Double-Clad Antiresonant Hollow-Core Fiber and Its Comparison with Other Fibers for Multiphoton Micro-Endoscopy
Abstract
:1. Introduction
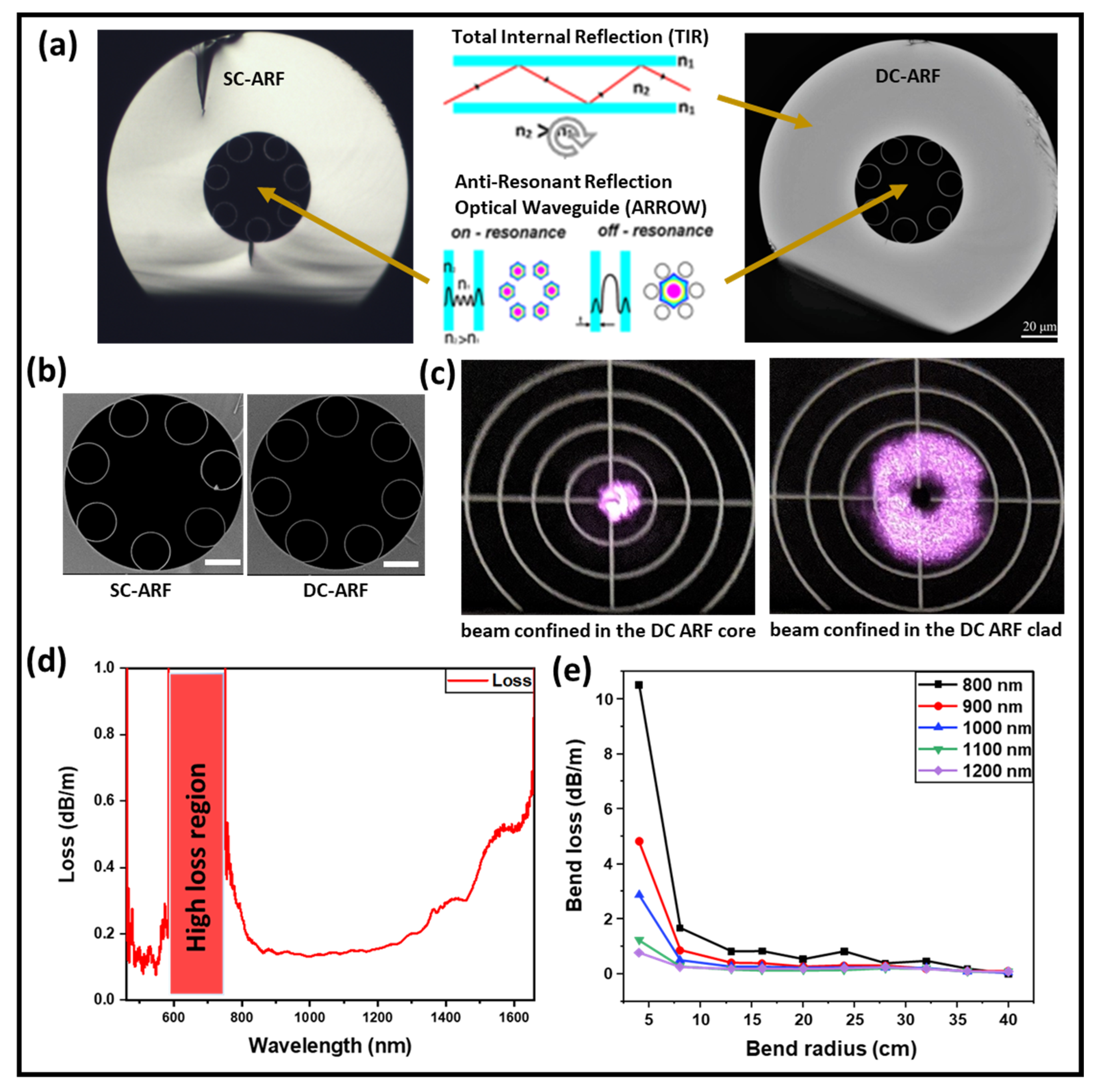
2. Hollow-Core Double-Clad Anti-Resonant Fiber (DC-ARF) Fabrication and Loss Characterization
3. Suitability of Fiber Characteristics for Multiphoton Endoscopy: Comparison of DC-ARF with SC-ARF and an SCF
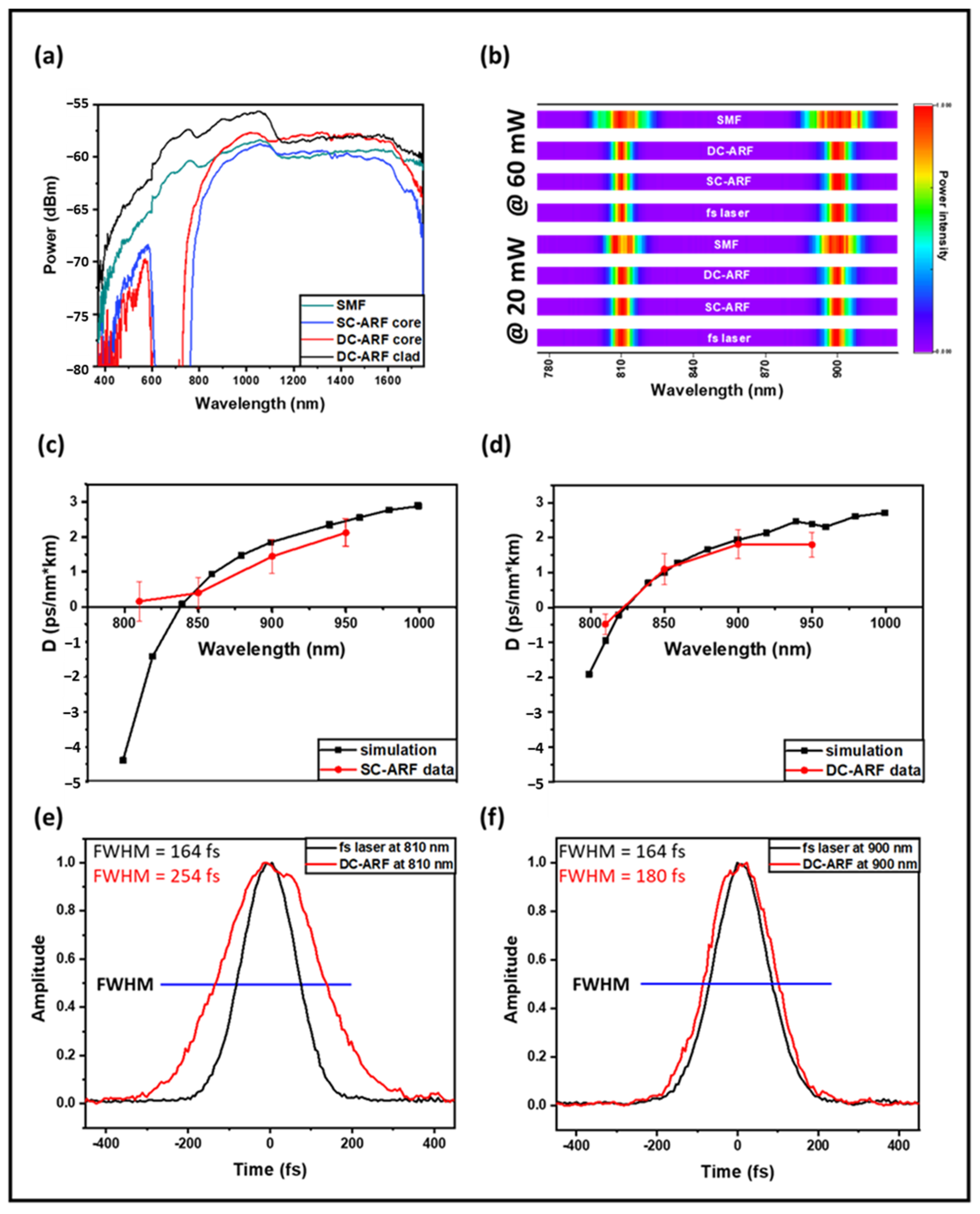
4. Optical Transmission
