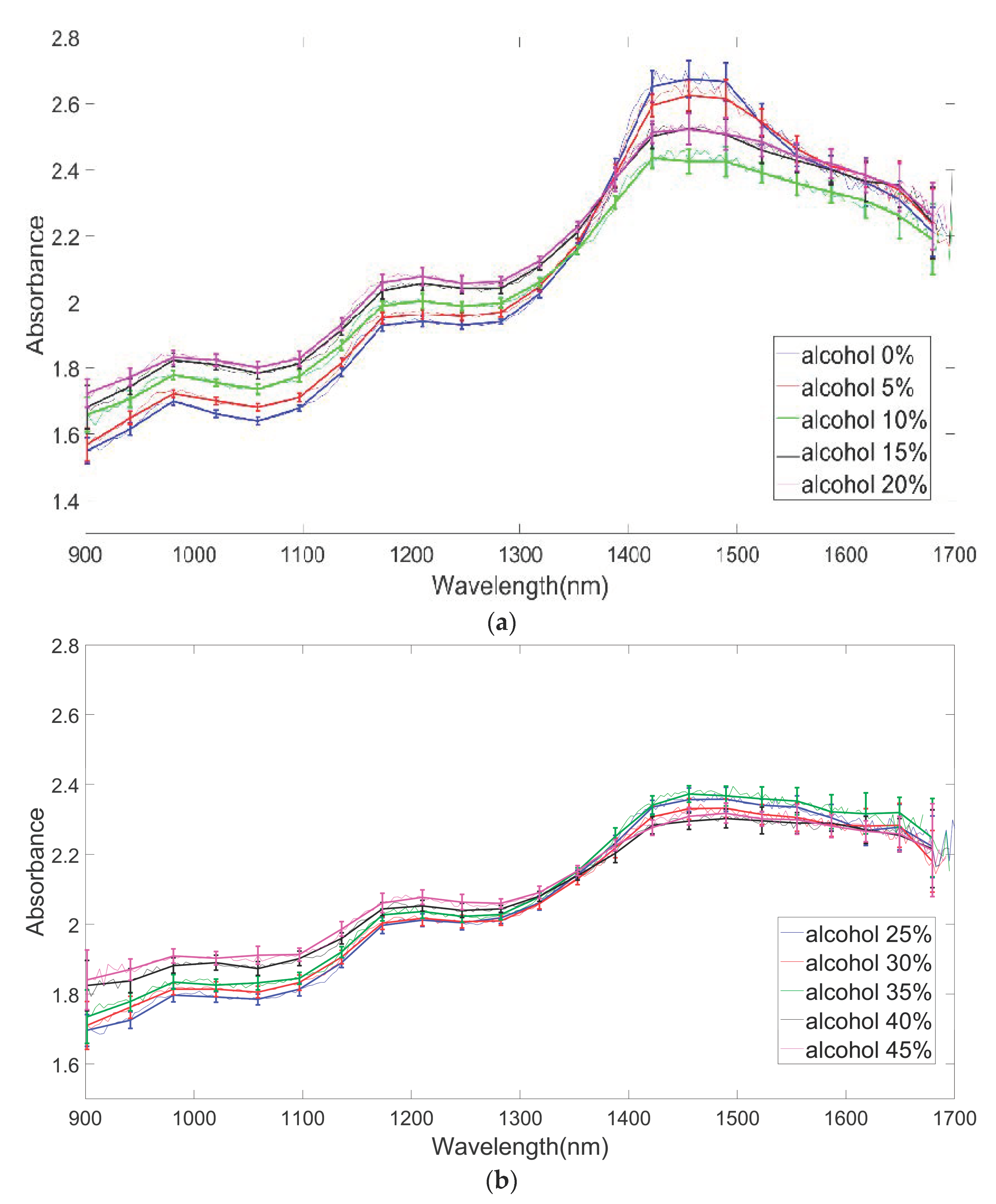
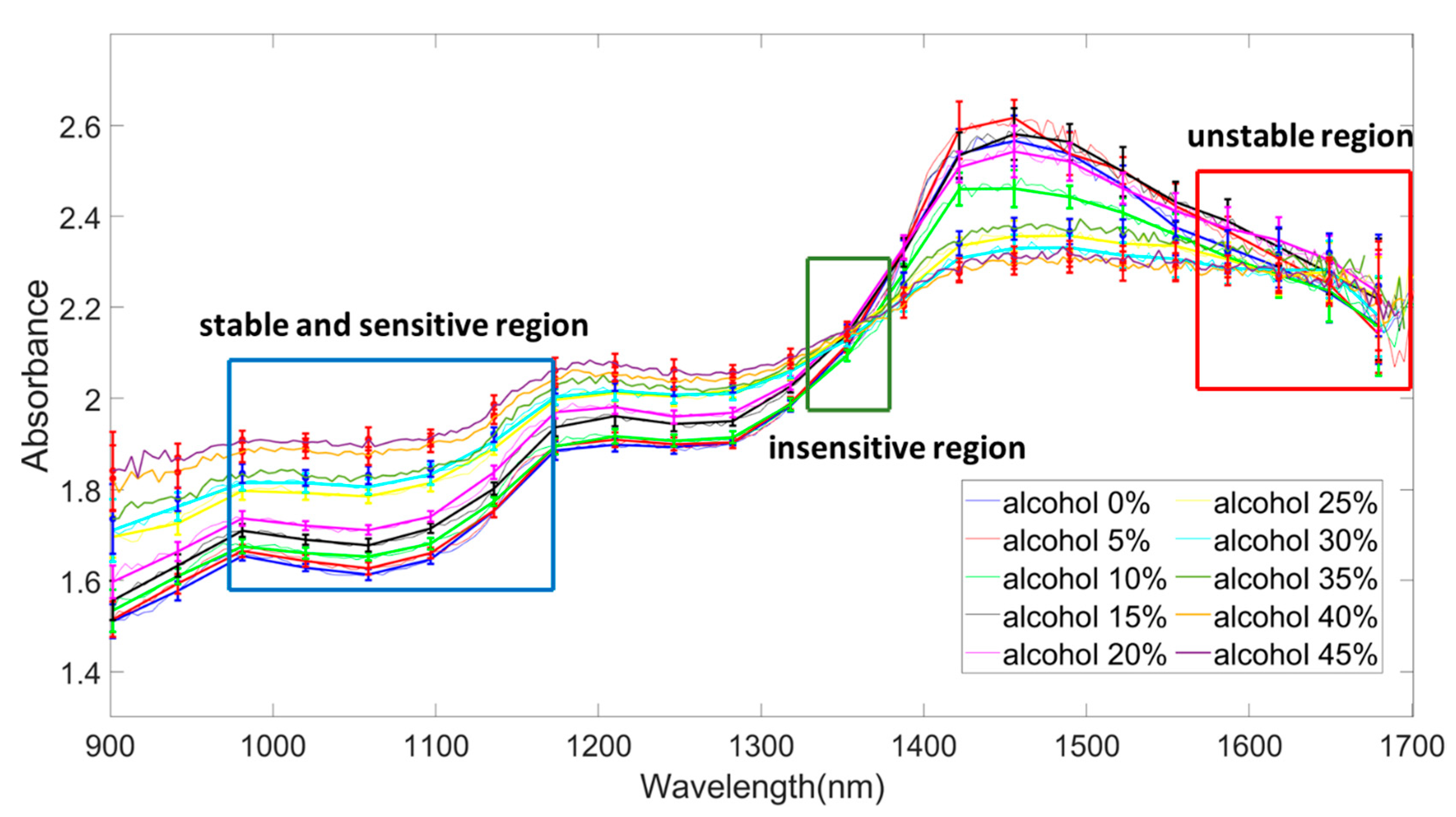
4.1. Optical Signals as a Function of Wavelength: Determination of Stable, Unstable, and Sensitive Region
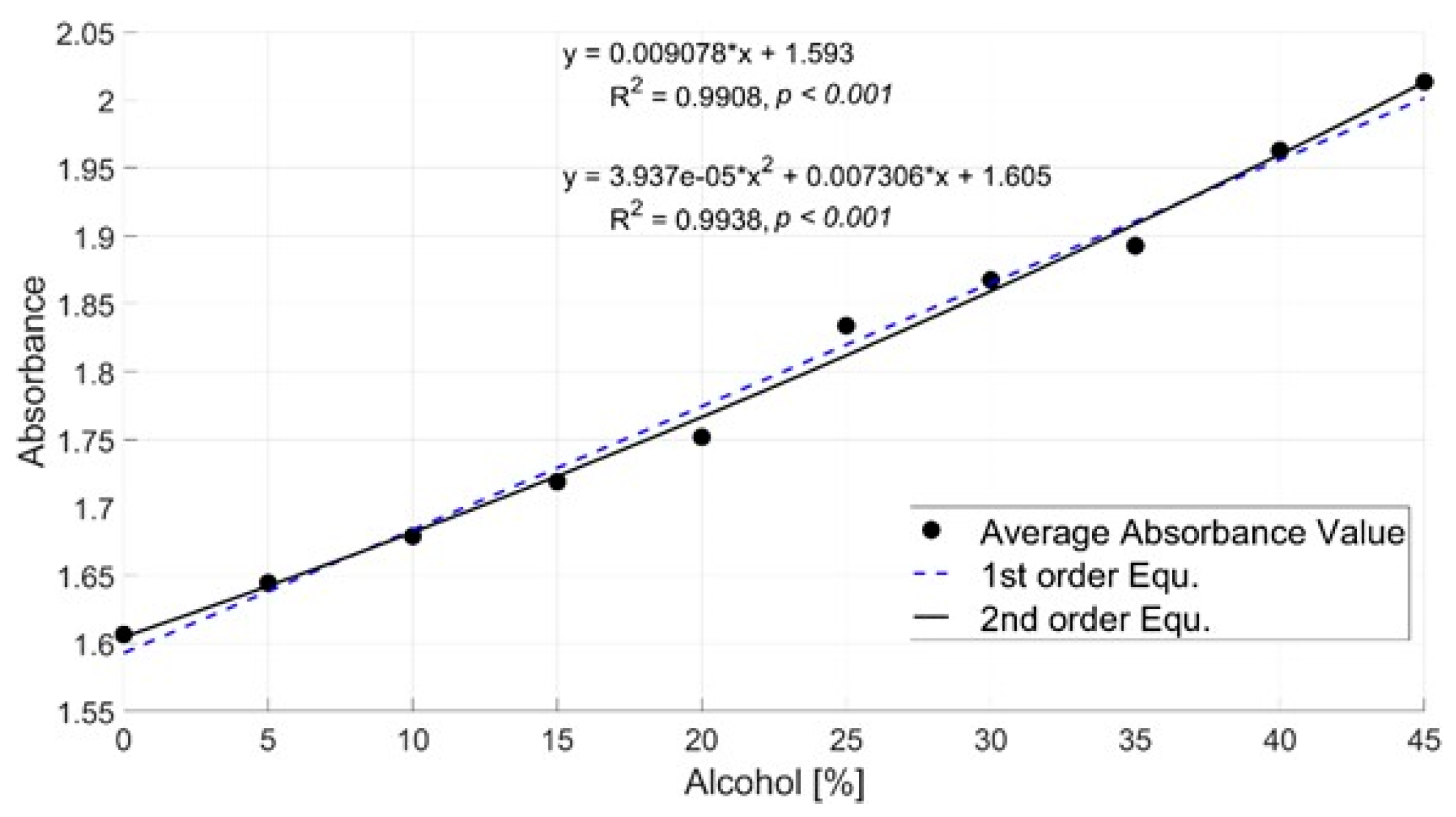
4.2. Alcohol Concentration Sensing through Mathematical Modeling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papalimperi, A.H.; Athanaselis, S.A.; Mina, A.D.; Papoutsis, I.I.; Spiliopoulou, C.A.; Papadodima, S.A. Incidence of fatalities of road traffic accidents associated with alcohol consumption and the use of psychoactive drugs: A 7-year survey (2011–2017). Exp. Ther. Med. 2019, 18, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Behnood, A.; Mannering, F.L. The effects of drug and alcohol consumption on driver injury severities in single-vehicle crashes. Traffic Inj. Prev. 2017, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Vissers, L.; Houwing, S.; Wegman, F. Alcohol-Related Road Casualties in Official Crash Statistics; ITF: International Transport Forum: Paris, France, 2018. [Google Scholar]
- Oscar-Berman, M.; Marinković, K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007, 17, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, C.; Zhang, C.; Ren, Y.; Guo, L.; Wang, T.; Chen, F.; Li, Y.; Zhang, X.; Wang, H. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed. Pharmacother. 