The Analytical Validity of Stride Detection and Gait Parameters Reconstruction Using the Ankle-Mounted Inertial Measurement Unit Syde®
Abstract
1. Introduction
- High variability of data depending on the state and motivation of the participant at the time of data collection;
- Access to a fixed number of activities chosen by the experimenter;
2. Materials and Methods
2.1. Motion Recording Materials
2.1.1. IMU-Based Wearable Device
- Raw data are written on the docking station after the sensors are reattached;
- Station uploads the raw data to a remote secure cloud;
- Usable data are extracted against the sensors’ calibration files;
- Trajectory is estimated at the stride level from the calibrated inertial data;
- Gait parameters are estimated from the reconstructed trajectory.
2.1.2. Motion Capture
2.2. Experimental Protocol
- Normal walk: The participants were asked to walk a distance of 80 m. The walking route consisted of 10 two-way trips between two markers on the ground separated by 4 m, as shown in Figure 3. The instruction given to the participant was to “walk at your own comfortable walking pace”, as it is similarly seen in pace instructions in standard assessments of walking, such as the 10 MWT at a comfortable pace [27].
- Fast walk: The participants had the same setting and distance as in the normal walking exercise but were instructed to walk "as fast as they could" as is similarly seen in the instructions of gait pace in the 10 MWT [27].
