Accuracy, Repeatability, and Reproducibility of a Hand-Held Structured-Light 3D Scanner across Multi-Site Settings in Lower Limb Prosthetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. 3D Scanners
2.2. Limb Models
- Transfemoral socket for a long residual limb, with ischial containment producing undercuts/indentations around the proximal brim, referred to as “TF Ischial”;
- Transfemoral socket for a long residual limb, with ischial containment but no undercuts/indentations around the proximal brim. Since this socket was based on hydrostatic casting with the Symphonie Aqua System (Romedis GmbH, Neubeuern, Germany) [23], it will be referred to as “TF Aqua”;
- Transtibial socket for a mid-length residual limb, with a total surface bearing design and a cylindrical shape, referred to as “TT Cylindrical”;
- Transtibial socket for a long residual limb, with patellar tendon bearing design and a conical shape, referred to as “TT Conical”.
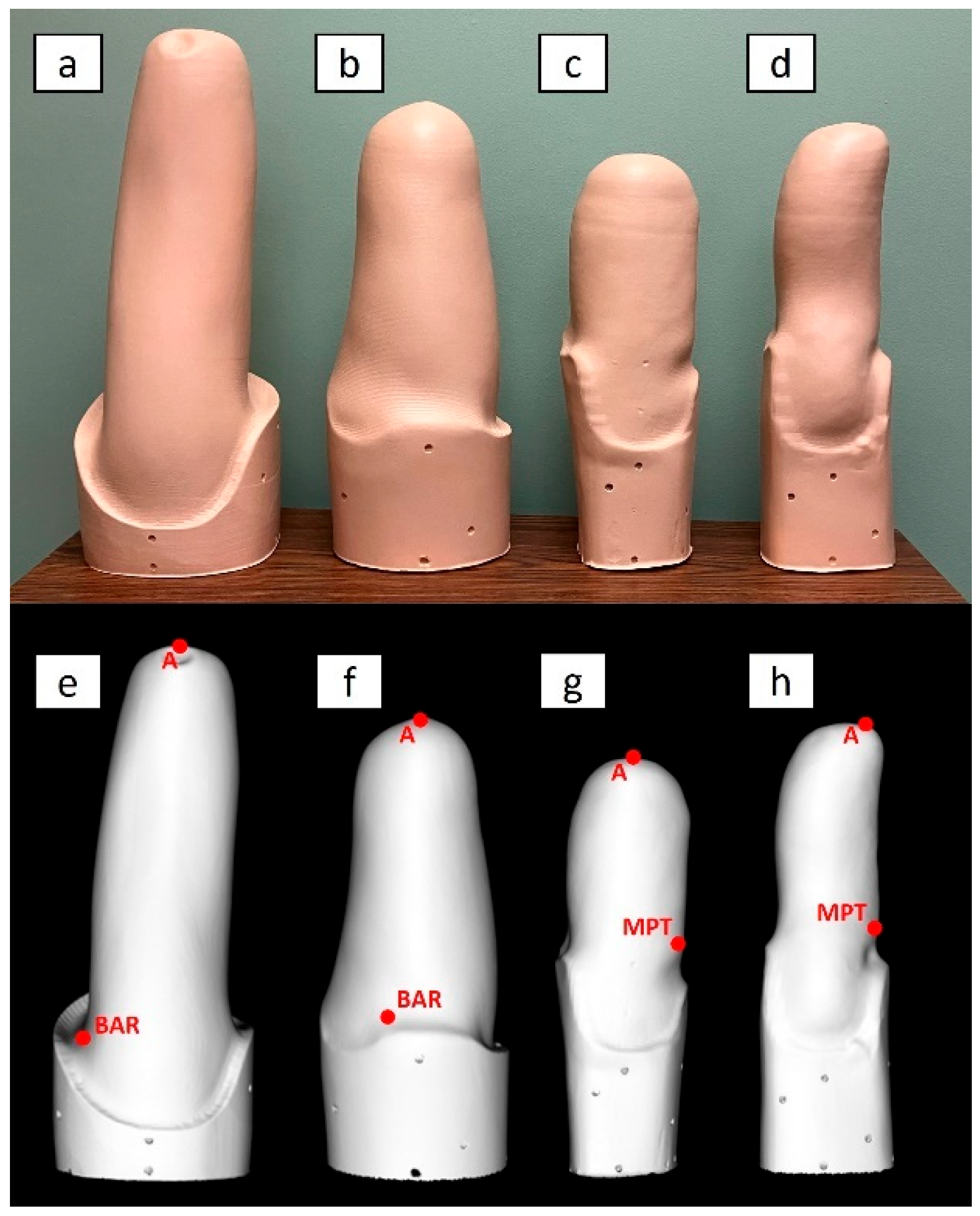
2.3. Testing Sites and Operators
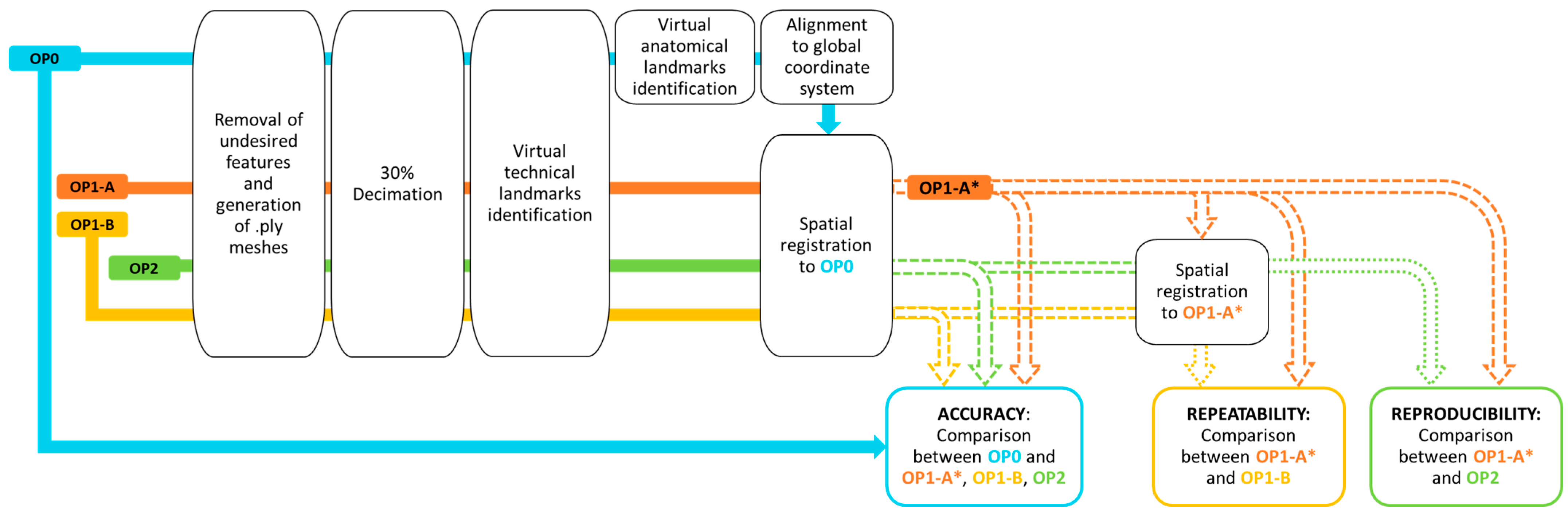
2.4. Scan Processing
- (1)
