Evaluation of Hand Action Classification Performance Using Machine Learning Based on Signals from Two sEMG Electrodes
Abstract
1. Introduction
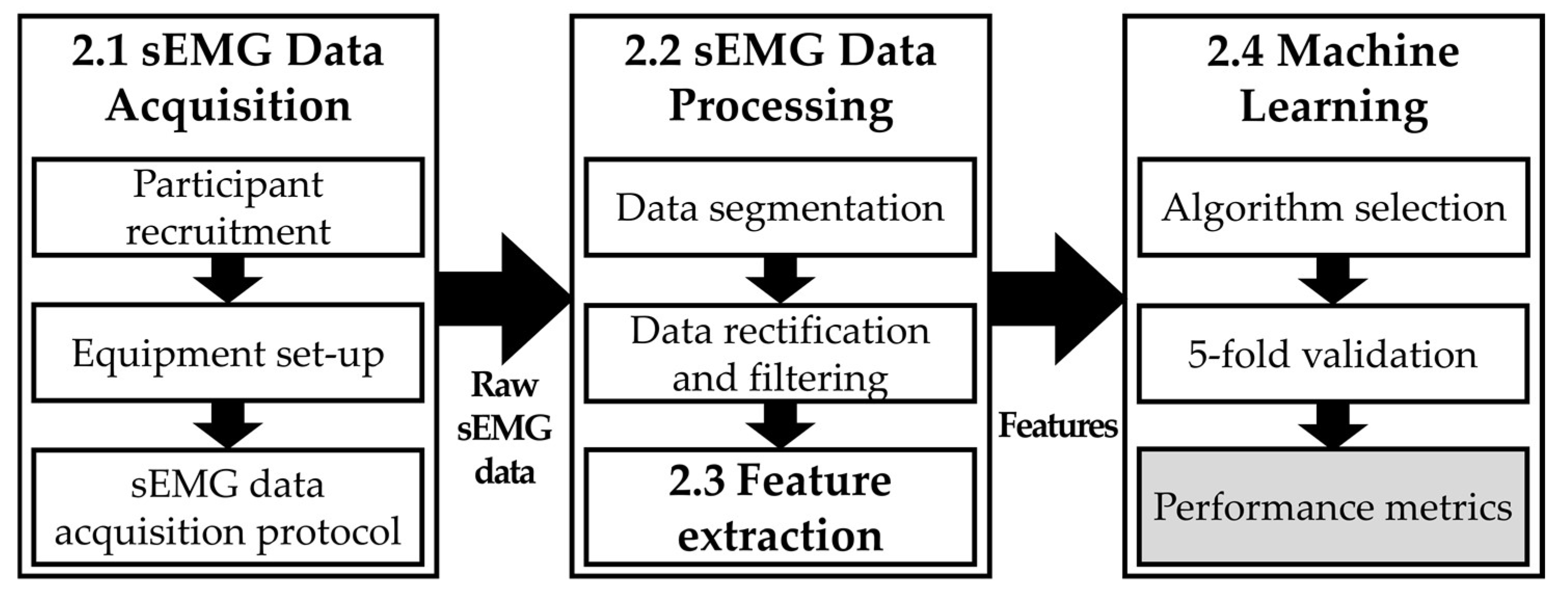
2. Materials and Method
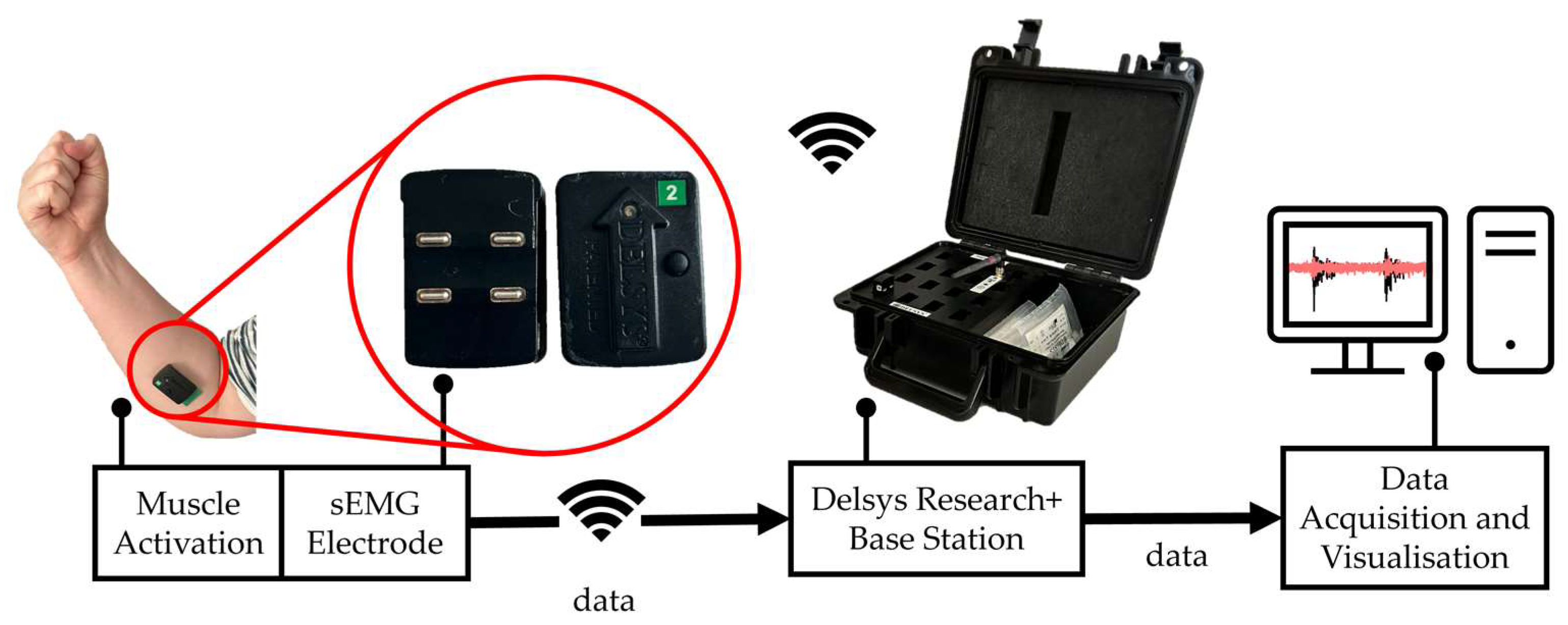
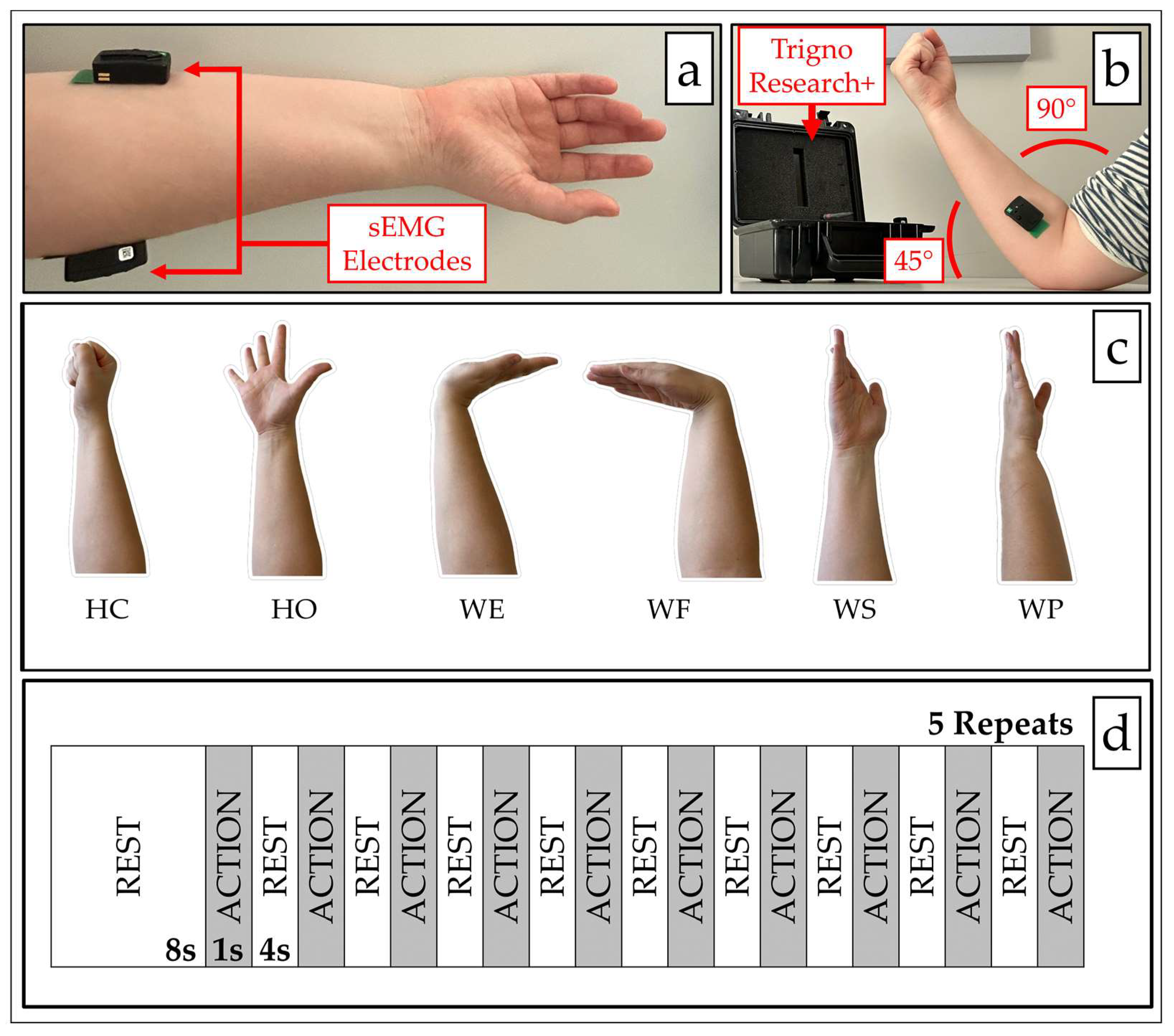
2.1. sEMG Data Acquisition
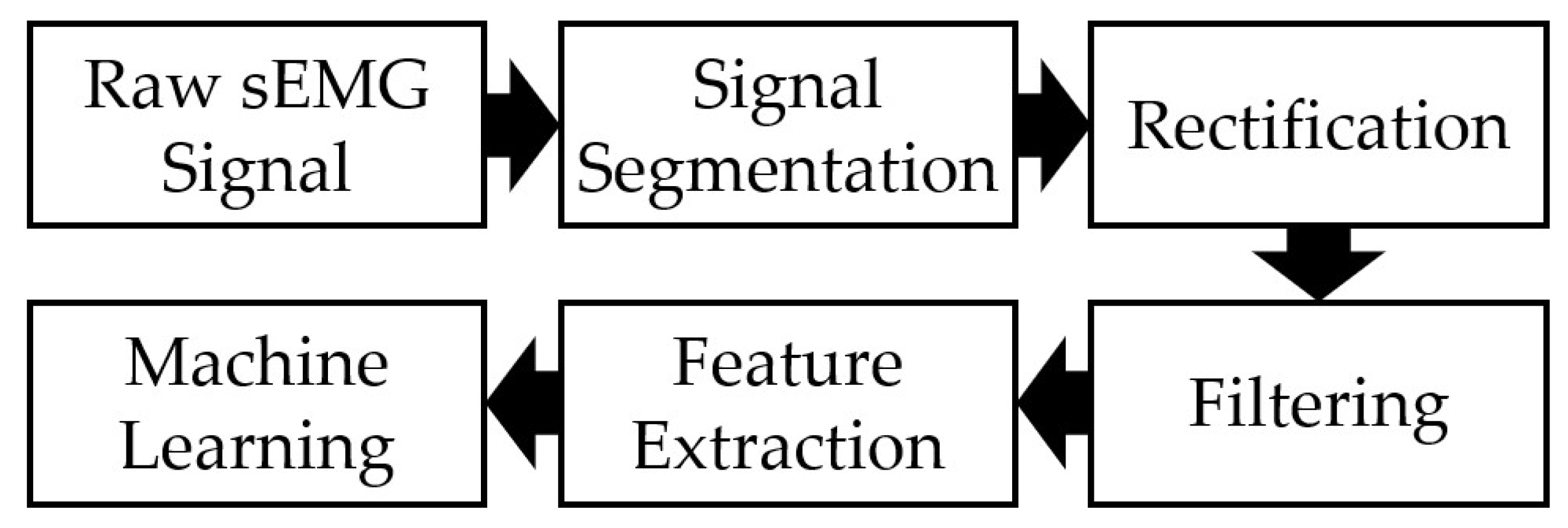
2.2. sEMG Data Processing
2.3. Feature Extraction
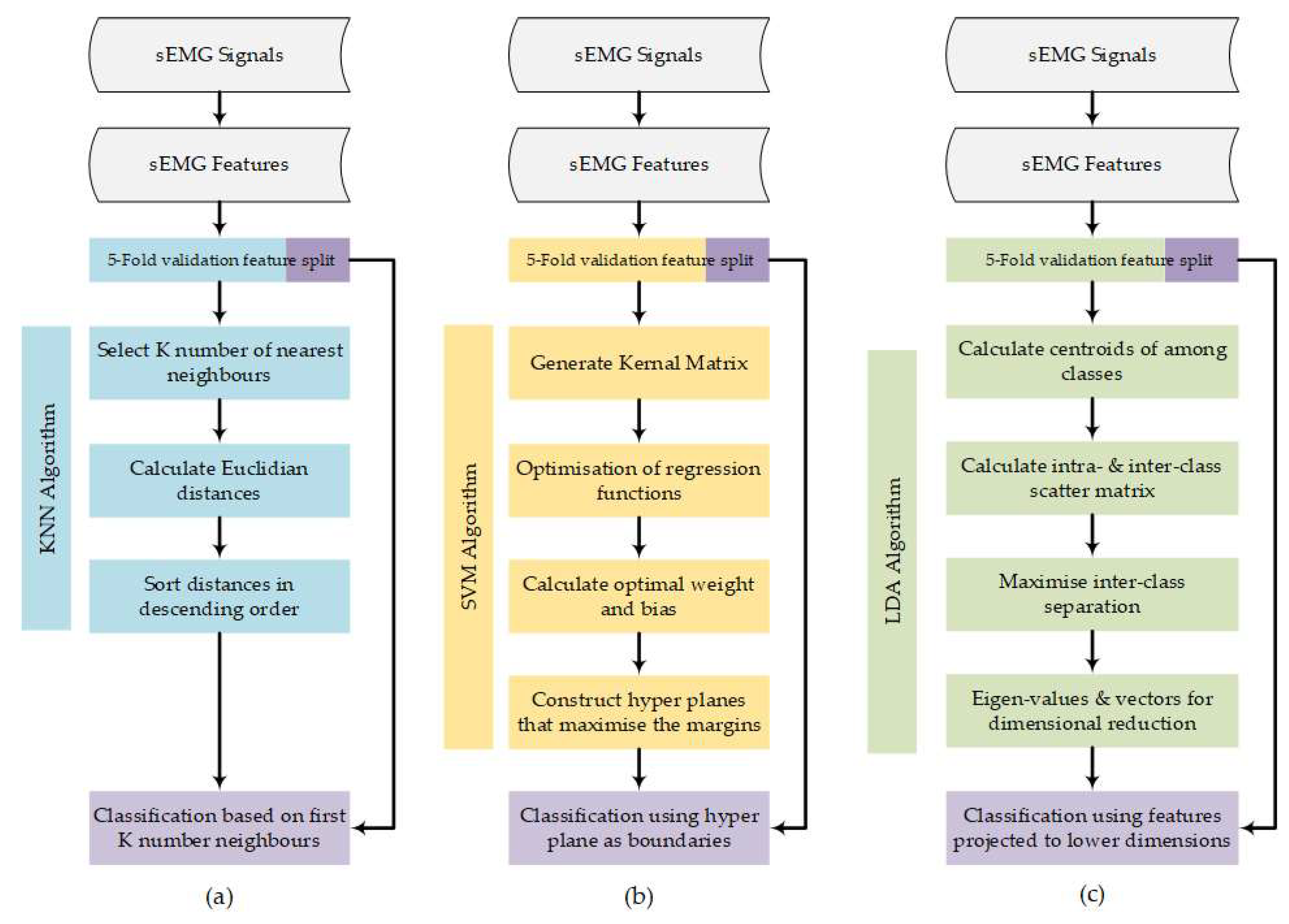
2.4. Machine Learning
3. Results
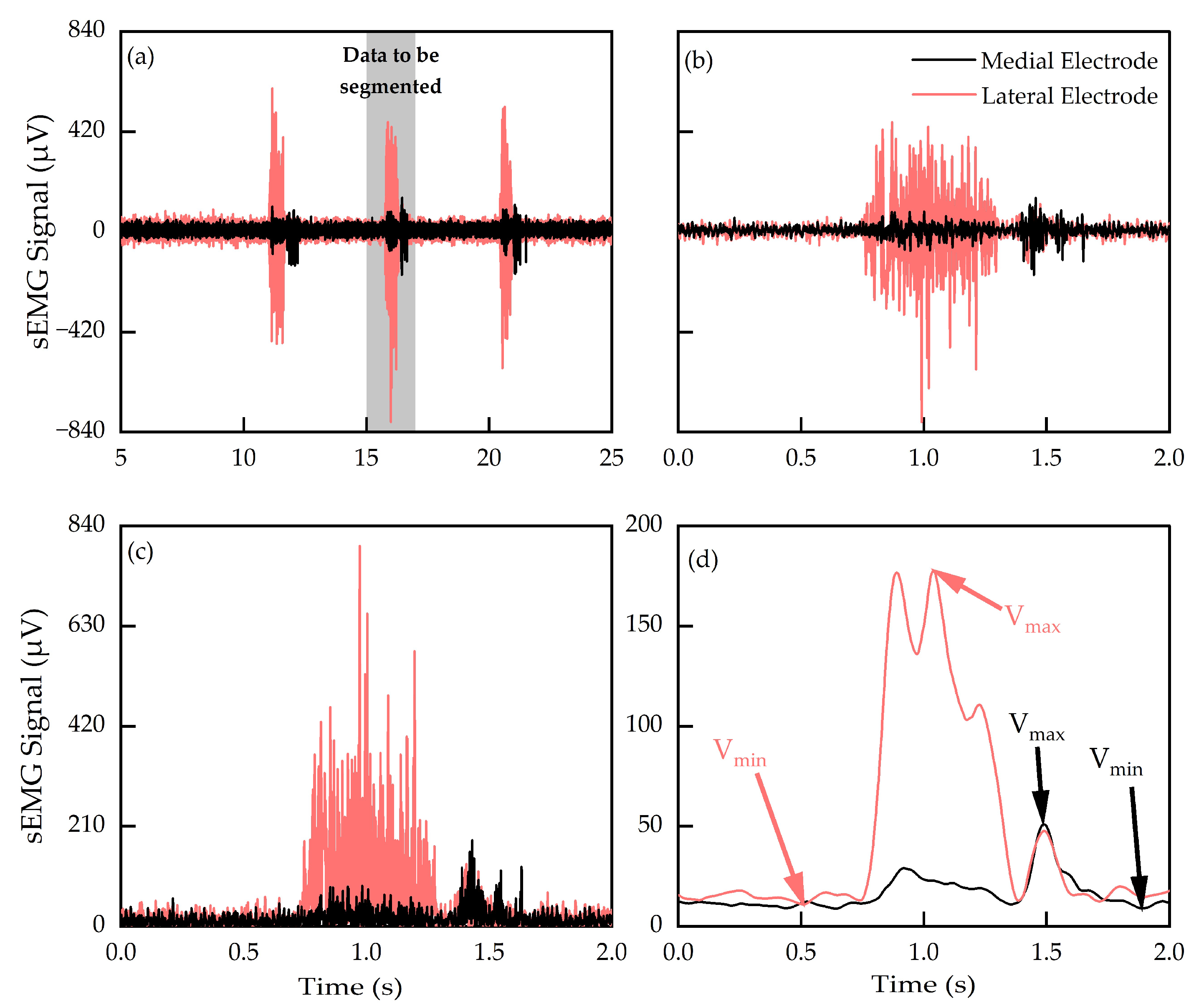
3.1. Processed sEMG Signals