- Dual-task walk: As inspired by a real-life condition, where "the multifaceted nature of the stimuli coming from the surrounding world requires responses that often imply the simultaneous performance of a motor and a cognitive task, i.e., dual-task" as presented by Pedullà et al. (2022) [28]. We choose to perform a dual task of walking and cognitive activity to change the patients’ walking behavior, such as walking speed, and get closer to real-life conditions, as shown in studies [29,30]: same setting, distance, and pace as in the normal walk exercise, but the participants were asked to perform a cognitive task at the same time. The task was to recite as many animal names as possible while walking.
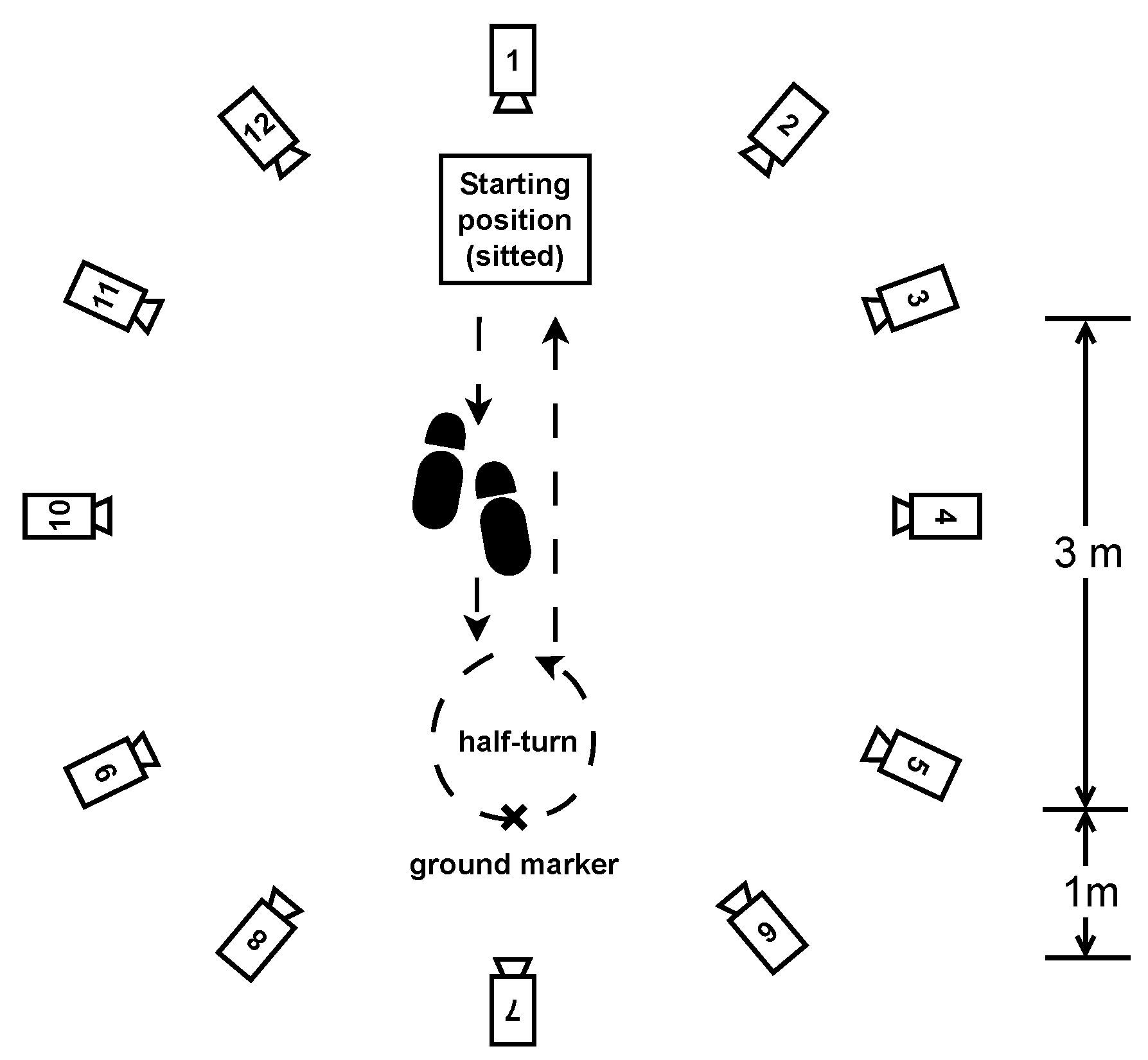
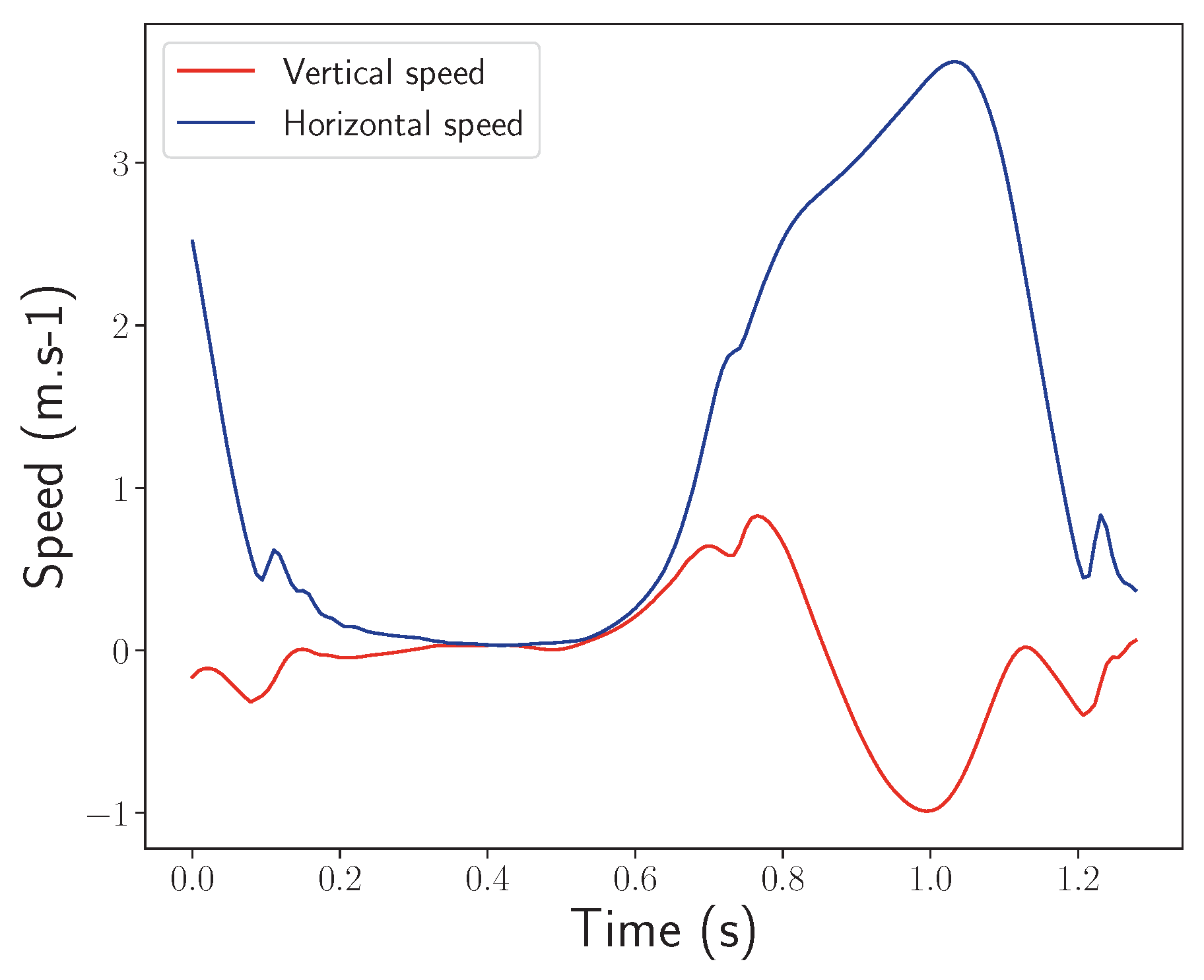
- Half-turns: The same setting and pace as in the normal walk exercise, but this time, the participants had to walk for one minute and make a half-turn each time the experimenter clapped their hands. Seven hand claps occurred at predetermined and heterogeneously distributed times within one minute. See Figure 4.
- Timed up-and-go: Each participant started the exercise sitting in a chair, stood up, walked 3 m at their natural pace, turned, walked back to the chair, and sat down. This exercise was repeated three times with little or no rest. See Figure 5.
2.3. Analysis Method
2.3.1. Gait Parameter Reconstruction from Inertial Data
2.3.2. Motion Capture Data Processing
2.3.3. Analytical Features Calculation
3. Results
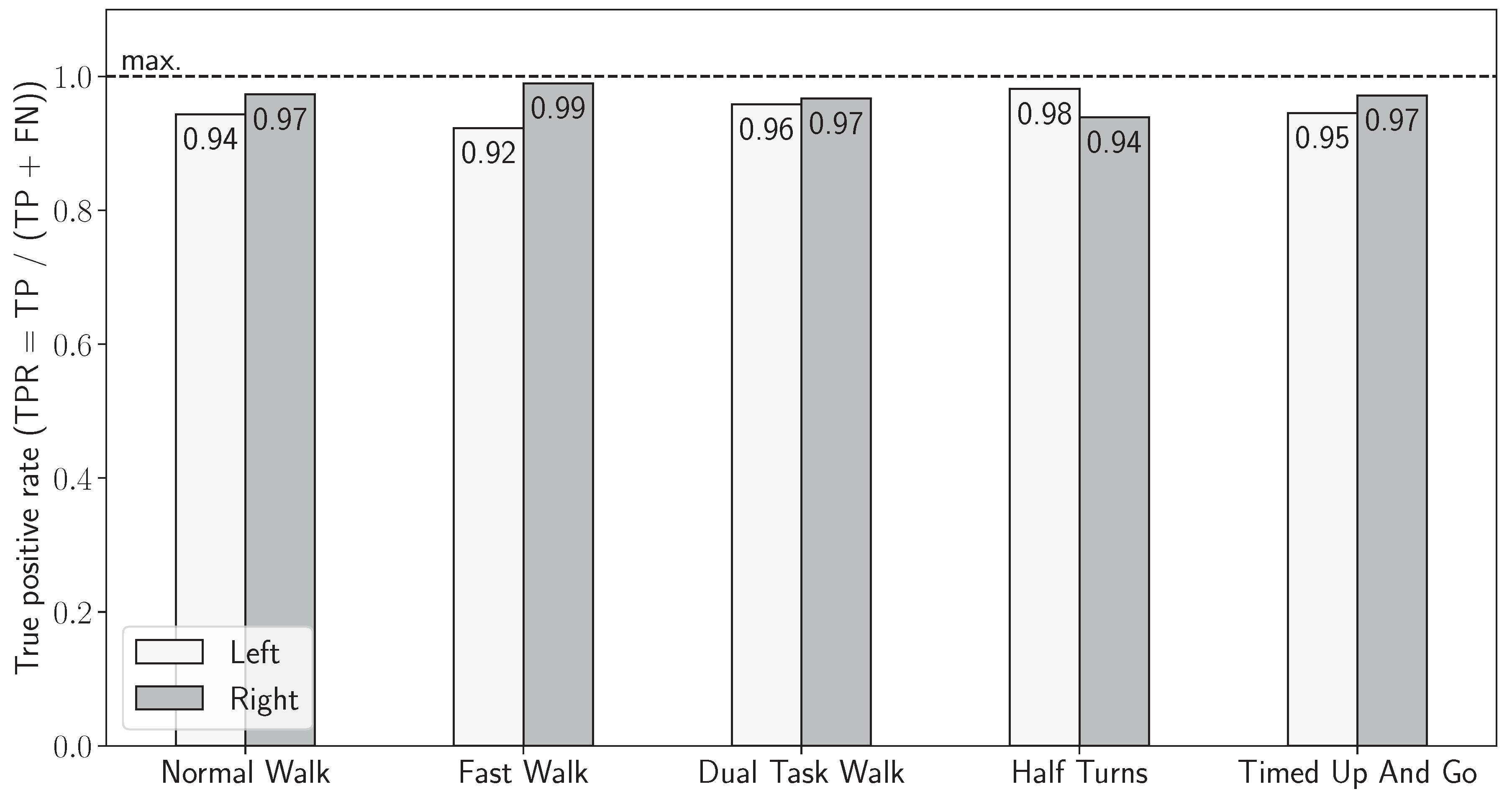
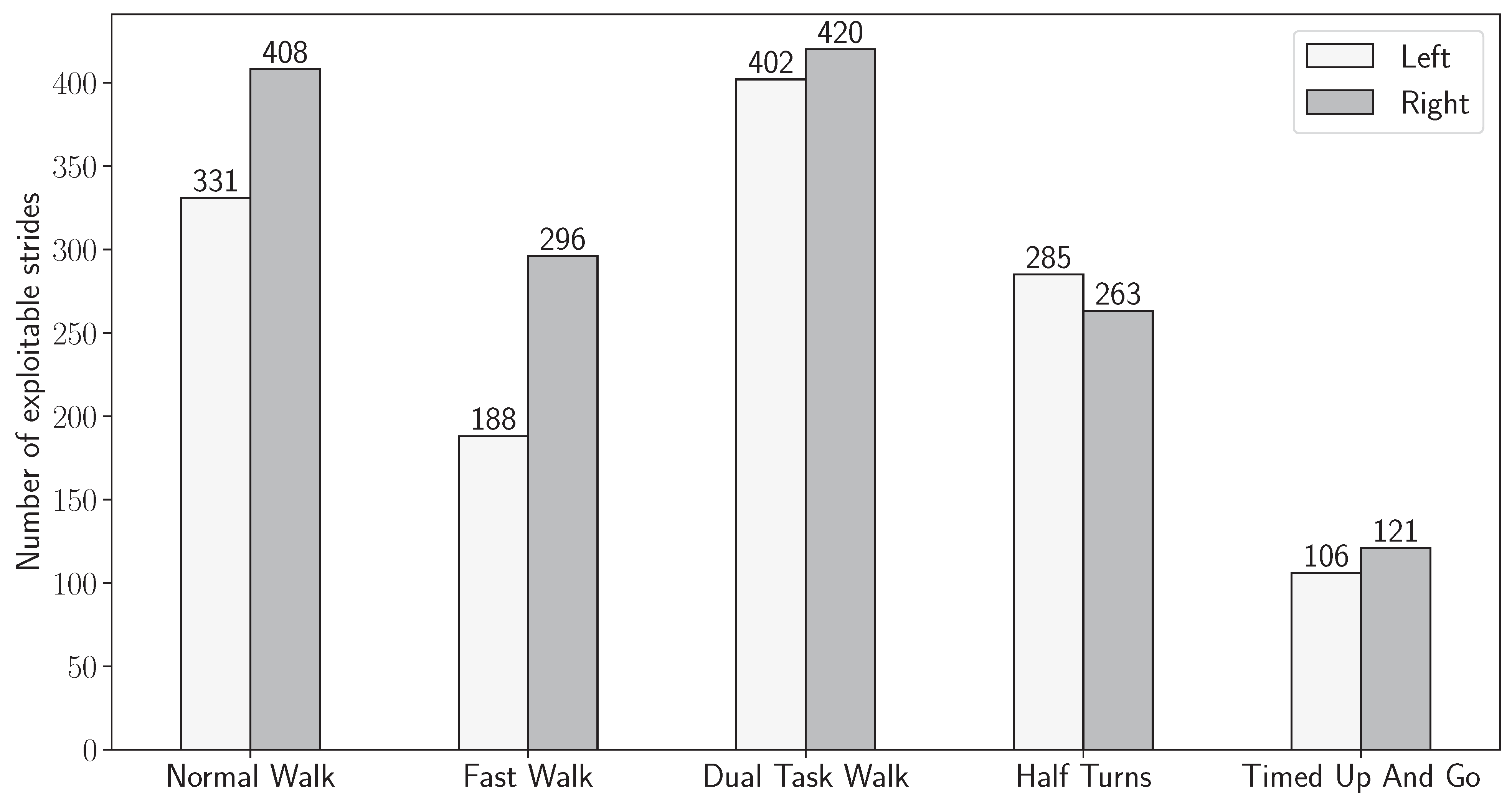
3.1. Validation of Sensor-Based Stride Detection
3.2. Joint Stride Detection
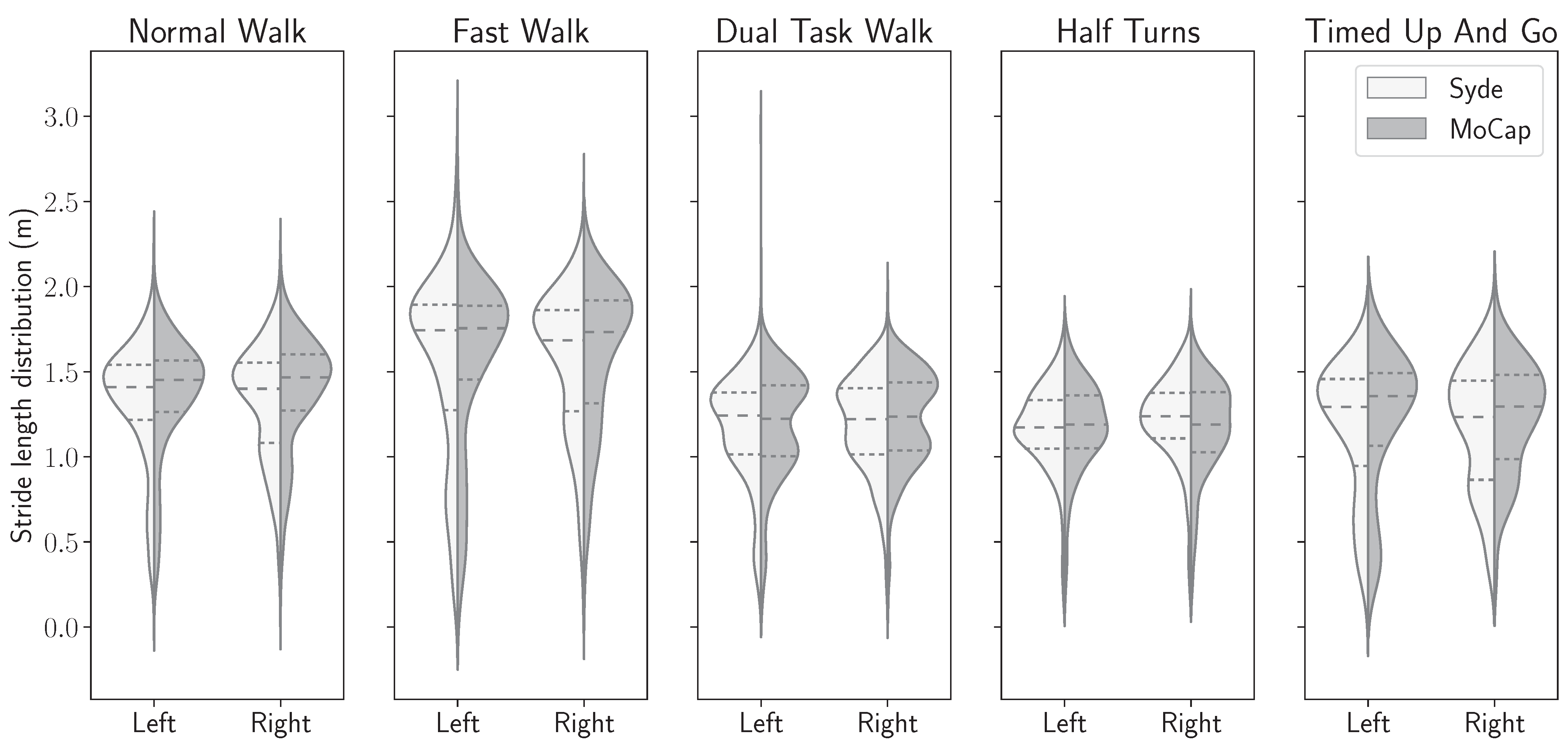
3.3. Gait Features Distribution in Both Systems
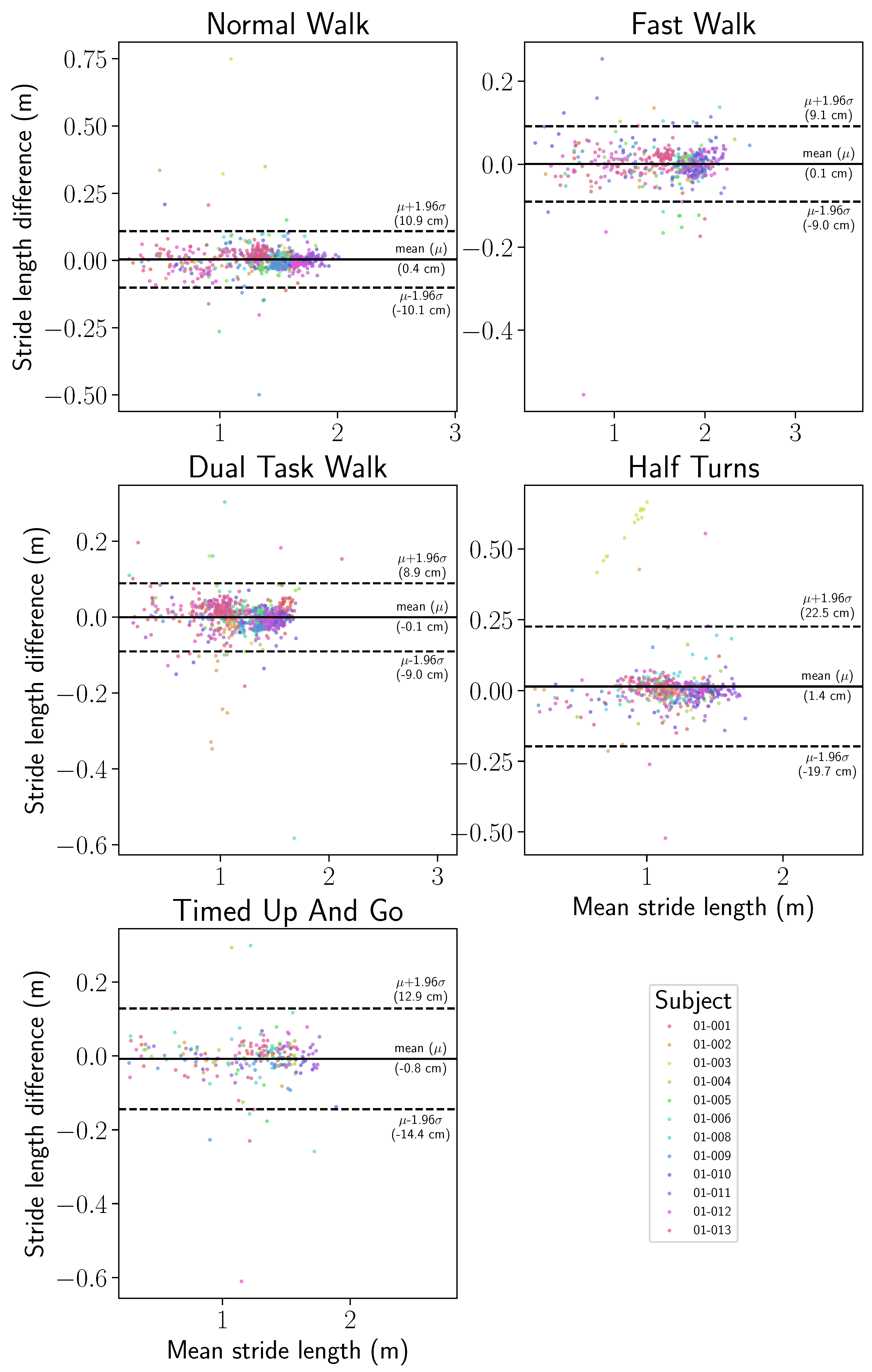
3.4. Precision of Motion Capture Reference
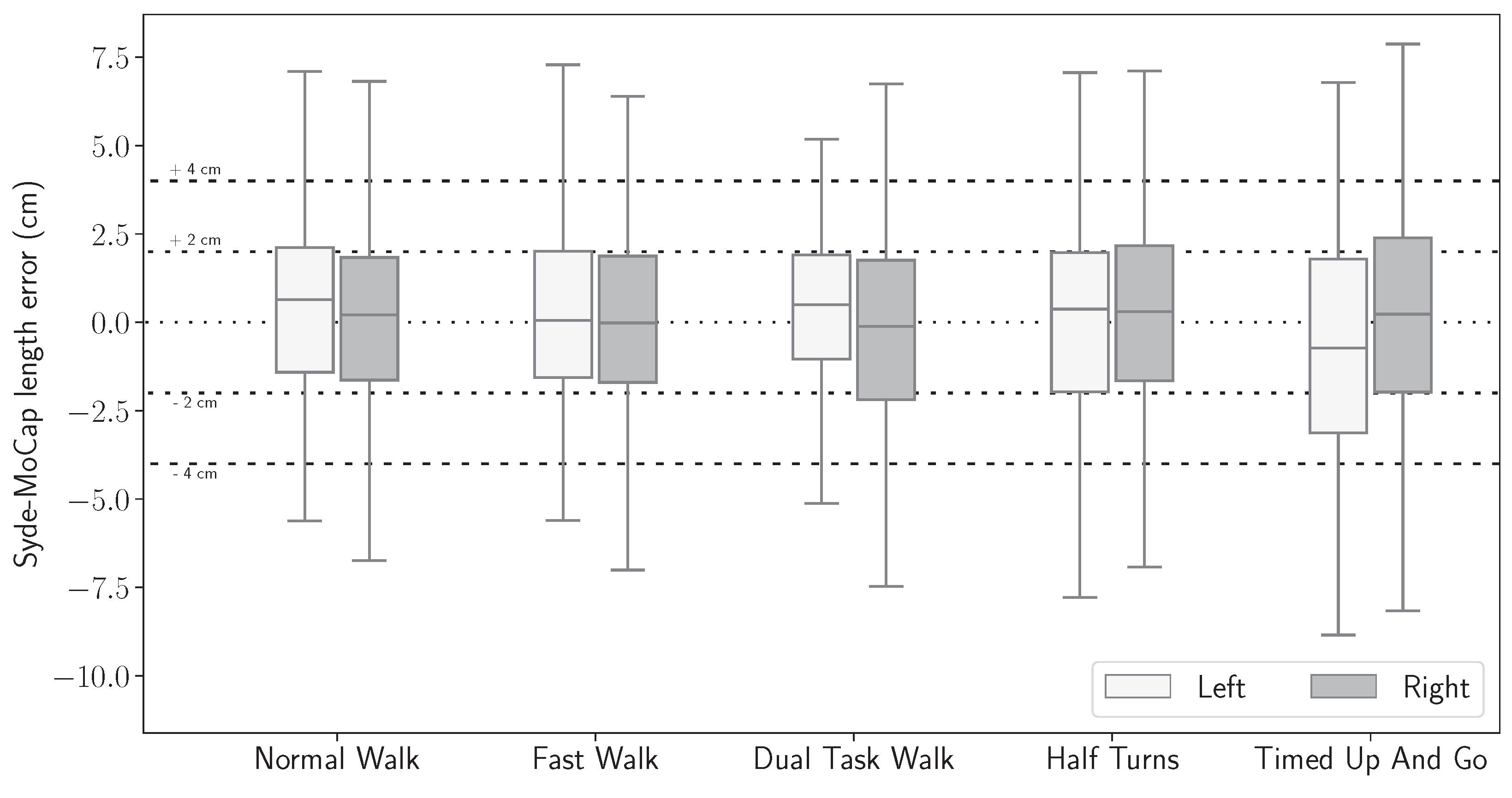
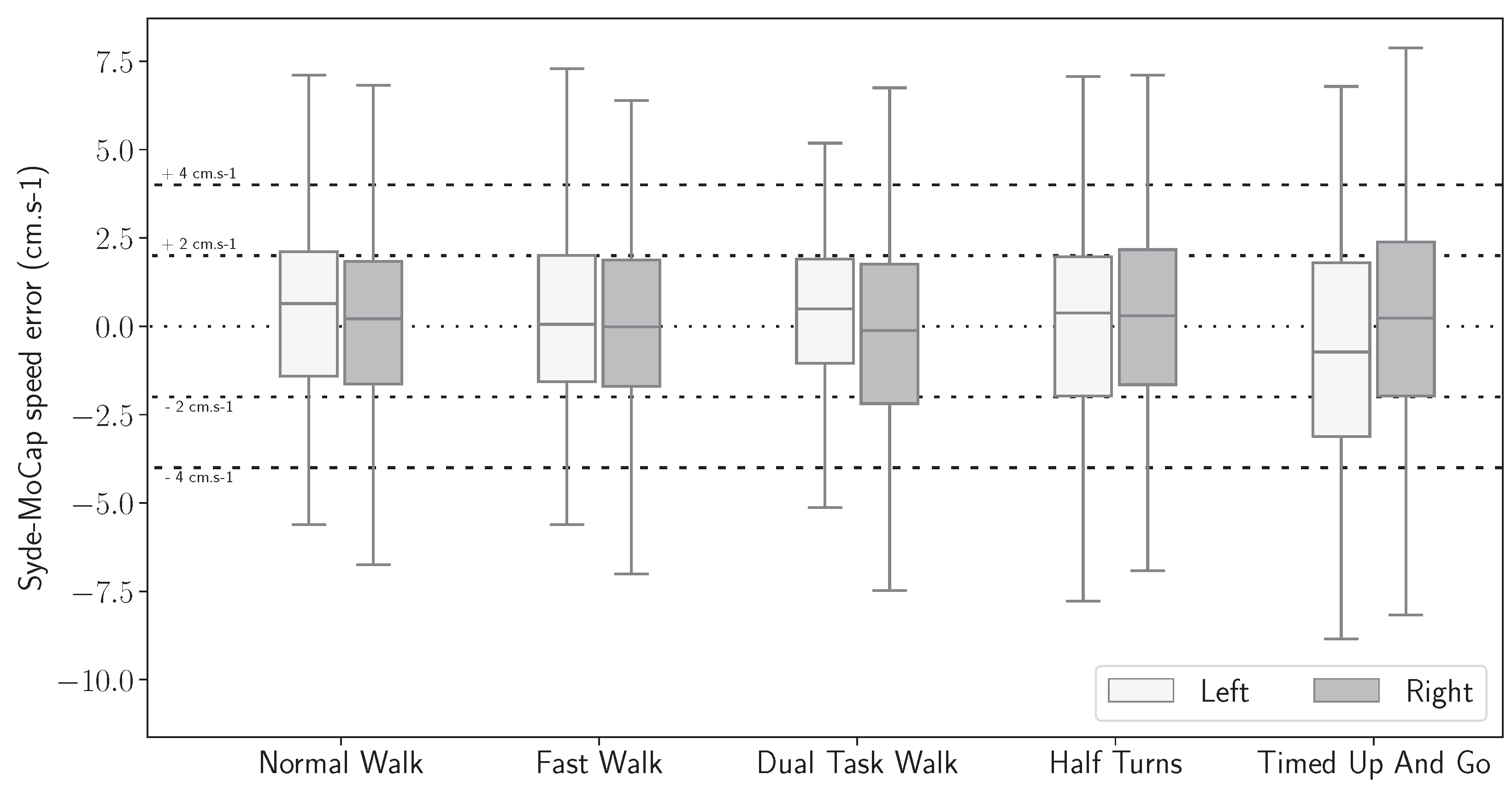