- Accuracy: scans from OP1-A, OP1-B, and OP2 (measured meshes) were spatially registered and compared to OP0 (reference mesh); the aligned scans of OP1-A on OP0 were referred to as OP1-A*.
- (2)
- Repeatability: scans from OP1-B (measured mesh) were spatially registered and compared to OP1-A* (reference mesh).
- (3)
- Reproducibility: scans from OP2 (measured mesh) were spatially registered and compared to OP1-A* (reference mesh).
2.5. Outcome Measures and Their Interpretation
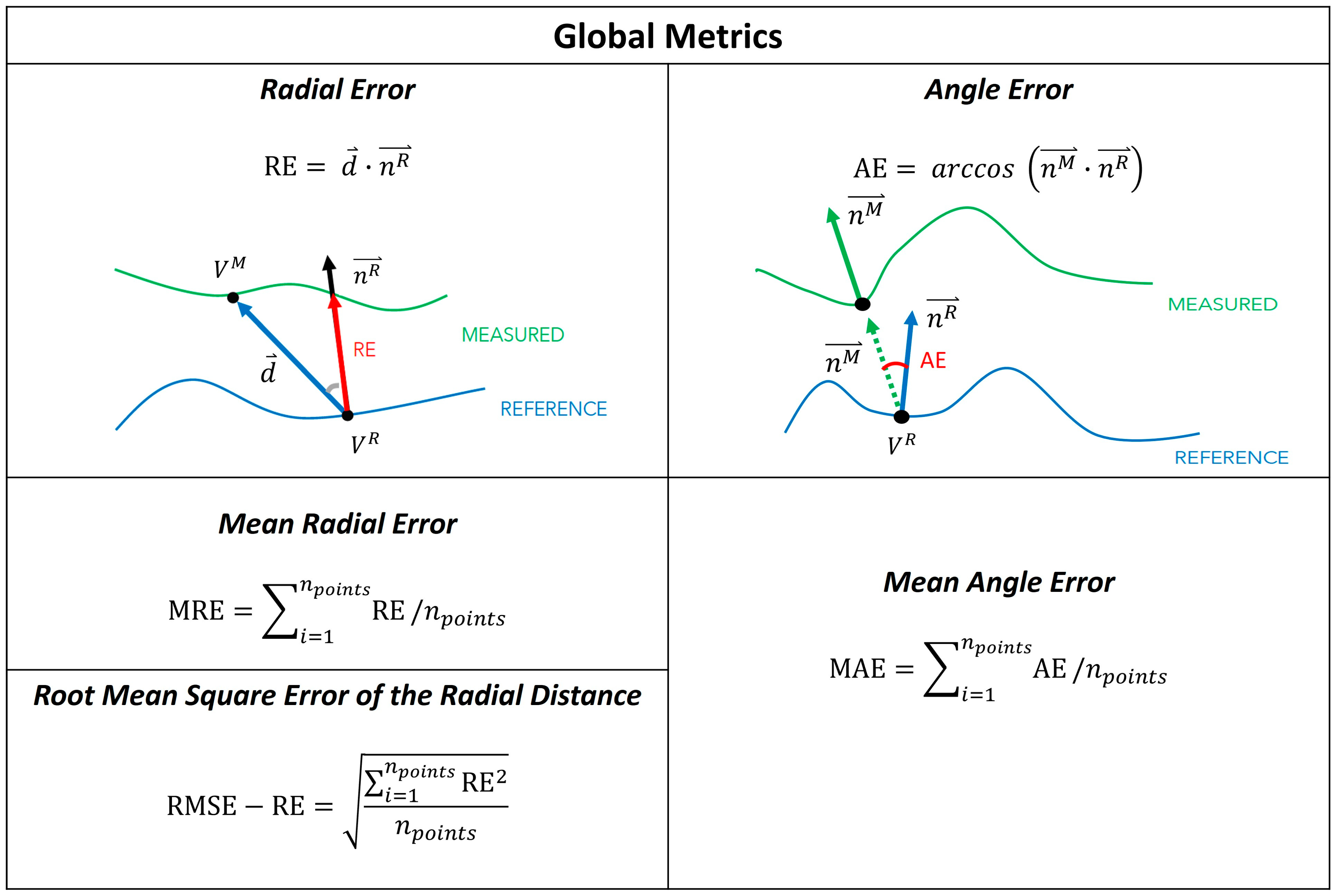
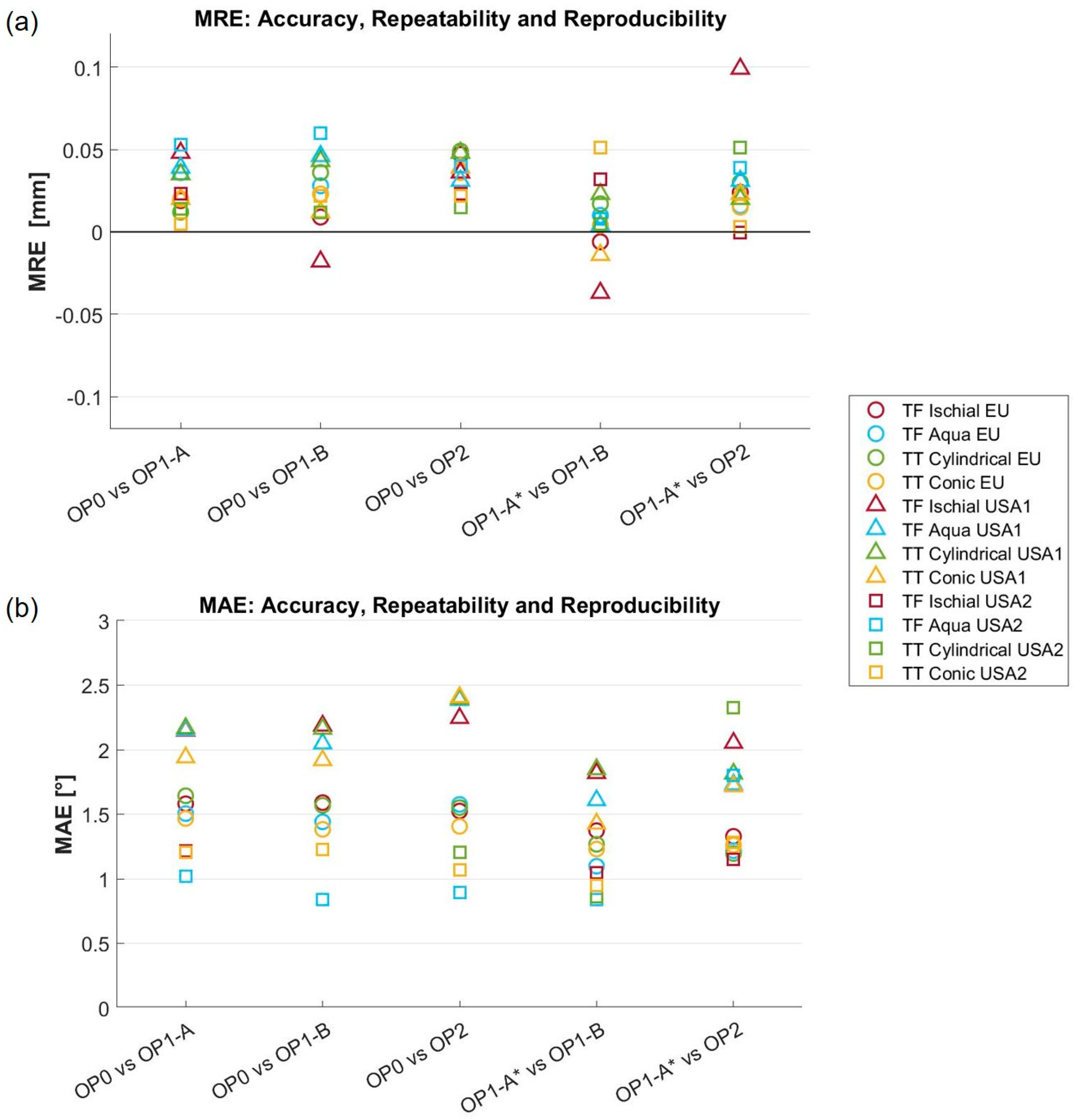