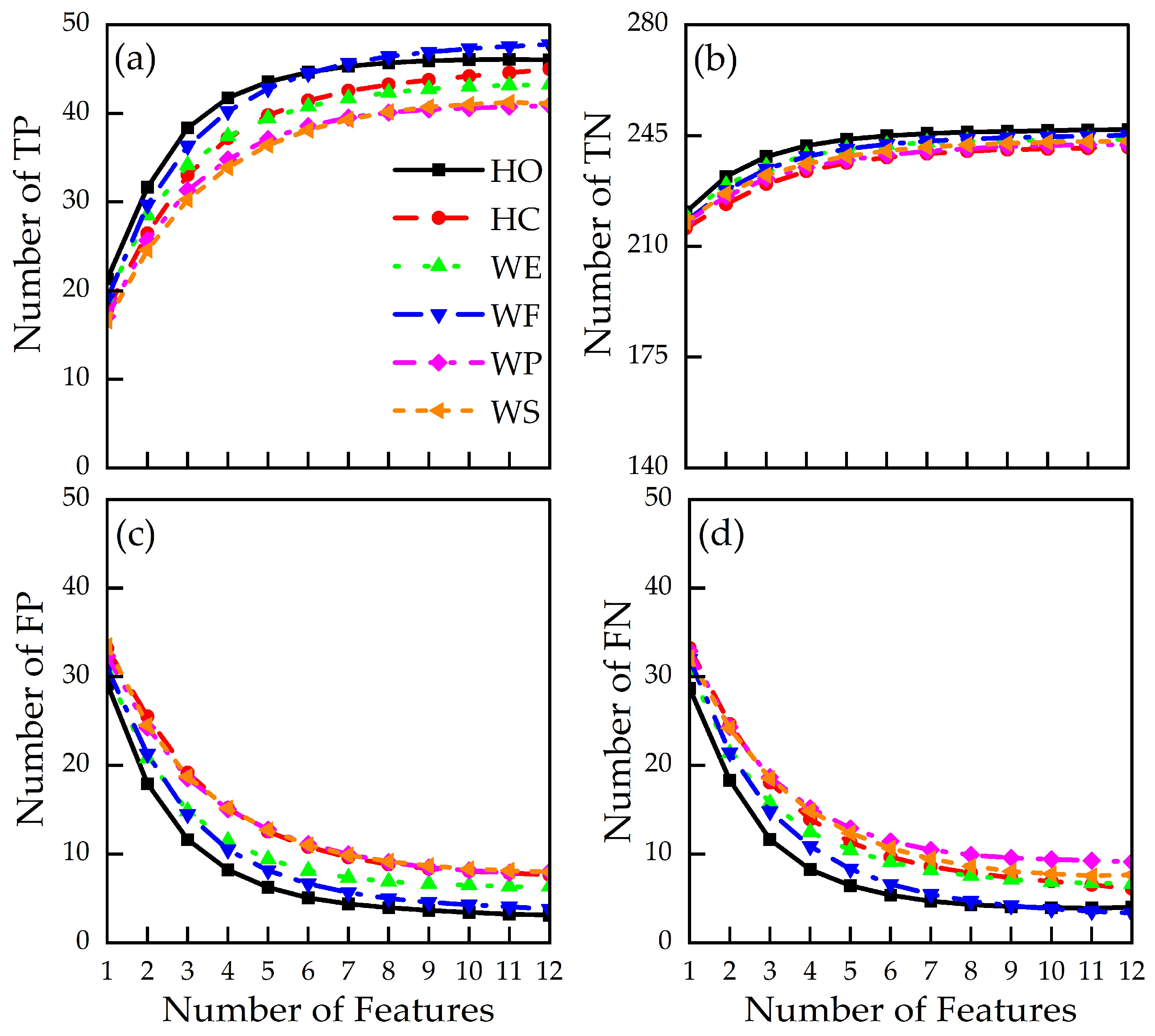
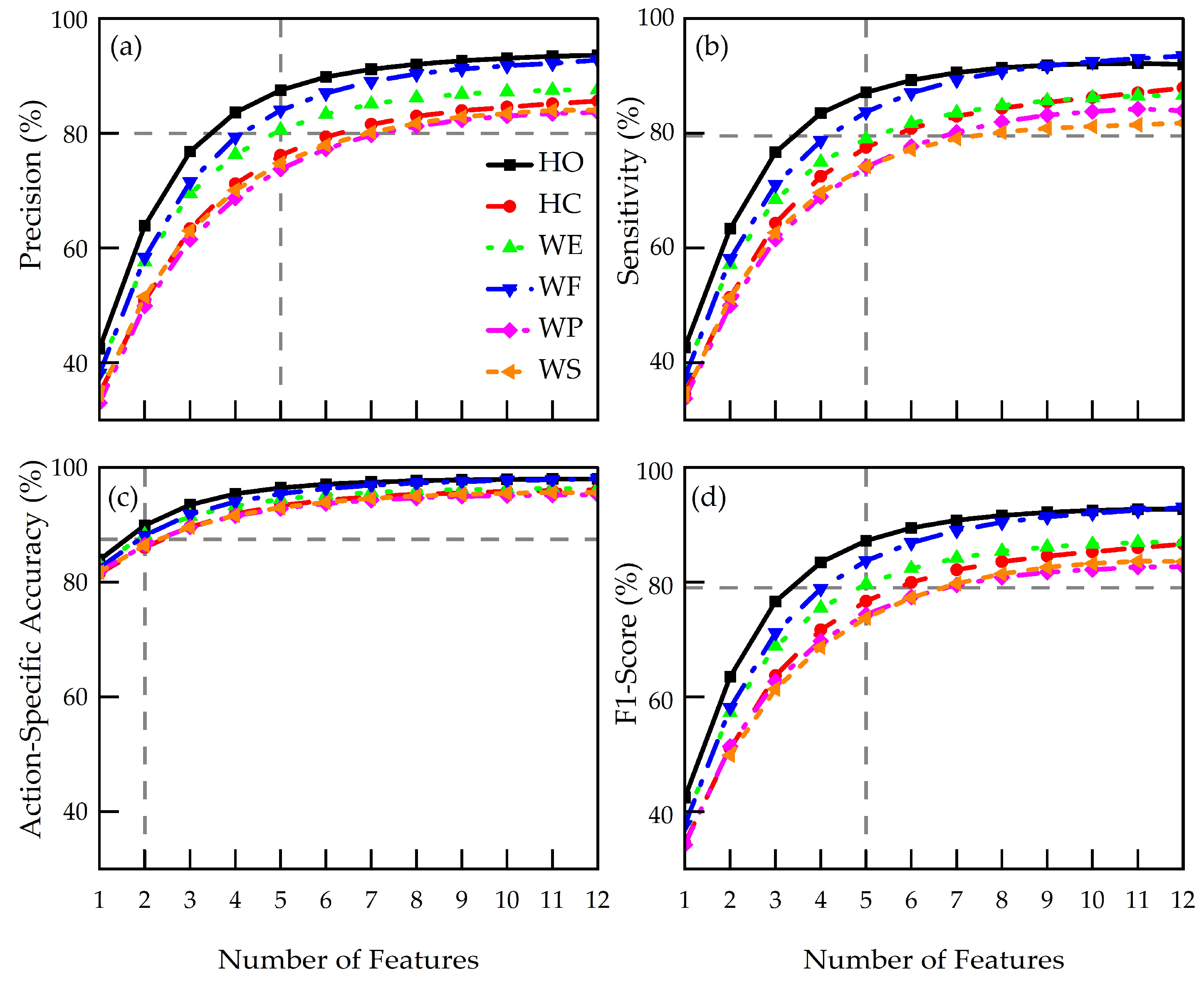
3.2. Action-Specific Performance Metrics
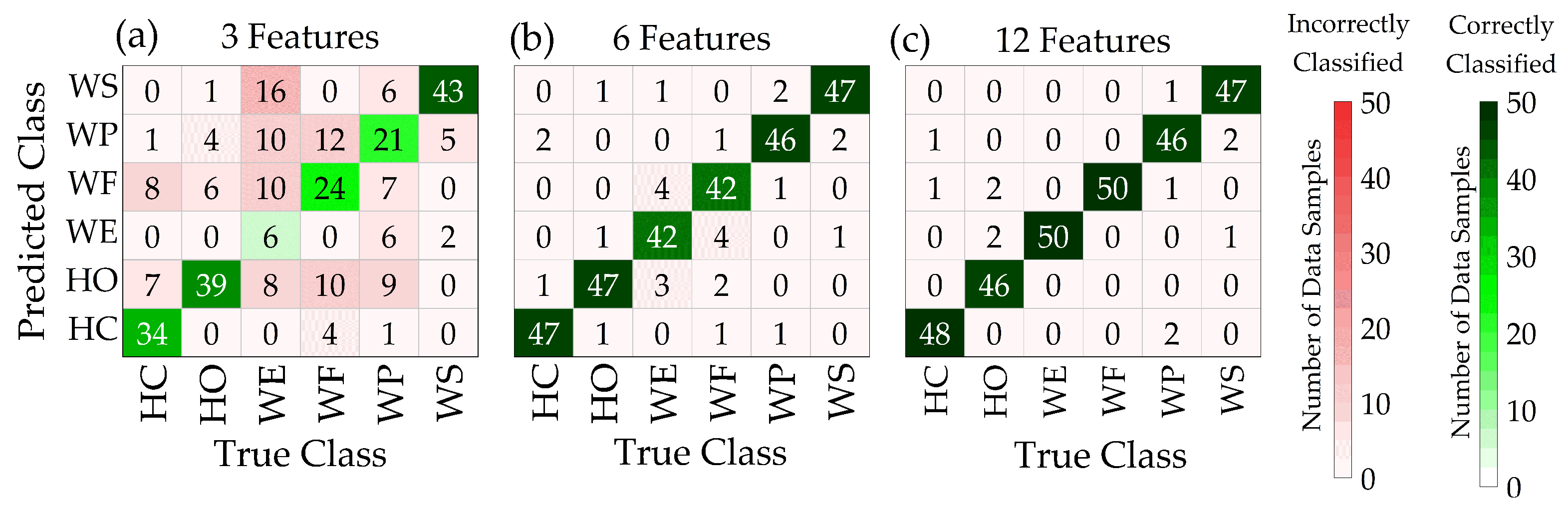
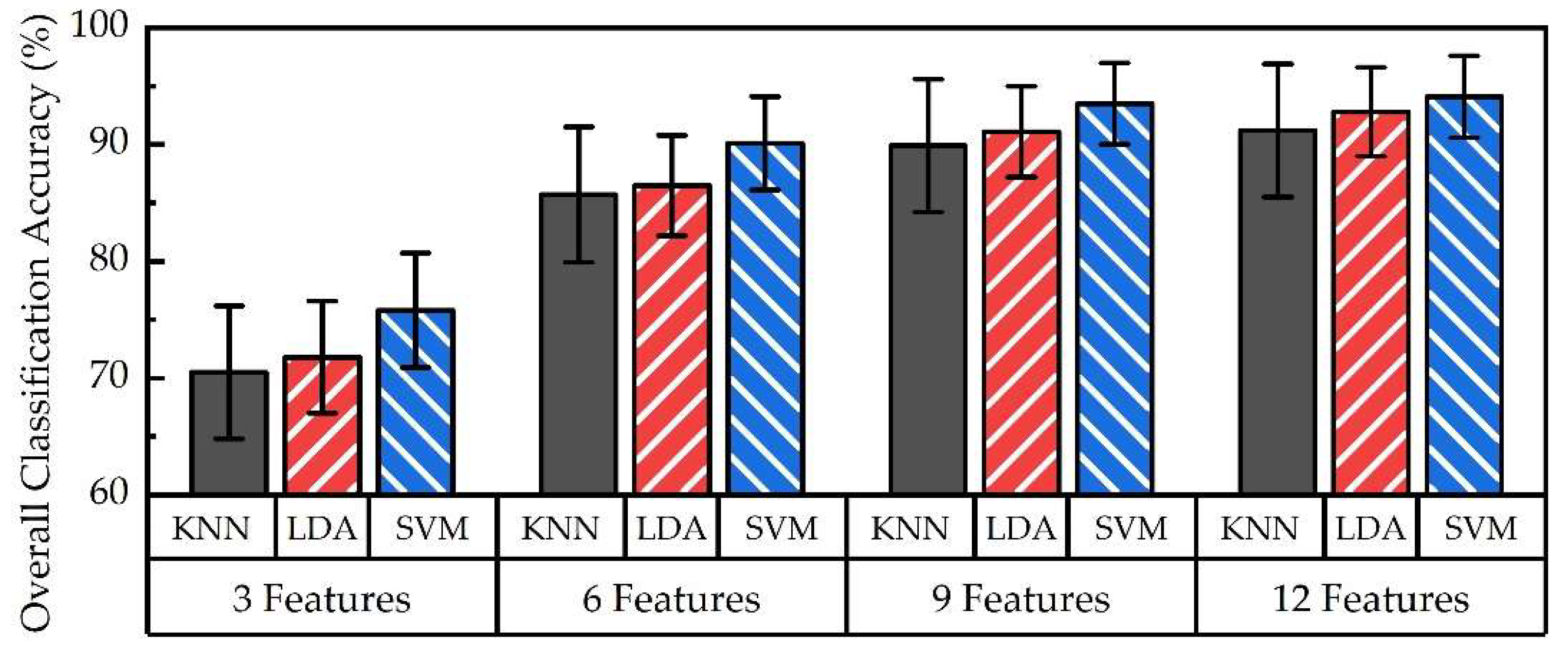
3.3. Overall Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cipriani, C.; Zaccone, F.; Micera, S.; Carrozza, M.C. On the shared control of an EMG-controlled prosthetic hand: Analysis of user-prosthesis interaction. IEEE Trans. Robot. 2008, 24, 170–184. [Google Scholar] [CrossRef]
