Energy Transfer-Based Recognition of Membrane Cholesterol by Controlling Intradistance of Linker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 20 nm Spherical AuNPs
2.2. β-Cyclodextrin Modification of AuNPs with Thiol Ligands
2.3. Fluorescence Turn-Off Quenching by Inclusion of Dye with β-Cyclodextrin
2.4. Preparation of Cholesterol Embedded Liposome Particles
2.5. Cholesterol Detection with Fluorescence Turn-On Recovery
2.6. Characterization
3. Results
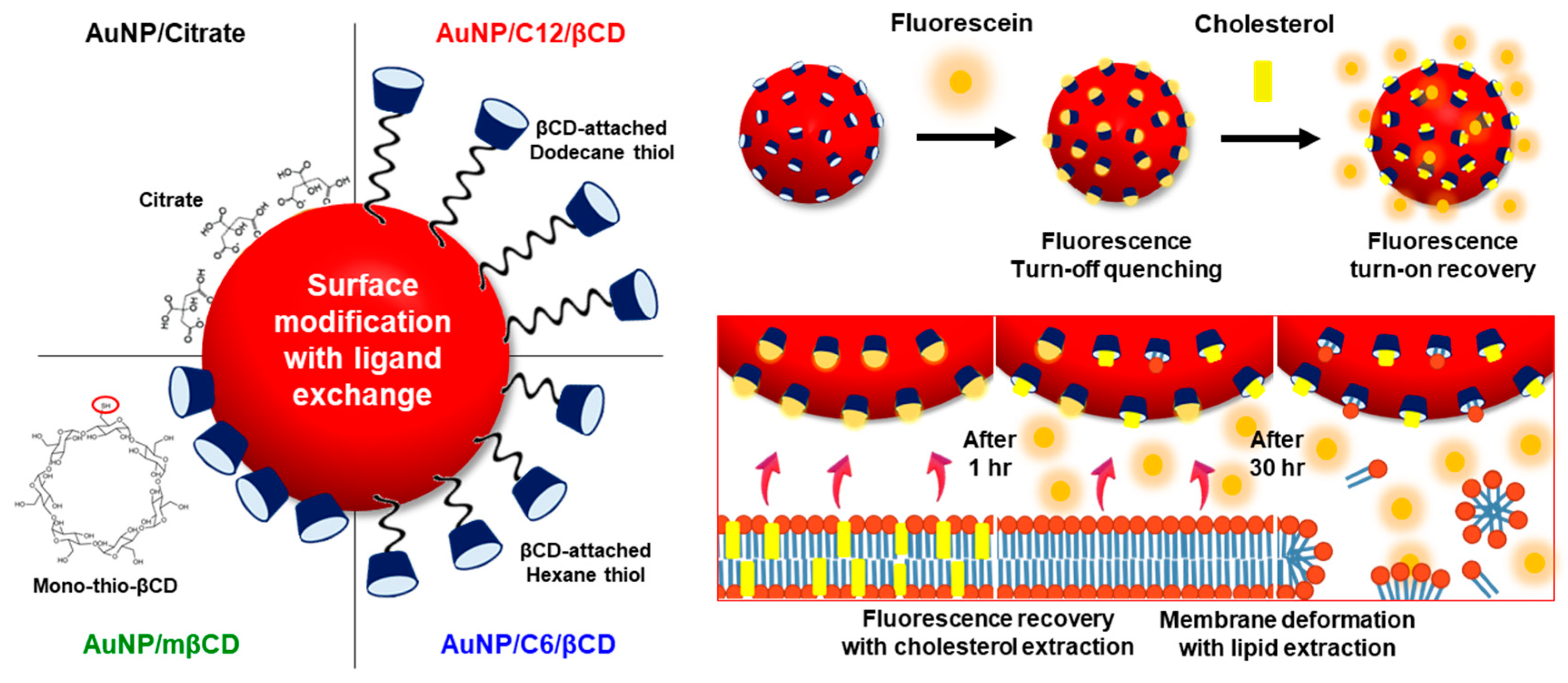
3.1. Fabrication of βCD-Modified AuNPs with Thiol Ligand Exchange
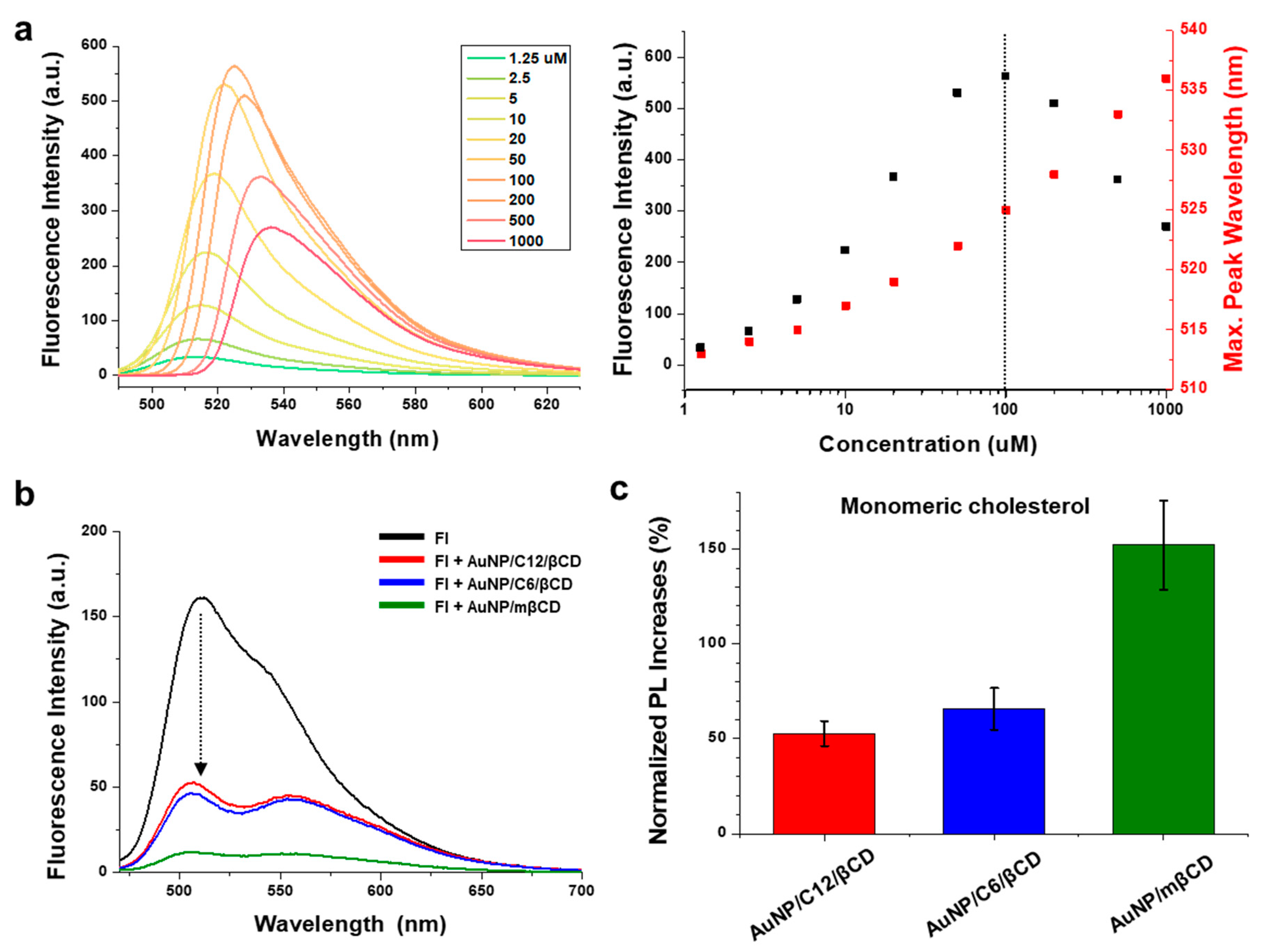
3.2. Fluorescence Turn-On Recovery of AuNP-βCD-Fluorescein Complexes for the Detection of Monomeric Cholesterol
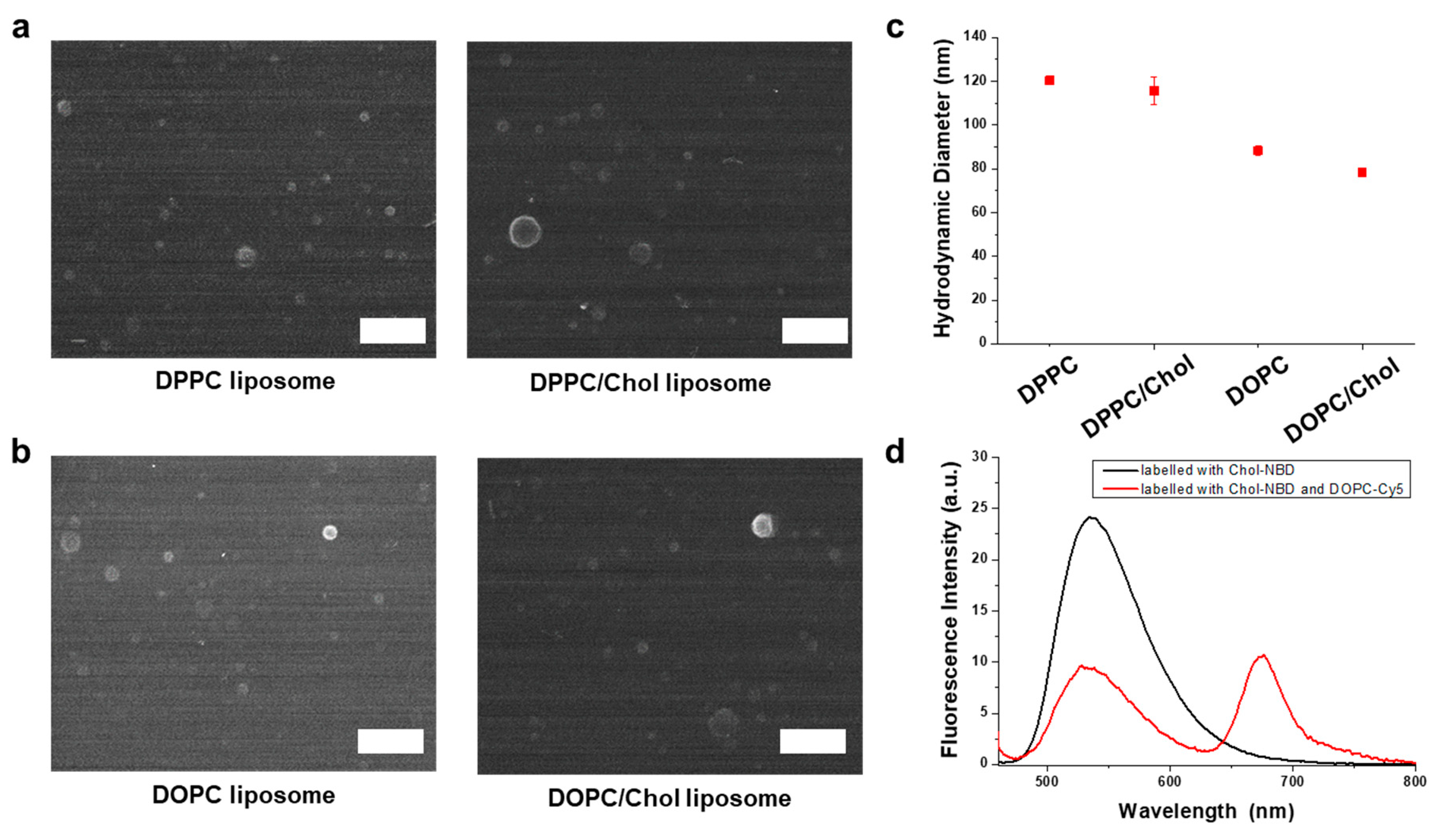
3.3. Fabrication of Liposome Nanoparticles Containing Cholesterol as a Membrane Cholesterol Model
3.4. Fluorescence Turn-On Recovery of AuNP-βCD-Fluorescein Complexes for Recognition of Membrane Cholesterol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sau, T.K.; Rogach, A.L.; Jäckel, F.; Klar, T.A.; Feldmann, J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv Mater 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir ACS J. Surf. Colloids 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, J.; Wu, X.; Piao, X.; Lv, D.; Zhang, G.; Ahn, D.J.; Cui, C. Obviously Enhanced Fluorescent Signal of Core-Shell Nanostructures through Simultaneous Regulation of Spectral Overlap and Shell Thickness for Imaging and Photothermal Therapy of Ovarian Cancer Cells. Part. Part. Syst. Charact. 