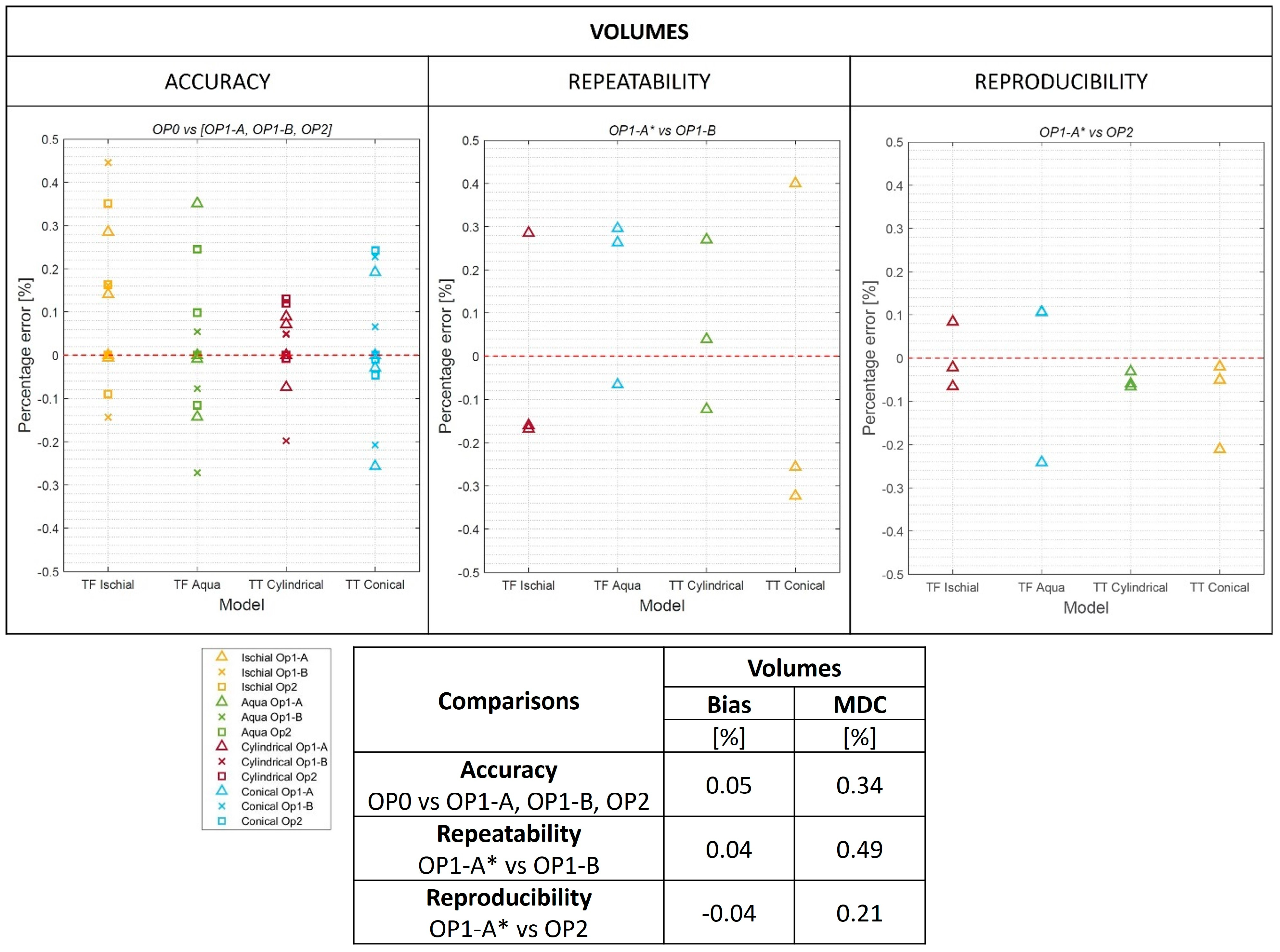
2.6. Global Metrics
- The mean radial error (MRE) [19], which provides a measure of the distance between the reference and measured mesh. For each point in the reference mesh (), the radial error (RE) is defined as the signed distance (in mm) to its closest point on the measured mesh (). The MRE is the average of the radial error over the whole set of reference mesh points (), holes excluded. This measure has also been referred to as the scan surface height by Dickinson et al. [15].