3.5. Gait Features Error Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Block, V.A.; Pitsch, E.; Tahir, P.; Cree, B.A.; Allen, D.D.; Gelfand, J.M. Remote physical activity monitoring in neurological disease: A systematic review. PLoS ONE 2016, 11, e0154335. [Google Scholar] [CrossRef]
- Kohn, C.G.; Baker, W.L.; Sidovar, M.F.; Coleman, C.I. Walking speed and health-related quality of life in multiple sclerosis. Patient-Patient-Centered Outcomes Res. 2014, 7, 55–61. [Google Scholar] [CrossRef]
- Schmid, A.; Duncan, P.W.; Studenski, S.; Lai, S.M.; Richards, L.; Perera, S.; Wu, S.S. Improvements in speed-based gait classifications are meaningful. Stroke 2007, 38, 2096–2100. [Google Scholar] [CrossRef]
- Brodie, M.A.; Lord, S.R.; Coppens, M.J.; Annegarn, J.; Delbaere, K. Eight-week remote monitoring using a freely worn device reveals unstable gait patterns in older fallers. IEEE Trans. Biomed. Eng. 2015, 62, 2588–2594. [Google Scholar] [CrossRef]
- Le Moing, A.G.; Seferian, A.M.; Moraux, A.; Annoussamy, M.; Dorveaux, E.; Gasnier, E.; Hogrel, J.Y.; Voit, T.; Vissière, D.; Servais, L. A movement monitor based on magneto-inertial sensors for non-ambulant patients with Duchenne muscular dystrophy: A pilot study in controlled environment. PLoS ONE 2016, 11, e0156696. [Google Scholar] [CrossRef]
- Annoussamy, M.; Seferian, A.M.; Daron, A.; Péréon, Y.; Cances, C.; Vuillerot, C.; De Waele, L.; Laugel, V.; Schara, U.; Gidaro, T.; et al. Natural history of Type 2 and 3 spinal muscular atrophy: 2-year NatHis-SMA study. Ann. Clin. Transl. Neurol. 2021, 8, 359–373. [Google Scholar] [CrossRef]
