Improving the Selectivity of Metal Oxide Semiconductor Sensors for Mustard Gas Simulant 2-Chloroethyl Ethyl Sulfide by Combining the Laminated Structure and Temperature Dynamic Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Layer Materials
2.1.1. Preparation of (Pt + Rh)@WO3 Slurry
2.1.2. Preparation of (Pt + Pd + Rh)@Al2O3 Slurry
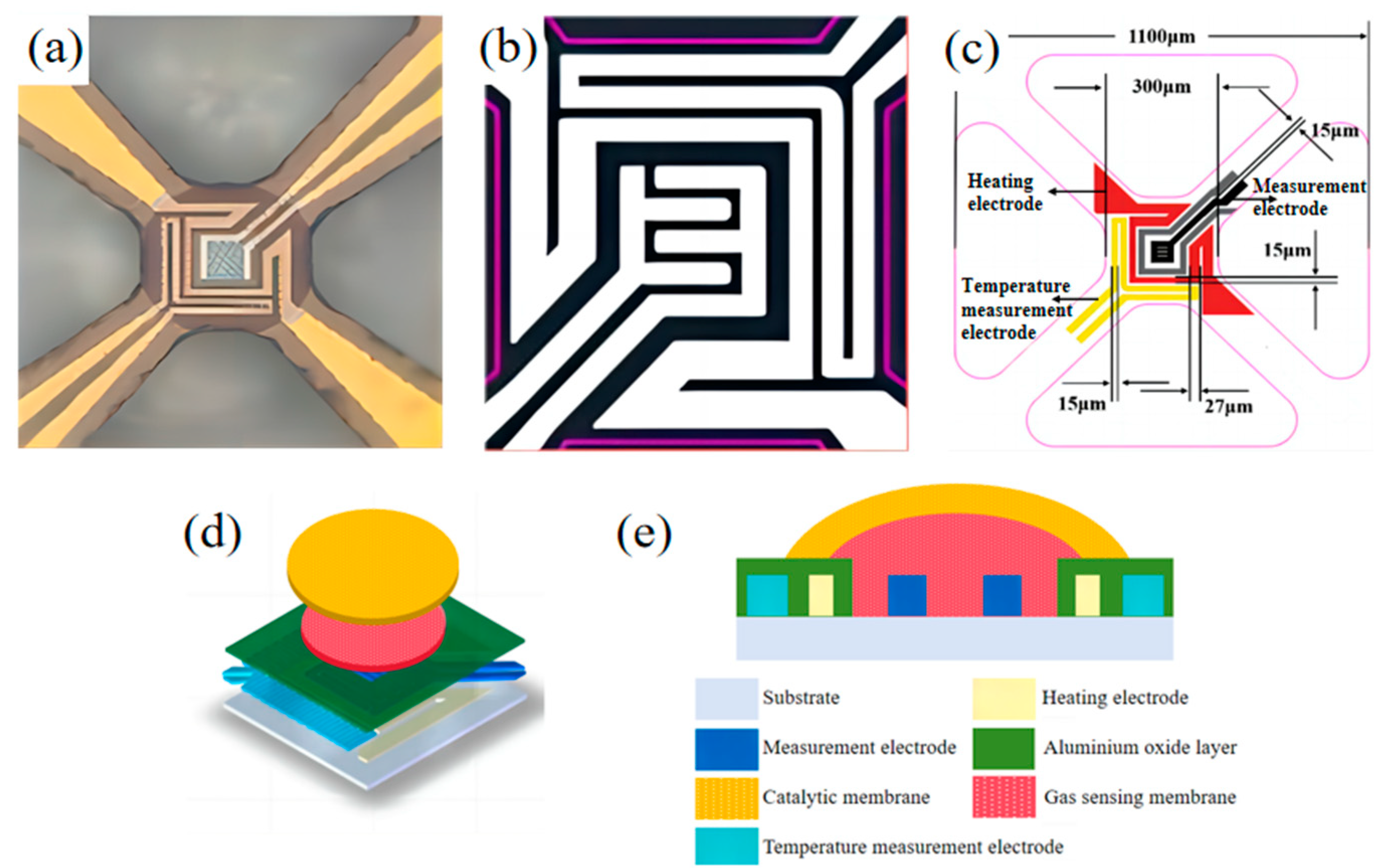