- The root mean square error of the radial distance (RMSE-RE) was also computed for comparison with Seminati et al. [12].
- The mean angle error (MAE), which provides a measure of the shape differences between the reference and measured mesh [19]. The angle error (AE) is the deviation of the normal unit vectors at closest points, measured in degrees. In detail, let us assume that is the normal to the reference mesh at its point , and is the normal vector to the measured mesh at the closest point to on that mesh. The angle error is the angular deviation of and . MAE is the average of the angular error over the whole set of points of the reference mesh (), holes excluded.
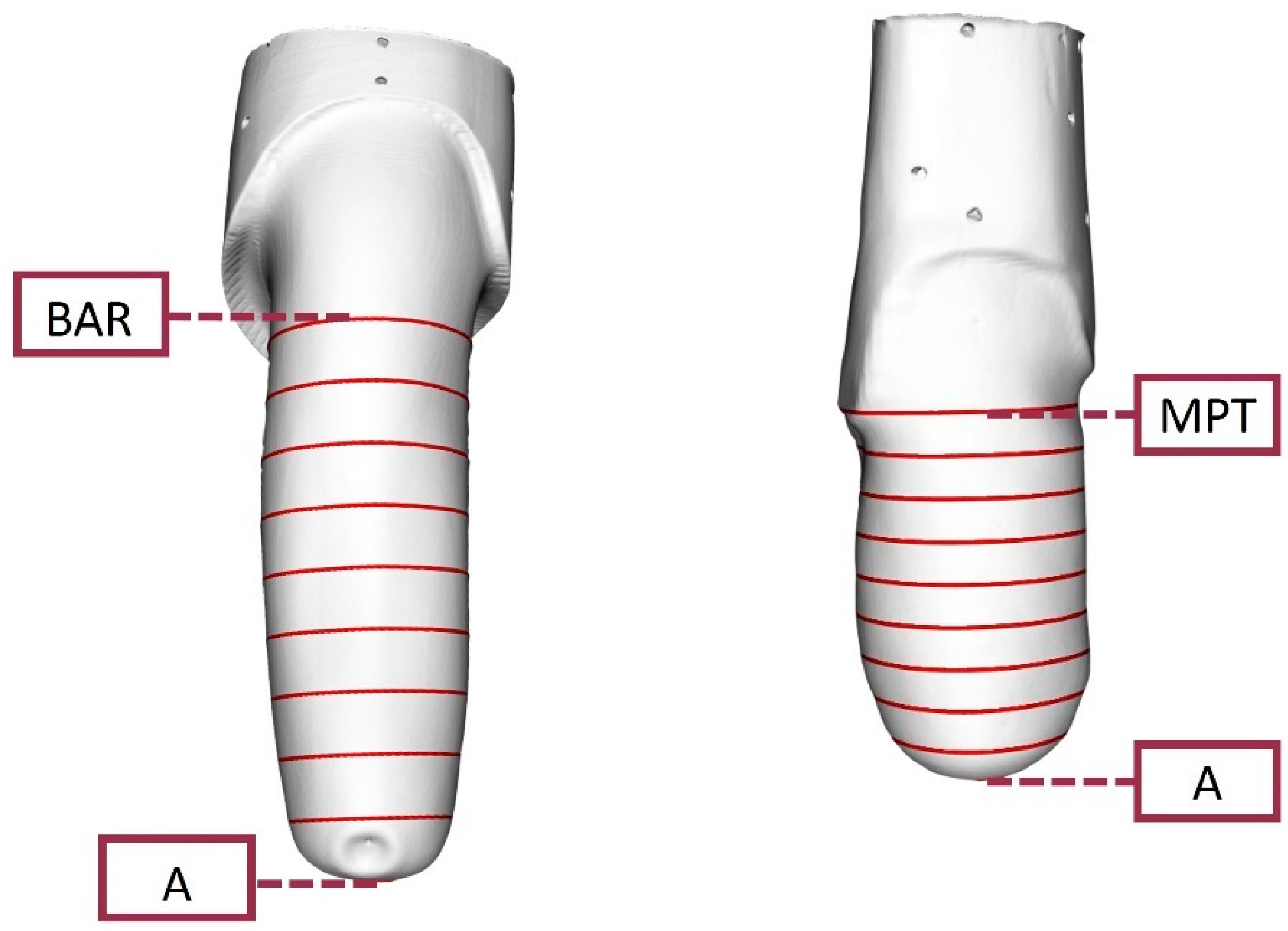
2.7. O&P-Relevant Metrics
- transtibial models, ran between landmark A and the Mid Patellar Tendon (MPT);
- transfemoral models, ran between landmark A and the midpoint Between the origin of Adductor longus and the ischial Ramus (BAR).