- Vienne, A.; Barrois, R.P.; Buffat, S.; Ricard, D.; Vidal, P.P. Inertial sensors to assess gait quality in patients with neurological disorders: A systematic review of technical and analytical challenges. Front. Psychol. 2017, 8, 817. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. White paper: “Walking speed: The sixth vital sign”. J. Geriatr. Phys. Ther. 2009, 32, 2–5. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef]
- Polhemus, A.M.; Bergquist, R.; de Basea, M.B.; Brittain, G.; Buttery, S.C.; Chynkiamis, N.; Dalla Costa, G.; Ortiz, L.D.; Demeyer, H.; Emmert, K.; et al. Walking-related digital mobility outcomes as clinical trial endpoint measures: Protocol for a scoping review. BMJ Open 2020, 10, e038704. [Google Scholar] [CrossRef]
- Jung, S.; Michaud, M.; Oudre, L.; Dorveaux, E.; Gorintin, L.; Vayatis, N.; Ricard, D. The use of inertial measurement units for the study of free living environment activity assessment: A literature review. Sensors 2020, 20, 5625. [Google Scholar] [CrossRef]
- Oudre, L.; Barrois-Müller, R.; Moreau, T.; Truong, C.; Vienne-Jumeau, A.; Ricard, D.; Vayatis, N.; Vidal, P.P. Template-based step detection with inertial measurement units. Sensors 2018, 18, 4033. [Google Scholar] [CrossRef]
- Verdecchia, P.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Zampi, I.; Gattobigio, R.; Sacchi, N.; Porcellati, C. White coat hypertension and white coat effect similarities and differences. Am. J. Hypertens. 1995, 8, 790–798. [Google Scholar] [CrossRef]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; Jácome, M.A.; García-Sancho, C.; Cudeiro, J.; Arias, P. Spatiotemporal gait patterns during overt and covert evaluation in patients with Parkinson’s disease and healthy subjects: Is there a Hawthorne effect? J. Appl. Biomech. 2015, 31, 189–194. [Google Scholar] [CrossRef]