5. Non-Linear Spectral Effects
6. Dispersion
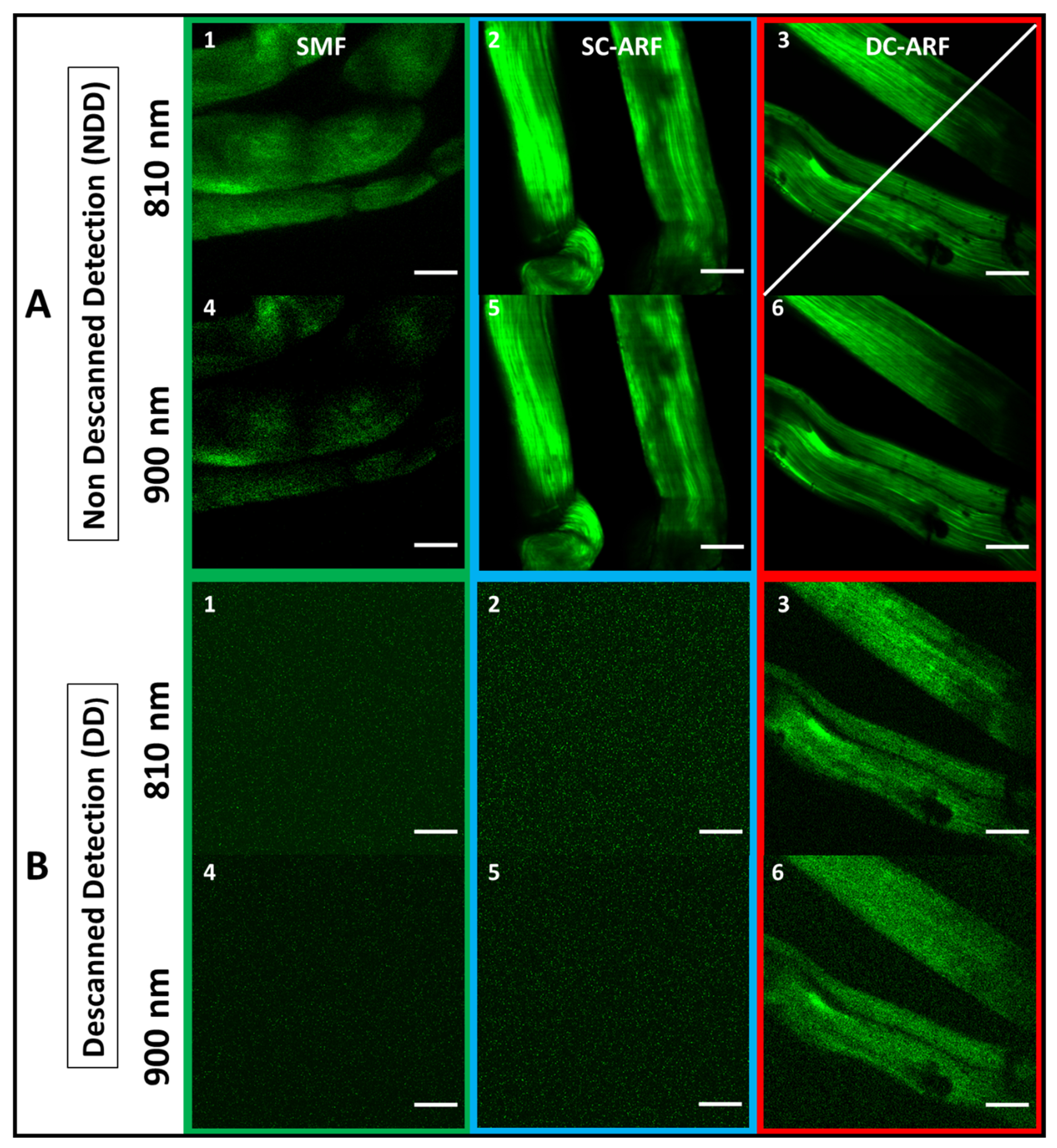
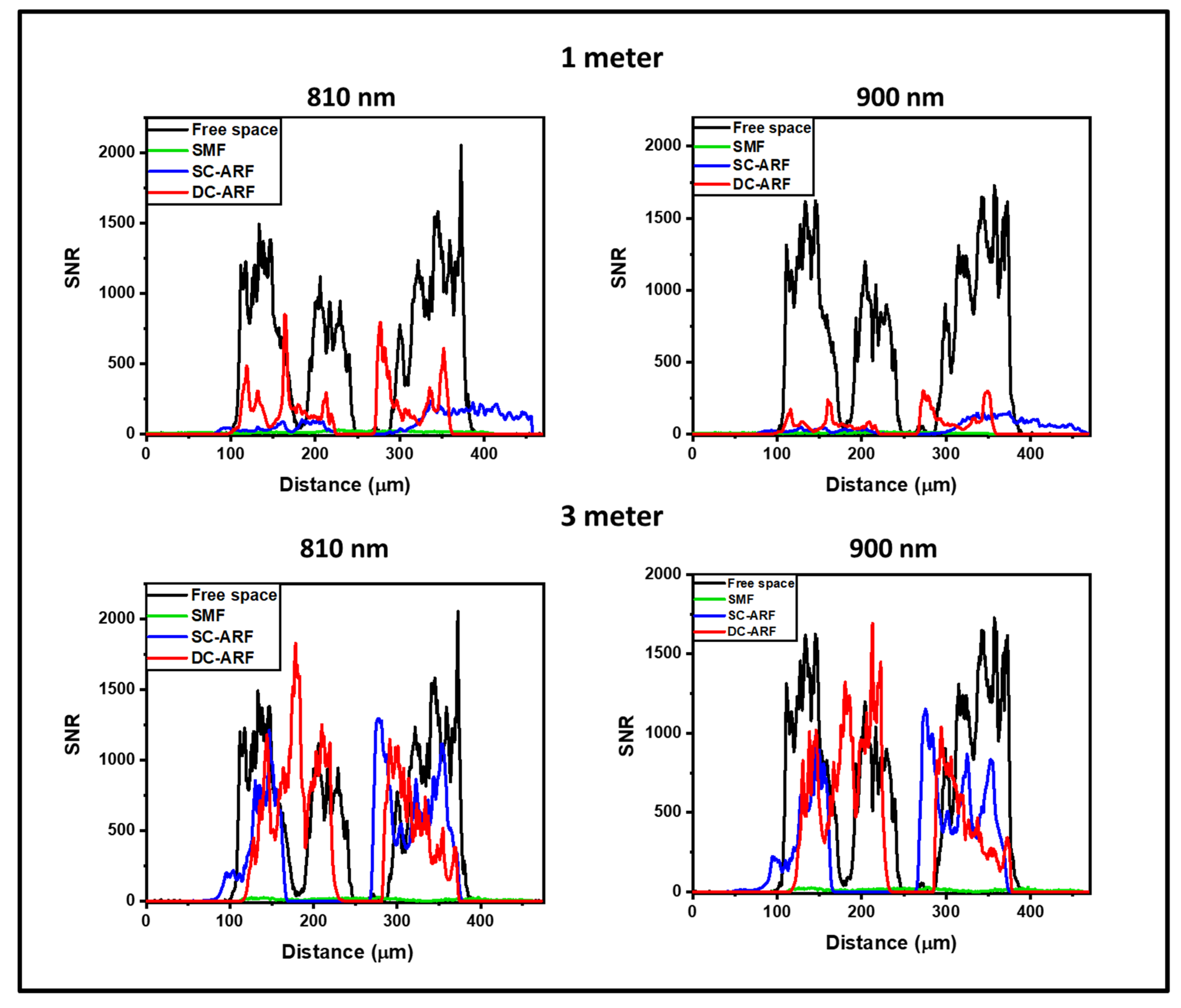
7. Comparison of SCF, SC-ARF, and DC-ARF in Non-de-Scanned (NDD) and De-Scanned (DD) Detection Scheme with Second Harmonic Generation Imaging
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulkadir, S. Histopathology Made Easy. Available online: https://www.academia.edu/40884313/Histopathology_Made_easy (accessed on 28 August 2021).