3. Results
3.1. Global Metrics
3.2. O&P-Relevant Metrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HD | High-Definition |
| OP0 | European independent Professional Operator |
| OP1 | Operator 1, with experience using the 3D scanner |
| OP1-A | Operator 1, first scan |
| OP1-A* | the aligned scans of OP1-A on OP0 |
| OP1-B | Operator 1, second scan |
| OP2 | Operator 2, with minimal prior experience using the 3D scanner |
| VDI/VDE | Association of German Engineers and Association for Electrical, Electronic, and Information Technologies |
| PETG | Polyethylene Terephthalate Glycol |
| USA1 | First United States site |
| USA2 | Second United States site |
| EU | European site |
| O&P | Orthotic and Prosthetic |
| MRE | mean radial error |
| VR | reference mesh |
| distance between meshes | |
| RE | radial error |
| VM | measured mesh |
| npoints | mesh points |
| RMSE-RE | root mean square error of the radial distance |
| MAE | mean angle error |
| AE | angle error |
| normal vector to the reference mesh | |
| normal vector to the measured mesh | |
| MCID | minimal clinically important difference |
| A | Most distal point of the model |
| MPT | Mid Patellar Tendon |
| BAR | Midpoint Between the origin of Adductor longus and the ischial Ramus |
| MDC | minimal detectable change |
| CR | Coefficient of Repeatability |
| TT | Transtibial |
| TF | Transfemoral |
References
- Vitali, A.; Togni, G.; Regazzoni, D.; Rizzi, C.; Molinero, G. A virtual environment to evaluate the arm volume for lymphedema affected patients. Comput. Methods Programs Biomed. 2021, 198, 105795. [Google Scholar] [CrossRef] [PubMed]
- Sidles, J.; Boone, D.; Harlan, J.; Burgess, E. Rectification maps: A new method for describing residual limb and socket shapes. J. Prosthet. Orthot. 1989, 1, 149–153. [Google Scholar] [CrossRef]
- Lemaire, E.; Johnson, F. A quantitative method for comparing and evaluating manual prosthetic socket modifications. IEEE Trans. Rehabil. Eng. 1996, 4, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, E.; Bexiga, P.; Johnson, F.; Solomonidis, S.; Paul, J. Validation of a quantitative method for defining CAD/CAM socket modifications. Prosthet. Orthot. Int. 1999, 23, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Fatone, S.; Johnson, W.; Tran, L.; Tucker, K.; Mowrer, C.; Caldwell, R. Quantification of rectifications for the Northwestern University Flexible Sub-Ischial Vacuum Socket. Prosthet. Orthot. Int. 2017, 41, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Steer, J.; Grudniewski, P.; Browne, M.; Worsley, P.; Sobey, A.; Dickinson, A. Predictive prosthetic socket design: Part 2-generating person-specific candidate designs using multi-objective genetic algorithms. Biomech. Model Mechanobiol. 2020, 19, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Steer, J.; Worsley, P.; Browne, M.; Dickinson, A. Predictive prosthetic socket design: Part 1-population-based evaluation of transtibial prosthetic sockets by FEA-driven surrogate modelling. Biomech. Model Mechanobiol. 2020, 19, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; Diment, L.; Morris, R.; Pearson, E.; Hannett, D.; Steer, J. Characterising residual limb morphology and prosthetic socket design based on expert clinician practice. Prosthesis 2021, 3, 280–299. [Google Scholar] [CrossRef]
- Raschke, S. Limb prostheses: Industry 1.0 to 4.0: Perspectives on technological advances in prosthetic care. Front. Rehabil. Sci. 2022, 3, 854404. [Google Scholar] [CrossRef]
- Mayo, A.; Gould, S.; Cimino, S.; Glasford, S.; Harvey, E.; Ratto, M.; Hitzig, S. A qualitative study on stakeholder perceptions of digital prosthetic socket fabrication for transtibial amputations. Prosthet. Orthot. Int. 2022, 46, 607–613. [Google Scholar] [CrossRef]
- Redaelli, D.F.; Barsanti, S.G.; Biffi, E.; Storm, F.A.; Colombo, G. Comparison of geometrical accuracy of active devices for 3D orthopaedic reconstructions. Int. J. Adv. Manuf. Technol. 2021, 114, 319–342. [Google Scholar] [CrossRef]