2023, 40, 2300083. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Templeton, A.C.; Wuelfing, W.P.; Murray, R.W. Monolayer-protected cluster molecules. Acc. Chem. Res. 2000, 33, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Bozich, J.S.; Lohse, S.E.; Torelli, M.D.; Murphy, C.J.; Hamers, R.J.; Klaper, R.D. Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environ. Sci. Nano 2014, 1, 260–270. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Pengo, P.; Broxterman, Q.B.; Kaptein, B.; Pasquato, L.; Scrimin, P. Synthesis of a stable helical peptide and grafting on gold nanoparticles. Langmuir 2003, 19, 2521–2524. [Google Scholar] [CrossRef]
- Schneider, B.H.; Dickinson, E.L.; Vach, M.D.; Hoijer, J.V.; Howard, L.V. Highly sensitive optical chip immunoassays in human serum. Biosens. Bioelectron. 2000, 15, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.; Rosenzweig, Z. Development of an aggregation-based immunoassay for anti-protein A using gold nanoparticles. Anal. Chem. 2002, 74, 1624–1648. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Srivastava, A. Synthesis and characterization of novel cationic lipid and cholesterol-coated gold nanoparticles and their interactions with dipalmitoylphosphatidylcholine membranes. Langmuir 2003, 19, 4439–4447. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Soman, C.; Sainkar, S.R.; Rao, M.; Sastry, M. On the preparation, characterization, and enzymatic activity of fungal protease-gold colloid bioconjugates. Bioconjugate Chem. 2001, 12, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.S.; Javier, A.; Jennings, T.; Fisher, M.; Hira, S.; Peterson, S.; Hopkins, B.; Reich, N.O.; Strouse, G.F. Nanometal surface energy transfer in optical rulers, breaking the FRET barrier. J. Am. Chem. Soc. 2005, 127, 3115–3119. [Google Scholar] [CrossRef]
- Chen, C.; Hildebrandt, N. Resonance energy transfer to gold nanoparticles: NSET defeats FRET. TrAC Trends Anal. Chem. 2020, 123, 115748. [Google Scholar] [CrossRef]
- Sen, T.; Patra, A. Recent advances in energy transfer processes in gold-nanoparticle-based assemblies. J. Phys. Chem. C 2012, 116, 17307–17317. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Förster, T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann. Der Phys. 1948, 437, 55–75. [Google Scholar] [CrossRef]
- Di Nunzio, M.R.; Douhal, A. Robust Inclusion Complex of Topotecan Comprised within a Rhodamine-Labeled β-Cyclodextrin: Competing Proton and Energy Transfer Processes. Pharmaceutics 2023, 15, 1620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, J.; Song, Y.; Zhang, J.; Han, Q. Determination of Fumonisin B1 by Aptamer-Based Fluorescence Resonance Energy Transfer. Sensors 2022, 22, 8598. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic Macromolecular Material Design-The Versatility of Cyclodextrin-Based Host-Guest Chemistry. Angew. Chem. (Int. Ed. Engl.) 2017, 56, 8350–8369. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Fenyvesi, É. Cyclodextrin-lipid complexes: Cavity size matters. Struct. Chem. 2017, 28, 479–492. [Google Scholar] [CrossRef]