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Butt, H.-J. Near-infrared photochemistry at interfaces based on upconverting nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 23585–23596. [Google Scholar] [CrossRef] [PubMed]
- New, G. Introduction to Non-Linear Optics; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Parodi, V.; Jacchetti, E.; Osellame, R.; Cerullo, G.; Polli, D.; Raimondi, M.T. Nonlinear Optical Microscopy: From Fundamentals to Applications in Live Bioimaging. Front. Bioeng. Biotechnol. 2020, 8, 585363. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.; Gu, M. Fibre-optic two-photon scanning fluorescence microscopy. J. Microsc. 2002, 208, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Légaré, F.; Evans, C.L.; Ganikhanov, F.; Xie, X.S. Towards CARS Endoscopy. Opt. Express 2006, 14, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Liu, G.; Chen, Z.; Tromberg, B.J.; Potma, E.O. Fiber delivered probe for efficient CARS imaging of tissues. Opt. Express 2010, 18, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Naji, M.; Murugkar, S.; Alarcon, E.; Brideau, C.; Stys, P.; Anis, H. Portable, miniaturized, fibre delivered, multimodal CARS exoscope. Opt. Express 2013, 21, 17161–17175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, L.; Luo, P.; Yang, Y.; Hammoudi, A.A.; Wong, K.K.; Wong, S.T.C. Coherent anti-Stokes Raman scattering microscopy imaging with suppression of four-wave mixing in optical fibers. Opt. Express 2011, 19, 7960–7970. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, X.; McCormick, D.T.; Wong, K.; Wong, S.T.C. Multimodal non-linear endo-microscopy probe design for high resolution, label-free intraoperative imaging. Biomed. Opt. Express 2015, 6, 2283–2293. [Google Scholar] [CrossRef]
- Deladurantaye, P.; Paquet, A.; Paré, C.; Zheng, H.; Doucet, M.; Gay, D.; Poirier, M.; Cormier, J.F.; Mermut, O.; Wilson, B.C.; et al. Advances in engineering of high contrast CARS imaging endoscopes. Opt. Express 2014, 22, 25053–25064. [Google Scholar] [CrossRef]
- Jung, W.; Tang, S.; McCormic, D.T.; Xie, T.; Ahn, Y.; Su, J.; Tomov, I.V.; Krasieva, T.B.; Tromberg, B.J.; Chen, Z. Miniaturized probe based on a microelectromechanical system mirror for multiphoton microscopy. Opt. Lett. 2008, 33, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Myaing, M.T.; Ye, J.Y.; Norris, T.B.; Thomas, T.; Baker, J.R.; Wadsworth, W.J.; Bouwmans, G.; Knight, J.C.; Russell, P. Enhanced two-photon biosensing with double-clad photonic crystal fibers. Opt. Lett. 2003, 28, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Jung, W.; McCormick, D.; Xie, T.; Su, J.; Ahn, J.; Tromberg, B.J.; Chen, Z. Design and implementation of fiber-based multiphoton endoscopy with microelectromechanical systems scanning. J. Biomed. Opt. 2009, 14, 034005. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Boussioutas, A.; Jeremy, R.; Russell, S.; Gu, M. Second harmonic generation imaging via non-linear endomicroscopy. Opt. Express 2010, 18, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.-P.; Chan, M.-C.; Tsai, T.-H.; Guol, S.-H.; Chen, L.-J.; Sun, C.-K. Two-photon fluorescence microscope with a hollow-core photonic crystal fiber. Opt. Express 2004, 12, 6122–6128. [Google Scholar] [CrossRef]
- Flusberg, B.A.; Jung, J.C.; Cocker, E.D.; Anderson, E.P.; Schnitzer, M.J. In Vivo brain imaging using a portable 3.9 g two-photon fluorescence microendoscope. Opt. Lett. 2005, 30, 2272–2274. [Google Scholar] [CrossRef]