- Shema-Shiratzky, S.; Hillel, I.; Mirelman, A.; Regev, K.; Hsieh, K.L.; Karni, A.; Devos, H.; Sosnoff, J.J.; Hausdorff, J.M. A wearable sensor identifies alterations in community ambulation in multiple sclerosis: Contributors to real-world gait quality and physical activity. J. Neurol. 2020, 267, 1912–1921. [Google Scholar] [CrossRef]
- Polhemus, A.; Ortiz, L.D.; Brittain, G.; Chynkiamis, N.; Salis, F.; Gaßner, H.; Gross, M.; Kirk, C.; Rossanigo, R.; Taraldsen, K.; et al. Walking on common ground: A cross-disciplinary scoping review on the clinical utility of digital mobility outcomes. NPJ Digit. Med. 2021, 4, 149. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: Toward clinical and at home use. IEEE J. Biomed. Health Inform. 2015, 20, 838–847. [Google Scholar] [CrossRef]
- Godfrey, A.; Vandendriessche, B.; Bakker, J.P.; Fitzer-Attas, C.; Gujar, N.; Hobbs, M.; Liu, Q.; Northcott, C.A.; Parks, V.; Wood, W.A.; et al. Fit-for-purpose biometric monitoring technologies: Leveraging the laboratory biomarker experience. Clin. Transl. Sci. 2021, 14, 62–74. [Google Scholar] [CrossRef]
- Lilien, C.; Gasnier, E.; Gidaro, T.; Seferian, A.; Grelet, M.; Vissière, D.; Servais, L. Home-based monitor for gait and activity analysis. JoVE (J. Vis. Exp.) 2019, 150, e59668. [Google Scholar]
- Gidaro, T.; Gasnier, E.; Denis, S.; Lilien, C.; Grelet, M.; Rigaud, A.; Moraux, A.; Dorveaux, E.; Vissiere, D.; Servais, L. Assessment of lower limbs in FSHD: The ActiMyo as a new outcome for home-monitoring. Neuromuscul. Disord. 2016, 26, S168. [Google Scholar] [CrossRef]