- Huang, Z.; London, E. Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid interaction. Langmuir ACS J. Surf. Colloids 2013, 29, 14631–14638. [Google Scholar] [CrossRef]
- Yadav, H.M.; Park, J.-D.; Kang, H.-C.; Lee, J.-J. Recent development in nanomaterial-based electrochemical sensors for cholesterol detection. Chemosensors 2021, 9, 98. [Google Scholar] [CrossRef]
- Mondal, A.; Jana, N.R. Fluorescent detection of cholesterol using β-cyclodextrin functionalized graphene. Chem. Commun. (Camb. Engl.) 2012, 48, 7316–7318. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; Wang, J.; Zhou, X.; Liu, Z. A new biosensor for glucose determination in serum based on up-converting fluorescence resonance energy transfer. Biosens. Bioelectron. 2011, 28, 414–420. [Google Scholar] [CrossRef]
- Flamigni, L. Inclusion of fluorescein and halogenated derivatives in. alpha-,. beta-, and gamma-cyclodextrins: A steady-state and picosecond time-resolved study. J. Phys. Chem. 1993, 97, 9566–9572. [Google Scholar] [CrossRef]
- Breslow, R.; Zhang, B. Cholesterol recognition and binding by cyclodextrin dimers. J. Am. Chem. Soc. 1996, 118, 8495–8496. [Google Scholar] [CrossRef]
- Goh, D.Y.; Ahn, D.J. Carbonate crystal growth controlled by interfacial interactions of artificial cell membranes. Biotechnol. Bioprocess Eng. 1997, 2, 109–112. [Google Scholar] [CrossRef]