- Engelbrecht, C.J.; Johnston, R.S.; Seibel, E.J.; Helmchen, F. Ultra-compact fiber-optic two-photon microscope for functional fluorescence imaging in vivo. Opt. Express 2008, 16, 5556–5564. [Google Scholar] [CrossRef]
- Göbel, W.; Axel Nimmerjahn, A.; Helmchen, F. Distortion-free delivery of nanojoule femtosecond pulses from a Ti:sapphire laser through a hollow-core photonic crystal fiber. Opt. Lett. 2004, 29, 1285–1287. [Google Scholar] [CrossRef]
- Brustlein, S.; Berto, P.; Hostein, R.; Ferrand, P.; Billaudeau, C.; Marguet, D.; Muir, A.; Knight, J.; Rigneault, H. Double-clad hollow core photonic crystal fibre for coherent Raman endoscope. Opt. Express 2011, 19, 12562–12568. [Google Scholar] [CrossRef]
- Mosley, P.; Huang, P.W.; Welch, M.G.; Mangan, B.J.; Wadsworth, W.J.; Knight, J.C. Ultrashort pulse compression and delivery in a hollow-core photonic crystal fiber at 540 nm wavelength. Opt. Lett. 2010, 35, 3589–3591. [Google Scholar] [CrossRef]
- Welch, M.G.; de Nobriga, C.E.; Correa, R.A.; Wadsworth, W.J.; Knight, J.C. Accurate measurement of the dispersion of hollow-core fibers using a scalable technique. Opt. Express 2009, 17, 9006–9011. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zeng, H.; Lui, H.; Skibina, J.S.; Steinmeyer, G.; Tang, S. Characterization and application of chirped photonic crystal fiber in multiphoton imaging. Opt. Express 2014, 22, 10366–10379. [Google Scholar] [CrossRef] [PubMed]
- Markos, C.H.; Travers, J.C.; Abdolvand, A.; Eggleton, B.J.; Bang, O. Hybrid photonic-crystal fiber. Rev. Mod. Phys. 2017, 89, 045003. [Google Scholar] [CrossRef]
- Lombardini, A.; Mytskaniuk, V.; Sivankutty, S.; Andresen, E.R.; Chen, X.; Wenger, J.; Fabert, M.; Joly, N.; Louradour, F.; Kudlinski, A.; et al. High-resolution multimodal flexible coherent Raman endoscope. Light Sci. Appl. 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wheeler, N.V.; Couny, F.; Roberts, P.J.; Benabid, F. Low loss broadband transmission in hypocycloid-core Kagome hollow-core photonic crystal fibre. Opt. Lett. 2011, 36, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.S.J.; Holzer, P.; Chang, W.; Abdolvand, A.; Travers, J.C. Hollow-core photonic crystal fibres for gas-based non-linear optics. Nat. Photonics 2014, 8, 278–286. [Google Scholar] [CrossRef]
- Poletti, F. Nested antiresonant nodeless hollow core fibre. Opt. Express 2014, 22, 23807–23828. [Google Scholar] [CrossRef]
- Bufetov, I.A.; Kosolapov, A.F.; Pryamikov, A.D.; Gladyshev, A.V.; Kolyadin, A.N.; Krylov, A.A.; Yatsenko, Y.P.; Biriukov, A.S. Revolver hollow core optical fibres. Fibres 2018, 6, 39. [Google Scholar] [CrossRef]
- Sakr, H.; Chen, Y.; Jasion, G.T.; Bradley, T.D.; Hayes, J.R.; Mulvad, H.; Davidson, I.A.; Numkam Fokoua, E.; Poletti, F. Hollow core optical fibres with comparable attenuation to silica fibres between 600 and 1100 nm. Nat. Commun. 2020, 11, 6030. [Google Scholar] [CrossRef]
- Hayes, J.R.; Sandoghchi, S.R.; Bradly, T.B.; Liu, Z.; Slavik, R.; Gouveia, M.A.; Wheeler, N.V.; Jasion, G.; Chen, Y.; Fokoua, E.N.; et al. Antiresonant hollow core fibre with an one octave spanning bandwidth for short haul data communications. J. Light Wave Technol. 2017, 35, 437–442. [Google Scholar] [CrossRef]