2023, 160, 114308. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.; Kraus, L.; Piontek, D.; Mueller, S.; Demmel, R. Direct and indirect effects of alcohol expectancies on alcohol-related problems. Psychol. Addict. Behav. 2014, 28, 20. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.A.; Lee, L. Alcohol-related traffic laws and drunk-driving fatal accidents. Accid. Anal. Prev. 2021, 161, 106358. [Google Scholar] [CrossRef]
- Levitt, S.D.; Porter, J. How dangerous are drinking drivers? J. Political Econ. 2001, 109, 1198–1237. [Google Scholar] [CrossRef]
- Manthey, J.; Hassan, S.A.; Carr, S.; Kilian, C.; Kuitunen-Paul, S.; Rehm, J. What are the economic costs to society attributable to alcohol use? A systematic review and modelling study. Pharmacoeconomics 2021, 39, 809–822. [Google Scholar] [CrossRef]
- Alcohol-Impaired Driving; NHTSA: Washington, NJ, USA, 2023; Rep. DOT-HS-813-450.
- Møller, M.; Haustein, S.; Prato, C.G. Profiling drunk driving recidivists in Denmark. Accid. Anal. Prev. 2015, 83, 125–131. [Google Scholar] [CrossRef]
- Kaur, A.; Williams, J.; Recker, R.; Rose, D.; Zhu, M.; Yang, J. Subsequent risky driving behaviors, recidivism, and crashes among drivers with a traffic violation: A scoping review. Accid. Anal. Prev. 2023, 192, 107234. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, L.; Fang, S.; Chang, H.; Yang, T.; Chang, R.; Hsing, T.; Huang, M. Examining factors associated with postintervention recidivism in DUI repeat offenders after alcohol treatment: One-year follow-up study. J. Subst. Abus. Treat. 2021, 130, 108426. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Hlastala, M.P. The alcohol breath test in practice: Effects of exhaled volume. J. Appl. Physiol. 2019, 126, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.S.; Carter, C.R.; Carter, R.J.; Del Valle, M.M.; Pena, J.R. Comparison of immediate and delayed blood alcohol concentration testing. J. Anal. Toxicol. 2015, 39, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Barba-Maza, L.M.; Sánchez-López, C. Development of a breathalyzer for car drivers, 2016 IEEE International Autumn Meeting on Power, Electronics and Computing (ROPEC). In Proceedings of the 2016 IEEE International Autumn Meeting on Power, Electronics and Computing (ROPEC), Ixtapa, Mexico, 9–11 November 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Campbell, A.S.; Kim, J.; Wang, J. Wearable electrochemical alcohol biosensors. Curr. Opin. Electrochem. 2018, 10, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mihretu, L.D.; Gebru, A.G.; Mekonnen, K.N.; Asgedom, A.G.; Desta, Y.H. Determination of ethanol in blood using headspace gas chromatography with flameionization detector (HS-GC-FID): Validation of a method. Cogent Chem. 2020, 6, 1760187. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor gas sensors: Dry synthesis and application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.M.; Prazeres, D.M.F.; Cabral, J.M.; Fonseca, L.P. Ethanol biosensors based on alcohol oxidase. Biosens. Bioelectron. 2005, 21, 235–247. [Google Scholar] [CrossRef]
- Podder, E.; Jibon, R.H.; Hossain, M.B.; Bulbul, A.A.; Biswas, S.; Kabir, M.A. Alcohol sensing through photonic crystal fiber at different temperature. Opt. Photonics J. 2018, 8, 309. [Google Scholar] [CrossRef]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 2016, 6, 27582. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, Y.; Hu, T.; Xu, C. A skin patch integrating swellable microneedles and electrochemical test strips for glucose and alcohol measurement in skin interstitial fluid. Bioeng. Transl. Med. 2023, 8, e10413. [Google Scholar] [CrossRef] [PubMed]
- Ridder, T.D.; Hendee, S.P.; Brown, C.D. Noninvasive alcohol testing using diffuse reflectance near-infrared spectroscopy. Appl. Spectrosc. 2005, 59, 181–189. [Google Scholar] [CrossRef]
- Ridder, T.D.; Ver Steeg, B.J.; Laaksonen, B.D. Comparison of spectroscopically measured tissue alcohol concentration to blood and breath alcohol measurements. J. Biomed. Opt. 2009, 14, 054039. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, Q.; Singh, R.; Zhang, W.; Marques, C.; Xie, Y.; Zhang, B.; Kumar, S. Graphene oxide/multiwalled carbon nanotubes assisted serial quadruple tapered structure-based LSPR sensor for glucose detection. IEEE Sens. J. 2022, 22, 16904–16911. [Google Scholar] [CrossRef]
- Pandey, P.S.; Raghuwanshi, S.K.; Kumar, S. Recent advances in two-dimensional materials-based Kretschmann configuration for SPR sensors: A review. IEEE Sens. J. 2021, 22, 1069–1080. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Bai, H.; Wang, G.; Hu, X.; Kumar, S.; Min, R. Biocompatible and biodegradable polymer optical fiber for biomedical application: A review. Biosensors 2021, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.A.; Gillis, K.D.; Glass, T.E. A high-affinity fluorescent sensor for catecholamine: Application to monitoring norepinephrine exocytosis. Angew. Chem. 2019, 131, 7693–7696. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Huo, F.; Yin, C. Fast-specific fluorescent probes to visualize norepinephrine signaling pathways and its flux in the epileptic mice brain. J. Am. Chem. Soc. 2023, 145, 3229–3237. [Google Scholar] [CrossRef]
- Butcher, G. Tour of the Electromagnetic Spectrum; Government Printing Office: Washington, DC, USA, 2016.