- Lilien, C.; Annoussamy, M.; Polleur, M.; Seferian, A.; Boespflug-Tanguy, O.; Gorni, K.; Eggenspieler, D.; Servais, L. ActiMyo (R): Upper limb activity in non-ambulant patients with spinal muscular atrophy treated with Spinraza. Neuromuscul. Disord. 2020, 30, S100. [Google Scholar] [CrossRef]
- Poleur, M.; Ulinici, A.; Daron, A.; Schneider, O.; Dal Farra, F.; Demonceau, M.; Annoussamy, M.; Vissière, D.; Eggenspieler, D.; Servais, L. DMD/BMD–OUTCOME MEASURES: EP. 124 Actimyo®: Normative data in a non-controlled environment. Neuromuscul. Disord. 2021, 31, S86–S87. [Google Scholar] [CrossRef]
- Annoussamy, M.; Eggenspieler, D.; Seferian, A.; Mercuri, E.; Straub, V.; Muntoni, F.; Scoto, M.; Poleur, M.; Daron, A.; Butoianu, N.; et al. FP. 12 Application for primary endpoint qualification of the 95th centile of stride velocity (SV95C) in Duchenne muscular dystrophy. Neuromuscul. Disord. 2022, 32, S65. [Google Scholar] [CrossRef]
- Dyne Therapeutics. A Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose Study Assessing Safety, Tolerability, Pharmacodynamics, Efficacy, and Pharmacokinetics of DYNE-251 Administered to Participants with Duchenne Muscular Dystrophy Amenable to Exon 51 Skipping; Clinical Trial Registration NCT05524883; Dyne Therapeutics: Waltham, MA, USA, 2023. [Google Scholar]
- Sarepta Therapeutics, Inc. A Phase 3 Multinational, Randomized, Double-Blind, Placebo-Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Subjects with Duchenne Muscular Dystrophy (EMBARK); Clinical Trial Registration NCT05096221; Sarepta Therapeutics, Inc.: Cambridge, MA, USA, 2023. [Google Scholar]