- Seminati, E.; Canepa Talamas, D.; Young, M.; Twiste, M.; Dhokia, V.; Bilzon, J. Validity and reliability of a novel 3D scanner for assessment of the shape and volume of amputees’ residual limb models. PLoS ONE 2017, 12, e0184498. [Google Scholar] [CrossRef] [PubMed]
- Seminati, E.; Young, M.; Canepa Talamas, D.; Twiste, M.; Dhokia, V.; Bilzon, J. Reliability of three different methods for assessing amputee residuum shape and volume: 3D scanners vs. circumferential measurements. Prosthet. Orthot. Int. 2022, 46, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; Steer, J.; Woods, C.; Worsley, P. Registering methodology for imaging and analysis of residual-limb shape after transtibial amputation. J. Rehabil. Res. Dev. 2016, 53, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; Donovan-Hall, M.; Kheng, S.; Bou, K.; Tech, A.; Steer, J.; Metcalf, C.; Worsley, P. Selecting appropriate 3D scanning technologies for prosthetic socket design and transtibial residual limb shape characterization. J. Prosthet. Orthot. 2022, 34, 33–43. [Google Scholar] [CrossRef]
- Kofman, R.; Beekman, A.; Emmelot, C.; Geertzen, J.; Dijkstra, P. Measurement properties and usability of non-contact scanners for measuring transtibial residual limb volume. Prosthet. Orthot. Int. 2018, 42, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Kofman, R.; Winter, R.; Emmelot, C.; Geertzen, J.; Dijkstra, P. Clinical usability, reliability, and repeatability of noncontact scanners in measuring residual limb volume in persons with transtibial amputation. Prosthet. Orthot. Int. 2022, 46, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Lemaire, E. A smartphone photogrammetry method for digitizing prosthetic socket interiors. Prosthet. Orthot. Int. 2017, 41, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.; Severance, M.; Allyn, K. Computer-socket manufacturing error: How much before it is clinically apparent? J. Rehabil. Res. Dev. 2012, 49, 567–582. [Google Scholar] [CrossRef]
- Mehmood, W.; Abd Razak, N.; Lau, M.; Chung, T.; Gholizadeh, H.; Abu Osman, N. Comparative study of the circumferential and volumetric analysis between conventional casting and three-dimensional scanning methods for transtibial socket: A preliminary study. Proc. Inst. Mech. Eng. Part H 2019, 233, 181–192. [Google Scholar] [CrossRef]
- Bartlett, J.; Frost, C. Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous variables. Ultrasound Obstet. Gynecol. 2008, 31, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Verein Deutscher Ingenieure, e.V. VDI/VDE 2634, Optical 3D Measurement Systems—Multiple View Systems Based on Area Scanning. 2008. Engl. VDI/VDE-Gesellschaft Mess- und Automatisierungstechnik: Dusseldorf, Germany. Available online: https://www.vdi.de/en/home/vdi-standards/details/vdivde-2634-blatt-3-optical-3d-measuring-systems-multiple-view-systems-based-on-area-scanning(accessed on 26 March 2024).
- Cutti, A.; Osti, G.; Migliore, G.; Cardin, D.; Venturoli, F.; Verni, G. Clinical Effectiveness of a Novel Hydrostatic Casting Method for Transfemoral Amputees: Results from the First 64 Patients. O&P News. October 2018. Available online: http://oandpnews.org/2018/10/31/september-october-2018-research-clinical-effectiveness-of-a-novel-hydrostatic-casting-method-for-transfemoral-amputees-results-from-the-first-64-patients/ (accessed on 26 March 2024).
- Fatone, S.; Cutti, A.G. Limb Model Scans [Dataset]; Forthcoming: Dryad, WA, USA, 2024. [Google Scholar] [CrossRef]
- Cutti, A.; Fatone, S.; Hansen, A.; Group, R.L.S.C. Technique to assess shape captured by two casting approaches. Prosthet. Orthot. Int. 2023, 47, 272. [Google Scholar]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An open-source Mesh processing tool. Computing 2008, 1, 129–136. Available online: https://diglib.eg.org/handle/10.2312/LocalChapterEvents.ItalChap.ItalianChapConf2008.129-136 (accessed on 26 March 2024).