- Won, T.K.; Roh, J.; Ahn, D.J. Fabrication of long-lasting multilayers of diacetylene@ silica nanoparticles patterned on solids for sensory figures. J. Ind. Eng. Chem. 2022, 114, 77–83. [Google Scholar] [CrossRef]
- Seo, J.S.; Liu, H.; Cho, Y.H.; Jung, W.H.; Kim, S.; Ahn, D.J. Triple-Peak Photoluminescence of DNA-Hybrid Alq3 Crystals Emitting a Depressed Single Peak upon Bio-Recognition. ACS Appl. Mater. Interfaces 2023, 15, 29406–29412. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cho, Y.H.; Park, J.H.; Ahn, D.J. Light-emitting crystals of aptamer-hybrid organic semiconductor signaling on human cells expressing EpCAM. J. Ind. Eng. Chem. 2022, 116, 268–275. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 1–14. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.H.; Won, T.K.; Ahn, D.J. Energy Transfer-Based Recognition of Membrane Cholesterol by Controlling Intradistance of Linker. Sensors 2024, 24, 2315. https://doi.org/10.3390/s24072315
Cho YH, Won TK, Ahn DJ. Energy Transfer-Based Recognition of Membrane Cholesterol by Controlling Intradistance of Linker. Sensors. 2024; 24(7):2315. https://doi.org/10.3390/s24072315
Chicago/Turabian StyleCho, Yong Ho, Tae Kyung Won, and Dong June Ahn. 2024. "Energy Transfer-Based Recognition of Membrane Cholesterol by Controlling Intradistance of Linker" Sensors 24, no. 7: 2315. https://doi.org/10.3390/s24072315