- Committee for Medicinal Products for Human Use. Qualification Opinion for Stride Velocity 95th Centile as Primary Endpoint in Studies in Ambulatory Duchenne Muscular Dystrophy Studies; Qualification Opinion EMADOC-1700519818-1127132; European Medicines Agency: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Moore, J.L.; Potter, K.; Blankshain, K.; Kaplan, S.L.; O’Dwyer, L.C.; Sullivan, J.E. A core set of outcome measures for adults with neurologic conditions undergoing rehabilitation: A clinical practice guideline. J. Neurol. Phys. Ther. 2018, 42, 174. [Google Scholar] [CrossRef]
- Pedullà, L.; Tacchino, A.; Podda, J.; Bragadin, M.M.; Bonzano, L.; Battaglia, M.; Bove, M.; Brichetto, G.; Ponzio, M. The patients’ perspective on the perceived difficulties of dual-tasking: Development and validation of the Dual-task Impact on Daily-living Activities Questionnaire (DIDA-Q). Mult. Scler. Relat. Disord. 2020, 46, 102601. [Google Scholar] [CrossRef]
- Ebersbach, G.; Dimitrijevic, M.R.; Poewe, W. Influence of concurrent tasks on gait: A dual-task approach. Percept. Mot. Ski. 1995, 81, 107–113. [Google Scholar] [CrossRef]
- Nohelova, D.; Bizovska, L.; Vuillerme, N.; Svoboda, Z. Gait variability and complexity during single and dual-task walking on different surfaces in outdoor environment. Sensors 2021, 21, 4792. [Google Scholar] [CrossRef]
- Vissiere, D.; Hillion, M.; Dorveaux, E.; Jouy, A.; Grelet, M. Method for Estimating the Movement of a Pieton. FR FR3042266A1, 14 April 2017. [Google Scholar]
- VissiÈRe, D.; Grelet, M. Analyse de la Foulee D’un Pieton en Marche. WO WO2019243609A1, 26 December 2019. [Google Scholar]
- Optitrack. Quick Start Guide: Precision Capture. Available online: https://docs.optitrack.com/quick-start-guides/quick-start-guide-precision-capture (accessed on 31 January 2024).