- Nasouri, B.; Murphy, T.E.; Berberoglu, H. Near infrared laser penetration and absorption in human skin. In Mechanisms for Low-Light Therapy IX: 1–2 February 2014, San Francisco, CA, USA; SPIE: Bellingham, WA, USA, 2014; pp. 67–78. [Google Scholar] [CrossRef]
- Tsai, S.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B Biol. 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Bolognia, M.D.; Jean, L.; Jorizzo, M.D.; Joseph, L.; Rapini, M.D.; Ronald, P. Dermatology, 2nd ed.; Mosby Elsevier: London, UK, 2008. [Google Scholar]
- Sakudo, A. Near-infrared spectroscopy for medical applications: Current status and future perspectives. Clin. Chim. Acta 2016, 455, 181–188. [Google Scholar] [CrossRef]
- Höpe, A. Diffuse reflectance and transmittance. In Experimental Methods in the Physical Sciences; Elsevier: Amsterdam, The Netherlands, 2014; Volume 46, pp. 179–219. [Google Scholar] [CrossRef]
- Evers, D.J.; Nachabe, R.; Vranken Peeters, M.; van der Hage, J.A.; Oldenburg, H.S.; Rutgers, E.J.; Lucassen, G.W.; Hendriks, B.H.; Wesseling, J.; Ruers, T.J. Diffuse reflectance spectroscopy: Towards clinical application in breast cancer. Breast Cancer Res. Treat. 2013, 137, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zonios, G.; Dimou, A. Modeling diffuse reflectance from semi-infinite turbid media: Application to the study of skin optical properties. Opt. Express 2006, 14, 8661–8674. [Google Scholar] [CrossRef] [PubMed]
- Dahm, D.J. Explaining some light scattering properties of milk using representative layer theory. J. Near Infrared Spectrosc. 2013, 21, 323–339. [Google Scholar] [CrossRef]
- Albor-Ramírez, E.; Reyes-Alberto, M.; Vidal-Flores, L.M.; Gutierrez-Herrera, E.; Padilla-Castañeda, M.A. Agarose Gel Characterization for the Fabrication of Brain Tissue Phantoms for Infrared Multispectral Vision Systems. Gels 2023, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Presura, C.N.; Urbach, H.P.; Bhattacharya, N. Dynamic light scattering from pulsatile flow in the presence of induced motion artifacts. Biomed. Opt. Express 2014, 5, 2145–2156. [Google Scholar] [CrossRef][Green Version]
- Vaz, P.G.; Humeau-Heurtier, A.; Figueiras, E.; Correia, C.; Cardoso, J. Effect of static scatterers in laser speckle contrast imaging: An experimental study on correlation and contrast. Phys. Med. Biol. 2017, 63, 015024. [Google Scholar] [CrossRef]
- Thompson, O.B.; Andrews, M.K. Tissue perfusion measurements: Multiple-exposure laser speckle analysis generates laser Doppler–like spectra. J. Biomed. Opt. 2010, 15, 027015. [Google Scholar] [CrossRef]


| (a) | ||||||
|---|---|---|---|---|---|---|
| The %Error by Alcohol Concentration is calculated as follows: %Error = (STD/Absorbance Mean Value) ∗ 100 | ||||||
| Concentration | 0% | 5% | 10% | 15% | 20% | |
| Wavelength | ||||||
| 1032.64 nm to 1067.45 nm (Stable Region) | ||||||