- Tam, S.; Boukadoum, M.; Campeau-Lecours, A.; Gosselin, B. Intuitive real-time control strategy for high-density myoelectric hand prosthesis using deep and transfer learning. Sci. Rep. 2021, 11, 11275. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.K.; Jelfs, B.; Sui, X.H.; Arjunan, S.P. Prosthetic hand control: A multidisciplinary review to identify strengths, shortcomings, and the future. Biomed. Signal Process. Control 2019, 53, 101588. [Google Scholar] [CrossRef]
- MyoBoy. Available online: https://shop.ottobock.us/Prosthetics/Upper-Limb-Prosthetics/Myo-Hands-and-Components/Myo-Software/MyoBoy/p/757M11~5X-CHANGE (accessed on 22 January 2024).
- Johnson, S.S.; Mansfield, E. Prosthetic training: Upper limb. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Salminger, S.; Stino, H.; Pichler, L.H.; Gstoettner, C.; Sturma, A.; Mayer, J.A.; Szivak, M.; Aszmann, O.C. Current rates of prosthetic usage in upper-limb amputees—Have innovations had an impact on device acceptance? Disabil. Rehabil. 2022, 44, 3708–3713. [Google Scholar] [CrossRef] [PubMed]
- Resnik, L.; Borgia, M.; Clark, M. Function and Quality of Life of Unilateral Major Upper Limb Amputees: Effect of Prosthesis Use and Type. Arch. Phys. Med. Rehabil. 2020, 101, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Smail, L.C.; Neal, C.; Wilkins, C.; Packham, T.L. Comfort and function remain key factors in upper limb prosthetic abandonment: Findings of a scoping review. Disabil. Rehabil. Assist. Technol. 2021, 16, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Chadwell, A.; Kenney, L.; Thies, S.; Head, J.; Galpin, A.; Baker, R. Addressing unpredictability may be the key to improving performance with current clinically prescribed myoelectric prostheses. Sci. Rep. 2021, 11, 3300. [Google Scholar] [CrossRef] [PubMed]
- NHS England (Ed.) Clinical Commissioning Policy Multi-Grip Prosthetic Hand (All Ages) (2009) [220801P]; NHS England: Leeds, UK, 2022. [Google Scholar]
- Ottobock Myoelectric Myoplus. Available online: https://www.ottobock.com/en-au/Prosthetics/UpperLimb_MyoPlus (accessed on 22 January 2024).
- COAPT. Available online: https://coaptengineering.com/ (accessed on 22 January 2024).