- Schroeder, W.; Martin, K.; Lorensen, B.; Kitware Inc. The Visualization Toolkit: An Object-Oriented Approach to 3D Graphics; Kitware: New York, NY, USA, 2006; Available online: https://books.google.it/books?id=rx4vPwAACAAJ (accessed on 26 March 2024).
- Qt Company Ltd. Qt for Python. Available online: https://doc.qt.io/qtforpython-6/index.html#documentation (accessed on 21 June 2023).
- Geil, M. Consistency and accuracy of measurement of lower-limb amputee anthropometrics. J. Rehabil. Res. Dev. 2005, 42, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.; Fatone, S. Residual limb volume change: Systematic review of measurement and management. J. Rehabil. Res. Dev. 2011, 48, 949–986. [Google Scholar] [CrossRef]
- Bland, J.; Altman, D. Applying the right statistics: Analyses of measurement studies. Ultrasound Obstet. Gynecol. 2003, 22, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bland, M. An Introduction to Medical Statistics, 3rd ed.; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Lilja, M.; Öberg, T. Proper time for definitive transtibial prosthetic fitting. J. Prosthet. Orthot. 1997, 9, 90. Available online: https://journals.lww.com/jpojournal/Fulltext/1997/00920/Proper_Time_for_Definitive_Transtibial_Prosthetic.9.aspx (accessed on 26 March 2024). [CrossRef]
- Sanders, J.; Youngblood, R.; Hafner, B.; Cagle, J.; McLean, J.; Redd, C.; Dietrich, C.; Ciol, M.; Allyn, K. Effects of socket size on metrics of socket fit in trans-tibial prosthesis users. Med. Eng. Phys. 2017, 44, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Lilja, M.; Johansson, S.; Öberg, T. Relaxed versus activated stump muscles during casting for trans-tibial prostheses. Prosthet. Orthot. Int. 1999, 23, 13–20. [Google Scholar] [CrossRef]



| Model | Total Height [mm] | Height [mm] TF: (A—BAR) TT: (A—MPT) | Volume [dL] TF: (A—BAR) TT: (A—MPT) |
|---|---|---|---|
| TF Ischial | 507.2 | 318.1 | 33.9 |
| TF Aqua | 442.1 | 269.2 | 36.3 |
| TT Conical | 429.4 | 213.8 | 14.7 |
| TT Cylindrical | 396.3 | 195.3 | 19.5 |
| Comparison | Radial Error (RE) [mm] | ||
|---|---|---|---|
| RMSE Min | RMSE Max | ||
| Accuracy | OP0 vs. OP1-A | 0.06 | 0.25 |
| OP0 vs. OP1-B | 0.06 | 0.21 | |
| OP0 vs. OP2 | 0.06 | 0.27 | |
| Repeatability | OP1-A* vs. OP1-B | 0.04 | 0.16 |
| Reproducibility | OP1-A* vs. OP2 | 0.04 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutti, A.G.; Santi, M.G.; Hansen, A.H.; Fatone, S., on behalf of the Residual Limb Shape Capture Group. Accuracy, Repeatability, and Reproducibility of a Hand-Held Structured-Light 3D Scanner across Multi-Site Settings in Lower Limb Prosthetics. Sensors 2024, 24, 2350. https://doi.org/10.3390/s24072350
Cutti AG, Santi MG, Hansen AH, Fatone S on behalf of the Residual Limb Shape Capture Group. Accuracy, Repeatability, and Reproducibility of a Hand-Held Structured-Light 3D Scanner across Multi-Site Settings in Lower Limb Prosthetics. Sensors. 2024; 24(7):2350. https://doi.org/10.3390/s24072350
Chicago/Turabian StyleCutti, Andrea Giovanni, Maria Grazia Santi, Andrew H. Hansen, and Stefania Fatone on behalf of the Residual Limb Shape Capture Group. 2024. "Accuracy, Repeatability, and Reproducibility of a Hand-Held Structured-Light 3D Scanner across Multi-Site Settings in Lower Limb Prosthetics" Sensors 24, no. 7: 2350. https://doi.org/10.3390/s24072350
APA StyleCutti, A. G., Santi, M. G., Hansen, A. H., & Fatone, S., on behalf of the Residual Limb Shape Capture Group. (2024). Accuracy, Repeatability, and Reproducibility of a Hand-Held Structured-Light 3D Scanner across Multi-Site Settings in Lower Limb Prosthetics. Sensors, 24(7), 2350. https://doi.org/10.3390/s24072350