| 1032.64 nm | 0.71% | 0.55% | 0.69% | 0.80% | 0.51% | |
| 1036.39 nm | 0.70% | 0.57% | 0.64% | 0.57% | 0.62% | |
| 1040.13 nm | 0.65% | 0.65% | 0.52% | 0.82% | 0.58% | |
| 1043.87 nm | 0.50% | 0.65% | 0.63% | 0.77% | 0.74% | |
| 1047.6 nm | 0.42% | 0.70% | 0.63% | 0.64% | 0.77% | |
| 1051.33 nm | 0.58% | 0.66% | 0.81% | 0.71% | 0.74% | |
| 1055.06 nm | 0.51% | 0.71% | 0.68% | 0.76% | 0.71% | |
| 1058.78 nm | 0.72% | 0.80% | 0.50% | 0.81% | 0.62% | |
| 1062.5 nm | 0.69% | 0.72% | 0.57% | 0.67% | 0.70% | |
| 1067.45 nm | 0.65% | 0.92% | 0.54% | 0.77% | 0.73% | |
| 1627.161 nm to 1655.038 nm (Unstable Region) | ||||||
| 1627.161 nm | 2.06% | 2.44% | 1.67% | 2.55% | 2.80% | |
| 1630.166 nm | 1.37% | 1.77% | 1.81% | 2.17% | 1.89% | |
| 1634.165 nm | 2.11% | 2.16% | 2.86% | 2.09% | 2.43% | |
| 1637.159 nm | 2.19% | 2.53% | 2.32% | 1.90% | 2.38% | |
| 1640.15 nm | 1.71% | 2.50% | 2.22% | 2.47% | 2.50% | |
| 1643.136 nm | 3.35% | 3.11% | 2.18% | 2.62% | 2.70% | |
| 1646.118 nm | 2.64% | 2.54% | 1.86% | 2.01% | 3.08% | |
| 1649.095 nm | 2.80% | 2.08% | 2.96% | 1.93% | 2.24% | |
| 1652.069 nm | 3.25% | 3.29% | 2.15% | 2.14% | 1.76% | |
| 1655.038 nm | 3.04% | 2.45% | 2.91% | 2.94% | 2.38% | |
| (b) | ||||||
| The %Error by Alcohol Concentration is calculated as follows: %Error = (STD/Absorbance Mean Value) ∗ 100 | ||||||
| Concentration | 25% | 30% | 35% | 40% | 45% | |
| Wavelength | ||||||
| 1032.64 nm to 1067.45 nm (Stable Region) | ||||||
| 1032.64 nm | 0.87% | 1.12% | 0.94% | 0.98% | 1.98% | |
| 1036.39 nm | 1.08% | 1.05% | 1.08% | 0.80% | 1.68% | |
| 1040.13 nm | 0.68% | 1.19% | 1.27% | 1.55% | 1.12% | |
| 1043.87 nm | 0.91% | 1.40% | 1.00% | 1.13% | 1.30% | |
| 1047.6 nm | 1.22% | 1.10% | 1.00% | 1.31% | 1.51% | |
| 1051.33 nm | 1.06% | 1.60% | 1.21% | 1.29% | 1.21% | |
| 1055.06 nm | 1.20% | 1.21% | 1.09% | 1.63% | 1.68% | |
| 1058.78 nm | 1.18% | 1.17% | 1.13% | 1.72% | 1.90% | |
| 1062.5 nm | 1.12% | 1.33% | 1.42% | 1.09% | 1.86% | |
| 1067.45 nm | 1.05% | 1.23% | 1.42% | 1.33% | 1.43% | |
| 1627.161 nm to 1655.038 nm (Unstable Region) | ||||||
| 1627.161 nm | 3.12% | 4.42% | 3.47% | 4.69% | 6.66% | |
| 1630.166 nm | 1.98% | 4.54% | 3.40% | 4.48% | 4.33% | |
| 1634.165 nm | 3.61% | 4.16% | 3.41% | 2.89% | 5.14% | |
| 1637.159 nm | 2.99% | 4.33% | 3.42% | 3.15% | 7.73% | |
| 1640.15 nm | 5.69% | 4.05% | 3.16% | 5.90% | 4.69% | |
| 1643.136 nm | 3.78% | 3.69% | 3.66% | 3.83% | 6.75% | |
| 1646.118 nm | 4.75% | 2.83% | 4.44% | 4.77% | 5.03% | |
| 1649.095 nm | 3.48% | 4.92% | 2.65% | 4.60% | 4.87% | |
| 1652.069 nm | 2.53% | 4.46% | 4.78% | 4.52% | 6.76% | |
| 1655.038 nm | 3.76% | 3.81% | 6.31% | 4.04% | 4.84% | |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Wavelength | 1032.64 nm | 1036.39 nm | 1040.13 nm | 1043.87 nm | 1047.6 nm | |
| Concentration | ||||||
| 1032.64 nm to 1047.6 nm | ||||||