| Subject | Gender | Age (years) | Height (cm) | Weight (kg) | BMI (kg·m−2) |
|---|---|---|---|---|---|
| 01 | M | 23 | 181.0 | 63 | 19.2 |
| 02 | M | 25 | 178.0 | 72 | 22.7 |
| 03 | M | 24 | 175.0 | 72 | 23.5 |
| 04 | M | 22 | 174.5 | 70 | 23.0 |
| 05 | M | 27 | 179.0 | 86 | 26.8 |
| 06 | F | 22 | 165.0 | 52 | 19.1 |
| 07 | M | 22 | 180.0 | 65 | 20.1 |
| 08 | M | 24 | 174.0 | 60 | 19.8 |
| 09 | F | 21 | 164.5 | 55 | 20.3 |
| 10 | M | 26 | 176.0 | 68 | 22.0 |
| 11 | M | 21 | 180.0 | 63 | 19.4 |
| 12 | F | 21 | 163.0 | 55 | 20.7 |
| Sensor Location | Left Ankle | Right Ankle | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise | Q1 | Median | Q3 | Q1 | Median | Q3 | Q1 | Median | Q3 |
| NW (cm) | 0.93 | 1.87 | 3.23 | 0.86 | 1.73 | 2.99 | 0.88 | 1.81 | 3.11 |
| FW (cm) | 0.93 | 1.72 | 3.01 | 0.86 | 1.81 | 3.05 | 0.87 | 1.79 | 3.03 |
| DTW (cm) | 0.69 | 1.55 | 2.95 | 0.98 | 1.98 | 3.20 | 0.83 | 1.74 | 3.05 |
| HT (cm) | 1.10 | 1.97 | 3.67 | 0.88 | 1.92 | 3.98 | 0.94 | 1.95 | 3.82 |
| TUG (cm) | 1.07 | 2.33 | 4.04 | 1.08 | 2.17 | 3.70 | 1.07 | 2.29 | 3.77 |
| Overall (cm) | 0.87 | 1.84 | 3.14 | 0.89 | 1.90 | 3.32 | 0.88 | 1.86 | 3.24 |
| Sensor Location | Left Ankle | Right Ankle | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise | Q1 | Median | Q3 | Q1 | Median | Q3 | Q1 | Median | Q3 |
| NW (cm·s−1) | 0.77 | 1.50 | 2.45 | 0.74 | 1.50 | 2.57 | 0.75 | 1.50 | 2.51 |
| FW (cm·s−1) | 0.77 | 1.58 | 2.69 | 0.88 | 1.78 | 3.05 | 0.84 | 1.71 | 2.87 |
| DTW (cm·s−1) | 0.54 | 1.21 | 2.09 | 0.77 | 1.55 | 2.48 | 0.64 | 1.38 | 2.26 |
| HT (cm·s−1) | 0.83 | 1.65 | 2.81 | 0.70 | 1.59 | 3.32 | 0.77 | 1.61 | 3.06 |
| TUG (cm·s−1) | 0.92 | 1.84 | 2.74 | 0.89 | 1.77 | 3.08 | 0.90 | 1.84 | 3.01 |
| Overall (cm·s−1) | 0.71 | 1.48 | 2.47 | 0.77 | 1.61 | 2.75 | 0.74 | 1.56 | 2.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaud, M.; Guérin, A.; Dejean de La Bâtie, M.; Bancel, L.; Oudre, L.; Tricot, A. The Analytical Validity of Stride Detection and Gait Parameters Reconstruction Using the Ankle-Mounted Inertial Measurement Unit Syde®. Sensors 2024, 24, 2413. https://doi.org/10.3390/s24082413
Michaud M, Guérin A, Dejean de La Bâtie M, Bancel L, Oudre L, Tricot A. The Analytical Validity of Stride Detection and Gait Parameters Reconstruction Using the Ankle-Mounted Inertial Measurement Unit Syde®. Sensors. 2024; 24(8):2413. https://doi.org/10.3390/s24082413
Chicago/Turabian StyleMichaud, Mona, Alexandre Guérin, Marguerite Dejean de La Bâtie, Léopold Bancel, Laurent Oudre, and Alexis Tricot. 2024. "The Analytical Validity of Stride Detection and Gait Parameters Reconstruction Using the Ankle-Mounted Inertial Measurement Unit Syde®" Sensors 24, no. 8: 2413. https://doi.org/10.3390/s24082413
APA StyleMichaud, M., Guérin, A., Dejean de La Bâtie, M., Bancel, L., Oudre, L., & Tricot, A. (2024). The Analytical Validity of Stride Detection and Gait Parameters Reconstruction Using the Ankle-Mounted Inertial Measurement Unit Syde®. Sensors, 24(8), 2413. https://doi.org/10.3390/s24082413