- Alizadeh-Meghrazi, M.; Sidhu, G.; Jain, S.; Stone, M.; Eskandarian, L.; Toossi, A.; Popovic, M. A Mass-Producible Washable Smart Garment with Embedded Textile EMG Electrodes for Control of Myoelectric Prostheses: A Pilot Study. Sensors 2022, 22, 666. [Google Scholar] [CrossRef]
- Farrell, T.R. Determining delay created by multifunctional prosthesis controllers. J. Rehabil. Res. Dev. 2011, 48, xxi–xxxviii. [Google Scholar] [CrossRef]
- Franzke, A.; Kristofferson, M.; Bongers, R.; Murgia, A.; Pobatschnig, B.; Unglaube, F.; van der Sluis, C. Users’ and therapists’ perceptions of myoelectric multi-function upper limb prostheses with conventional and pattern recognition control. PLoS ONE 2019, 14, e0220899. [Google Scholar] [CrossRef]
- Li, G.; Schultz, A.E.; Kuiken, T.A. Quantifying pattern recognition-based myoelectric control of multifunctional transradial prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 185–192. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.K.; Samui, S. An experimental study on upper limb position invariant EMG signal classification based on deep neural network. Biomed. Signal Process. Control 2020, 55, 101669. [Google Scholar] [CrossRef]
- Kaufmann, P.; Englehart, K.; Platzner, M. Fluctuating emg signals: Investigating long-term effects of pattern matching algorithms. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010, 2010, 6357–6360. [Google Scholar] [PubMed]
- Phinyomark, A.; Quaine, F.; Charbonnier, S.; Serviere, C.; Tarpin-Bernard, F.; Laurillau, Y. EMG feature evaluation for improving myoelectric pattern recognition robustness. Expert Syst. Appl. 2013, 40, 4832–4840. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, Z.; Qiu, M.; Zeng, M.; Luo, W. A two-dimensional matrix image based feature extraction method for classification of sEMG: A comparative analysis based on SVM, KNN and RBF-NN. J. X-ray Sci. Technol. 2017, 25, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, P.; Yu, H. Gesture Recognition Using Surface Electromyography and Deep Learning for Prostheses Hand: State-of-the-Art, Challenges, and Future. Front. Neurosci. 2021, 15, 621885. [Google Scholar] [CrossRef]
- Tavakoli, M.; Benussi, C.; Alhais Lopes, P.; Osorio, L.B.; de Almeida, A.T. Robust hand gesture recognition with a double channel surface EMG wearable armband and SVM classifier. Biomed. Signal Process. Control 2018, 46, 121–130. [Google Scholar] [CrossRef]
- Phukan, N.; Kakoty, N.M.; Shivam, P.; Gan, J.Q. Finger movements recognition using minimally redundant features of wavelet denoised EMG. Health Technol. 2019, 9, 579–593. [Google Scholar] [CrossRef]
- Tosin, M.C.; Machado, J.C.; Balbinot, A. SEMG-based upper limb movement classifier: Current scenario and upcoming challenges. J. Artif. Intell. Res. 2022, 75, 83–127. [Google Scholar] [CrossRef]
- Ekinci, E.; Garip, Z.; Serbest, K. Electromyography based hand movement classification and feature extraction using machine learning algorithms. Politek. Derg. 2023, 26, 1621–1633. [Google Scholar] [CrossRef]
- DELSYS Trigno Research+. Available online: https://delsys.com/trigno/research/ (accessed on 22 January 2024).
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Park, S.; Lim, T.H.; Lee, S.J. Upper-Limb Electromyogram Classification of Reaching-to-Grasping Tasks Based on Convolutional Neural Networks for Control of a Prosthetic Hand. Front. Neurosci. 2021, 15, 733359. [Google Scholar] [CrossRef] [PubMed]
- Ghapanchizadeh, H.; Ahmad, S.A.; Ishak, A.J. Recommended surface EMG electrode position for wrist extension and flexion. In Proceedings of the 2015 IEEE Student Symposium in Biomedical Engineering & Sciences (ISSBES), Shah Alam, Malaysia, 4 November 2015; pp. 108–112. [Google Scholar]
- Corbett, E.A.; Perreault, E.J.; Kuiken, T.A. Comparison of electromyography and force as interfaces for prosthetic control. J. Rehabil. Res. Dev. 2011, 48, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, J.; Boyd, W.J.; Martinez-Luna, C.; Dai, C.; Wang, H.; Wang, H.; Huang, X.; Farrell, T.R.; Clancy, E.A. Myoelectric Control Performance of Two Degree of Freedom Hand-Wrist Prosthesis by Able-Bodied and Limb-Absent Subjects. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Heerschop, A.; van der Sluis, C.K.; Otten, E.; Bongers, R.M. Looking beyond proportional control: The relevance of mode switching in learning to operate multi-articulating myoelectric upper-limb prostheses. Biomed. Signal Process. Control 2020, 55, 101647. [Google Scholar] [CrossRef]
- Kapelner, T.; Negro, F.; Aszmann, O.C.; Farina, D. Decoding Motor Unit Activity from Forearm Muscles: Perspectives for Myoelectric Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tapia, B.; Soto, I.; Martínez, D.M.; Arballo, N.C. Myoelectric Interfaces and Related Applications: Current State of EMG Signal Processing–A Systematic Review. IEEE Access 2020, 8, 7792–7805. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Nazmi, N.; Abdul Rahman, M.A.; Yamamoto, S.; Ahmad, S.A.; Zamzuri, H.; Mazlan, S.A. A Review of Classification Techniques of EMG Signals during Isotonic and Isometric Contractions. Sensors 2016, 16, 1304. [Google Scholar] [CrossRef]
- Rebala, G.; Ravi, A.; Churiwala, S. Clustering. In An Introduction to Machine Learning; Rebala, G., Ravi, A., Churiwala, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 67–76. [Google Scholar]