- Jaworski, P.; Yu, F.; Maire, R.J.R.; Wadsworth, W.J.; Knight, J.C.; Shephard, J.D.; Hand, D.P. Picosecond and nanosecond pulse delivery through a hollow-core Negative Curvature Fibre for micro-machining applications. Opt. Express 2013, 21, 22742–22753. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, P.; Yu, F.; Carter, R.M.; Knight, J.C.; Shephard, J.D.; Hand, D.P. High energy green nanosecond and picosecond pulse delivery through a negative curvature fibre for precision micro-machining. Opt. Express 2015, 23, 8498–8506. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, B.; Yu, F.; Stone, J.; Warren, S.; Paterson, C.; Neil, M.A.A.; French, P.M.W.; Knight, J. Dunsby Ch Tunable fiber-coupled multiphoton microscopy with a negative curvature fiber. J. Biophotonics 2016, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Myaing, M.T.; MacDonald, D.J.; Li, X. Fibre-optic scanning two-photon fluorescence endoscopy. Opt. Lett. 2006, 31, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Gu, M. Reduction of self-phase modulation in double-clad photonic crystal fiber for non-linear optical endoscopy. Opt. Lett. 2009, 34, 148–150. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, H.; Gordon, R.J. Development of a versatile two-photon endoscope for biological imaging. Biomed. Opt. Express 2010, 1, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Akins, M.L.; Murari, K.; Jiefeng Xi, J.; Li, M.-J.; Luby-Phelps, K.; Mahendroo, M.; Li, X. A compact fiber-optic SHG scanning endomicroscope and its application to visualize cervical remodeling during pregnancy. Proc. Natl. Acad. Sci. USA 2012, 10, 12878–12883. [Google Scholar] [CrossRef] [PubMed]
- Ducourthial, G.; Leclerc, P.; Mansuryan, T.; Fabert, M.; Brevier, J.; Habert, R.; Braud, F.; Batrin, R.; Vever-Bizet, C.H.; BourgHeckly, G.; et al. Development of a real-time flexible multiphoton microendoscope for label-free imaging in a live animal. Sci. Rep. 2015, 5, 18303. [Google Scholar] [CrossRef] [PubMed]
- Kudlinski, A.; Cassez, A.; Vanvincq, O.; Septier, D.; Pastre, A.; Habert, R.; Baudelle, K.; Douay, M.; Mytskaniuk, V.; Tsvirkun, V.; et al. Double clad tubular anti-resonant hollow core fiber for non-linear microendoscopy. Opt. Express 2020, 28, 15062–15070. [Google Scholar] [CrossRef]
- Michieletto, M.; Lyngsø, J.K.; Jakobsen, C.; Lægsgaard, J.; Bang, O.; Alkeskjold, T.T. Hollow-core fibers for high power pulse delivery. Opt. Express 2016, 24, 7103–7119. [Google Scholar] [CrossRef]
- Morales-Delgado, E.E.; Farahi, S.; Papadopoulos, I.N.; Psaltis, D.; Moser, C. Delivery of focused short pulses through a multimode fiber. Opt. Express 2015, 23, 9109–9120. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.; Partridge, M.C.; Hayes, J.; Chen, Y.; Bradley, T.; Sakr, H.; Rikimi, S.; Jasion, G.; Numkam, F.E.; Petrovich, M.; et al. Tubular anti-resonant hollow core fiber for visible Raman spectroscopy. In Proceedings of the Sixth International Workshop on Specialty Optical Fibers and Their Applications (WSOF 2019): Conference Digest, Charleston, SC, USA, 6–8 November 2019; SPIE: Bellingham, WA, USA, 2019; Volume 7, p. 11206. [Google Scholar]
- Setti, V.; Vincetti, L.; Argyros, A. Flexible tube lattice fibers for terahertz applications. Opt. Express 2013, 21, 3388–3399. [Google Scholar] [CrossRef] [PubMed]
- Belardi, W.; Knight, J.C. Effect of core boundary curvature on the confinement losses of hollow antiresonant fibers. Opt. Express 2013, 21, 21912–21917. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-F.; Wang, Y.-Y.; Liu, X.-L.; Ding, W.; Wang, P. Bending loss characterization in nodeless hollow-core anti-resonant fiber. Opt. Express 2016, 24, 14801–14811. [Google Scholar] [CrossRef] [PubMed]