| 0% | 1.61387 | 1.61216 | 1.60997 | 1.60964 | 1.60796 | |
| 5% | 1.65314 | 1.64823 | 1.67993 | 1.72509 | 1.75513 | |
| 10% | 1.685 | 1.67993 | 1.67992 | 1.67967 | 1.68234 | |
| 15% | 1.72717 | 1.72509 | 1.72367 | 1.72231 | 1.7199 | |
| 20% | 1.75906 | 1.75513 | 1.75436 | 1.75482 | 1.74988 | |
| 25% | 1.84298 | 1.84295 | 1.84251 | 1.83839 | 1.83809 | |
| 30% | 1.87355 | 1.87141 | 1.87539 | 1.87255 | 1.86992 | |
| 35% | 1.89596 | 1.89496 | 1.89522 | 1.8937 | 1.89469 | |
| 40% | 1.97608 | 1.96561 | 1.9639 | 1.97283 | 1.96597 | |
| 45% | 2.02508 | 2.01502 | 2.01193 | 2.01407 | 2.01243 | |
| R2 | 1st-order Equ. | 0.9884 | 0.9893 | 0.9897 | 0.9895 | 0.9897 |
| 2nd-order Equ. | 0.9922 | 0.9918 | 0.9914 | 0.9923 | 0.9923 | |
| p-value | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
| (b) | ||||||
| Wavelength | 1051.33 nm | 1055.06 nm | 1058.78 nm | 1062.5 nm | 1067.45 nm | |
| Concentration | ||||||
| 1051.33 nm to 1067.45 nm | ||||||
| 0% | 1.6064 | 1.60737 | 1.60746 | 1.61086 | 1.60862 | |
| 5% | 1.64452 | 1.64504 | 1.66594 | 1.6481 | 1.64808 | |
| 10% | 1.67878 | 1.67861 | 1.67762 | 1.68021 | 1.68566 | |
| 15% | 1.71902 | 1.71943 | 1.71904 | 1.72162 | 1.72089 | |
| 20% | 1.75184 | 1.7518 | 1.75159 | 1.75364 | 1.75488 | |
| 25% | 1.83386 | 1.83811 | 1.8399 | 1.84132 | 1.83963 | |
| 30% | 1.86756 | 1.86963 | 1.86946 | 1.87028 | 1.8696 | |
| 35% | 1.89271 | 1.89321 | 1.89781 | 1.891 | 1.8965 | |
| 40% | 1.96288 | 1.96401 | 1.96687 | 1.96939 | 1.9668 | |
| 45% | 2.01344 | 2.0106 | 2.01733 | 2.01363 | 2.01351 | |
| R2 | 1st-order Equ.. | 0.9908 | 0.9901 | 0.9898 | 0.9881 | 0.9908 |
| 2nd-order Equ. | 0.9938 | 0.9924 | 0.9928 | 0.991 | 0.9932 | |
| p-value | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Lee, W.; Sin, H.; Oh, S.; Choi, K.C.; Jun, J.-H. Non-Invasive Alcohol Concentration Measurement Using a Spectroscopic Module: Outlook for the Development of a Drunk Driving Prevention System. Sensors 2024, 24, 2252. https://doi.org/10.3390/s24072252
Cho Y, Lee W, Sin H, Oh S, Choi KC, Jun J-H. Non-Invasive Alcohol Concentration Measurement Using a Spectroscopic Module: Outlook for the Development of a Drunk Driving Prevention System. Sensors. 2024; 24(7):2252. https://doi.org/10.3390/s24072252
Chicago/Turabian StyleCho, Yechan, Wonjune Lee, Heock Sin, Suseong Oh, Kyo Chang Choi, and Jae-Hoon Jun. 2024. "Non-Invasive Alcohol Concentration Measurement Using a Spectroscopic Module: Outlook for the Development of a Drunk Driving Prevention System" Sensors 24, no. 7: 2252. https://doi.org/10.3390/s24072252
APA StyleCho, Y., Lee, W., Sin, H., Oh, S., Choi, K. C., & Jun, J.-H. (2024). Non-Invasive Alcohol Concentration Measurement Using a Spectroscopic Module: Outlook for the Development of a Drunk Driving Prevention System. Sensors, 24(7), 2252. https://doi.org/10.3390/s24072252