- Rebala, G.; Ravi, A.; Churiwala, S. Classification. In An Introduction to Machine Learning; Rebala, G., Ravi, A., Churiwala, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 57–66. [Google Scholar]
- Zhao, H.; Lai, Z.; Leung, H.; Zhang, X. Linear Discriminant Analysis. In Feature Learning and Understanding: Algorithms and Applications; Zhao, H., Lai, Z., Leung, H., Zhang, X., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–85. [Google Scholar]
- Yacouby, R.; Axman, D. Probabilistic Extension of Precision, Recall, and F1 Score for More Thorough Evaluation of Classification Models. In Proceedings of the First Workshop on Evaluation and Comparison of NLP Systems, Online, 8–12 November 2020; Association for Computational Linguistics: St. Stroudsburg, PA, USA, 2020; pp. 79–91. [Google Scholar]
- Fushiki, T. Estimation of prediction error by using K-fold cross-validation. Stat. Comput. 2009, 21, 137–146. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Jia, G.Y.; Lam, H.K.; Liao, J.K.; Wang, R. Classification of electromyographic hand gesture signals using machine learning techniques. Neurocomputing 2020, 401, 236–248. [Google Scholar] [CrossRef]
- Khan, M.U.; Khan, H.; Muneeb, M.; Abbasi, Z.; Abbasi, U.B.; Baloch, N.K. Supervised Machine Learning based Fast Hand Gesture Recognition and Classification Using Electromyography (EMG) Signals. In Proceedings of the 2021 International Conference on Applied and Engineering Mathematics (ICAEM), Taxila, Pakistan, 30–31 August 2021; pp. 81–86. [Google Scholar]
- Hicks, S.A.; Strümke, I.; Thambawita, V.; Hammou, M.; Riegler, M.A.; Halvorsen, P.; Parasa, S. On evaluation metrics for medical applications of artificial intelligence. Sci. Rep. 2022, 12, 5979. [Google Scholar] [CrossRef] [PubMed]
- Chadwell, A.; Kenney, L.; Thies, S.; Galpin, A.; Head, J. The Reality of Myoelectric Prostheses: Understanding What Makes These Devices Difficult for Some Users to Control. Front. Neurorobotics 2016, 10, 7. [Google Scholar] [CrossRef]
- Merletti, R.; Roy, S. Myoelectric and mechanical manifestations of muscle fatigue in voluntary contractions. J. Orthop. Sports Phys. Ther. 1996, 24, 342–353. [Google Scholar] [CrossRef]
- Stephens-Fripp, B.; Alici, G.; Mutlu, R. A Review of Non-Invasive Sensory Feedback Methods for Transradial Prosthetic Hands. IEEE Access 2018, 6, 6878–6899. [Google Scholar] [CrossRef]
- Krasoulis, A.; Vijayakumar, S.; Nazarpour, K. Multi-Grip Classification-Based Prosthesis Control with Two EMG-IMU Sensors. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 508–518. [Google Scholar] [CrossRef]
- Treadway, E.; Gillespie, B.; Bolger, D.; Blank, A.; O’Malley, M.; Davis, A. The role of auxiliary and referred haptic feedback in myoelectric control. In Proceedings of the 2015 IEEE World Haptics Conference (WHC), Evanston, IL, USA, 22–26 June 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 13–18. [Google Scholar]
- Joshi, A.A.; Aziz, R.M. A two-phase cuckoo search based approach for gene selection and deep learning classification of cancer disease using gene expression data with a novel fitness function. Multimed. Tools Appl. 2024. [Google Scholar] [CrossRef]


| Feature | Equation | Definition | |
|---|---|---|---|
| Vmean | (1) | The mean absolute voltage. | |
| Vmax | (2) | The maximum absolute voltage value. | |
| Vmin | (3) | The minimum absolute voltage value. | |
| VSD | (4) | The standard deviation of the absolute voltage. | |
| Skew | (5) | A measure of asymmetry. | |
| Kurt | (6) | A measure of tailedness. | |
| n: total number of sEMG signal samples; Vi: ith sEMG signal sample after rectification and filtering | |||
| Medial | ||||||
| Action | Vmean (µV) | Vmax (µV) | Vmin (µV) | VSD (µV) | Skew | Kurt |
| HO | 28.5 | 101.4 | 10.6 | 22.5 | 1.8 | 6.2 |
| HC | 38.7 | 155.5 | 11.7 | 34.9 | 1.8 | 6.5 |
| WF | 33.8 | 171.0 | 9.7 | 36.8 | 2.1 | 7.0 |
| WE | 19.0 | 81.8 | 9.0 | 12.7 | 2.4 | 10.2 |
| WS | 18.6 | 62.5 | 10.5 | 10.6 | 2.1 | 8.3 |
| WP | 23.8 | 121.6 | 9.2 | 21.7 | 2.0 | 7.9 |
| Lateral | ||||||
| Action | Vmean (µV) | Vmax (µV) | Vmin (µV) | VSD (µV) | Skew | Kurt |
| HO | 45.8 | 148.4 | 16.1 | 33.1 | 1.5 | 5.2 |
| HC | 51.9 | 153.2 | 23.2 | 28.6 | 1.6 | 5.7 |
| WF | 33.6 | 102.3 | 14.6 | 18.2 | 1.9 | 7.7 |
| WE | 53.8 | 204.5 | 13.8 | 52.1 | 1.6 | 5.1 |
| WS | 33.4 | 83.0 | 17.1 | 15.0 | 1.5 | 5.6 |
| WP | 37.2 | 109.4 | 17.2 | 19.6 | 1.6 | 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, H.O.; Devin, K.M.; Tang, J.; Jiang, L. Evaluation of Hand Action Classification Performance Using Machine Learning Based on Signals from Two sEMG Electrodes. Sensors 2024, 24, 2383. https://doi.org/10.3390/s24082383
Shaw HO, Devin KM, Tang J, Jiang L. Evaluation of Hand Action Classification Performance Using Machine Learning Based on Signals from Two sEMG Electrodes. Sensors. 2024; 24(8):2383. https://doi.org/10.3390/s24082383
Chicago/Turabian StyleShaw, Hope O., Kirstie M. Devin, Jinghua Tang, and Liudi Jiang. 2024. "Evaluation of Hand Action Classification Performance Using Machine Learning Based on Signals from Two sEMG Electrodes" Sensors 24, no. 8: 2383. https://doi.org/10.3390/s24082383
APA StyleShaw, H. O., Devin, K. M., Tang, J., & Jiang, L. (2024). Evaluation of Hand Action Classification Performance Using Machine Learning Based on Signals from Two sEMG Electrodes. Sensors, 24(8), 2383. https://doi.org/10.3390/s24082383