- Saleh, B.E.A.; Teich, M.C. Fundamentals of Photonics; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Boilard, T.; Vallée, R.; Bernier, M. Probing the dispersive properties of optical fibers with an array of femtosecond-written fiber Bragg gratings. Sci. Rep. 2022, 12, 4350. [Google Scholar] [CrossRef] [PubMed]
- Keikhosravi, A.; Bredfeldt, J.S.; Sagar, A.K.; Eliceiri, K.W. Second-harmonic generation imaging of cancer. Methods Cell Biol. 2014, 123, 531–546. [Google Scholar] [PubMed]
- Johnson, P.; Karvounis, A.; Johnson Singh, H.; Brereton, C.J.; Bourdakos, K.; Lunn, K.; Roberts, J.J.W.; Davies, D.E.; Muskens, O.L.; Jones, M.G.; et al. Super-resolved polarisation-enhanced second harmonic generation for direct imaging of nanoscale changes in collagen architecture. Optica 2021, 8, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.; Andriotis, O.G.; Roberts, J.J.; Lunn, K.; Tear, V.J.; Cao, L.; Ask, K.; Smart, D.E.; Bonfanti, A.; Johnson, P.; et al. Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. eLife 2018, 7, e36354. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Pologruto, T.A.; Sabatini, B.L.; Svoboda, K. ScanImage: Flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2003, 2, 13. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]


| (a) | ||||||
|---|---|---|---|---|---|---|
| 810 nm | 850 nm | 900 nm | 950 nm | |||
| SCF | 2659 | 2638.2 | 2553.2 | 2035.4 | ||
| SC-ARF | 168 | 156.3 | 133.2 | 117.2 | ||
| DC-ARF | 180 | 144 | 128 | 119 | ||
| (b) | Pulse Duration (fs) after 1 and 3 m of Fiber at 900 nm | |||||
| Initial Pulse Duration | SCF | SC-ARF | DC-ARF | |||
| 1 m | 3 m | 1 m | 3 m | 1 m | 3 m | |
| 10 fs | 26,595 | 79,754 | 2336 | 6987 | 1603 | 4789 |
| 100 fs | 2746 | 8043 | 287 | 660 | 249 | 548 |
| 116 fs | 2400 | 6972 | 277 | 599 | 245 | 503 |
| 1 ps | 1265 | 1795 | 1019 | 1056 | 1015 | 1045 |
| 10 ps | 10,158 | 10,473 | 10,008 | 10,024 | 10,006 | 10,019 |
| D (ps*nm−1*km−1) | ||||
|---|---|---|---|---|
| 810 nm | 850 nm | 900 nm | 950 nm | |
| SCF | −103 (±0.29) | −90.7 (±0.23) | −78.3 (±0.31) | −56 (±0.26) |
| SC-ARF | 0.163 (±0.56) | 0.404 (±0.45) | 1.45 (±0.48) | 2.13 (±0.39) |
| DC-ARF | −0.48 (±0.29) | 1.1 (±0.44) | 1.81 (±0.41) | 1.8 (±0.35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwaj, M.; Davidson, I.A.; Johnson, P.B.; Jasion, G.; Jung, Y.; Sandoghchi, S.R.; Herdzik, K.P.; Bourdakos, K.N.; Wheeler, N.V.; Mulvad, H.C.; et al. Double-Clad Antiresonant Hollow-Core Fiber and Its Comparison with Other Fibers for Multiphoton Micro-Endoscopy. Sensors 2024, 24, 2482. https://doi.org/10.3390/s24082482
Szwaj M, Davidson IA, Johnson PB, Jasion G, Jung Y, Sandoghchi SR, Herdzik KP, Bourdakos KN, Wheeler NV, Mulvad HC, et al. Double-Clad Antiresonant Hollow-Core Fiber and Its Comparison with Other Fibers for Multiphoton Micro-Endoscopy. Sensors. 2024; 24(8):2482. https://doi.org/10.3390/s24082482
Chicago/Turabian StyleSzwaj, Marzanna, Ian A. Davidson, Peter B. Johnson, Greg Jasion, Yongmin Jung, Seyed Reza Sandoghchi, Krzysztof P. Herdzik, Konstantinos N. Bourdakos, Natalie V. Wheeler, Hans Christian Mulvad, and et al. 2024. "Double-Clad Antiresonant Hollow-Core Fiber and Its Comparison with Other Fibers for Multiphoton Micro-Endoscopy" Sensors 24, no. 8: 2482. https://doi.org/10.3390/s24082482