2.2. Preparation of the Catalytic Layer/Gas-Sensitive Layer MOS Sensor
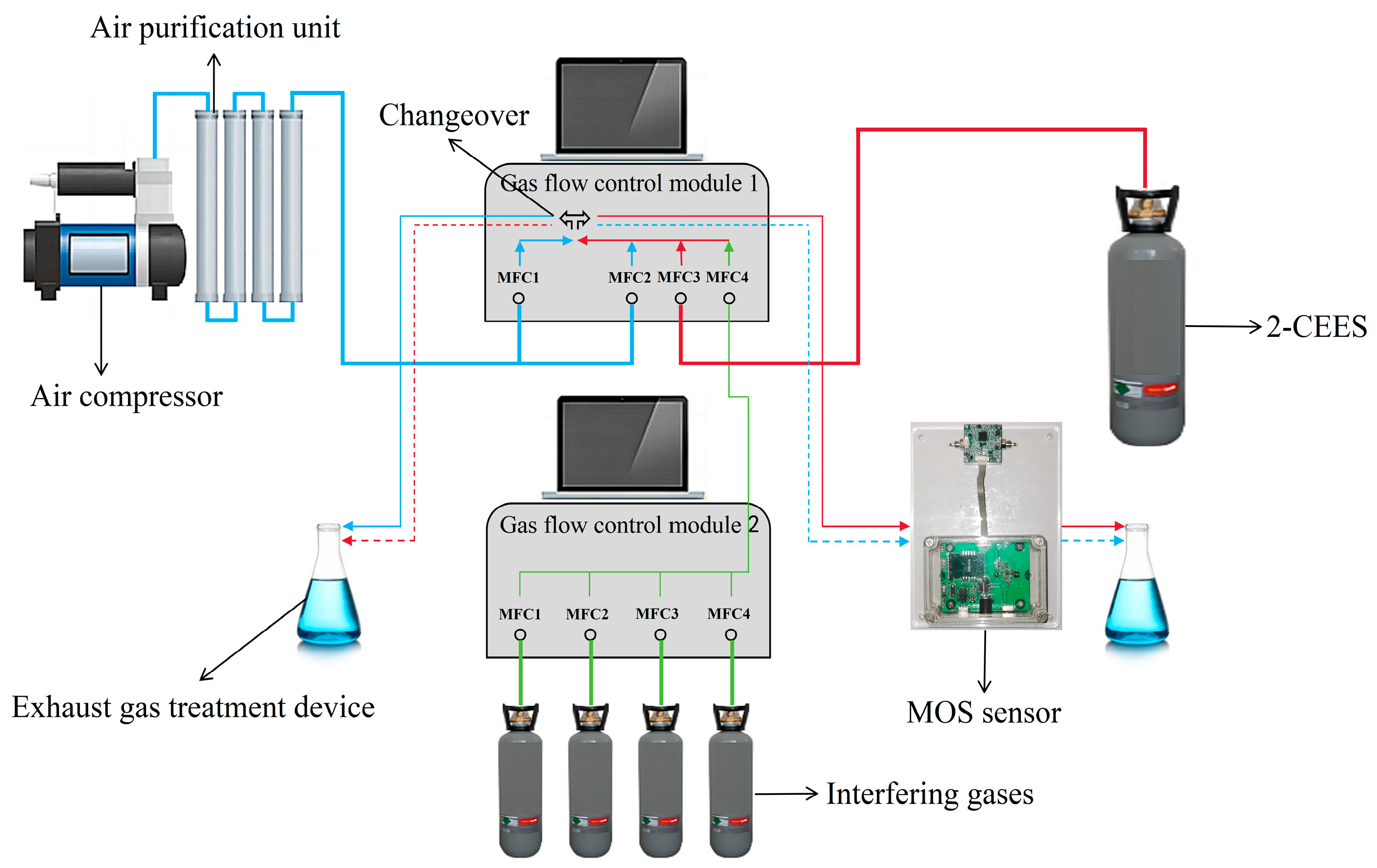
2.3. Test Platform
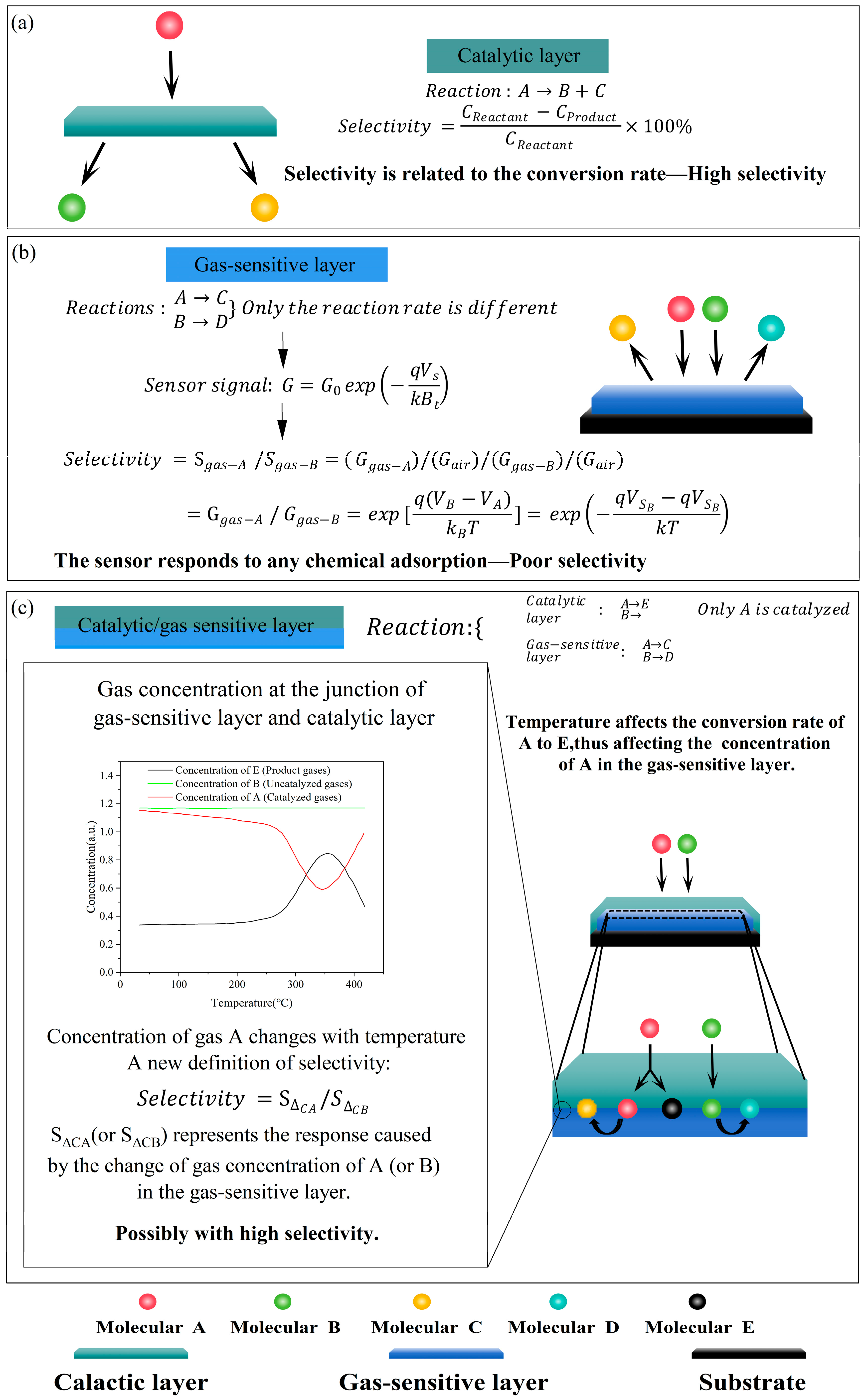
3. Results and Discussion
3.1. Characterization of Material
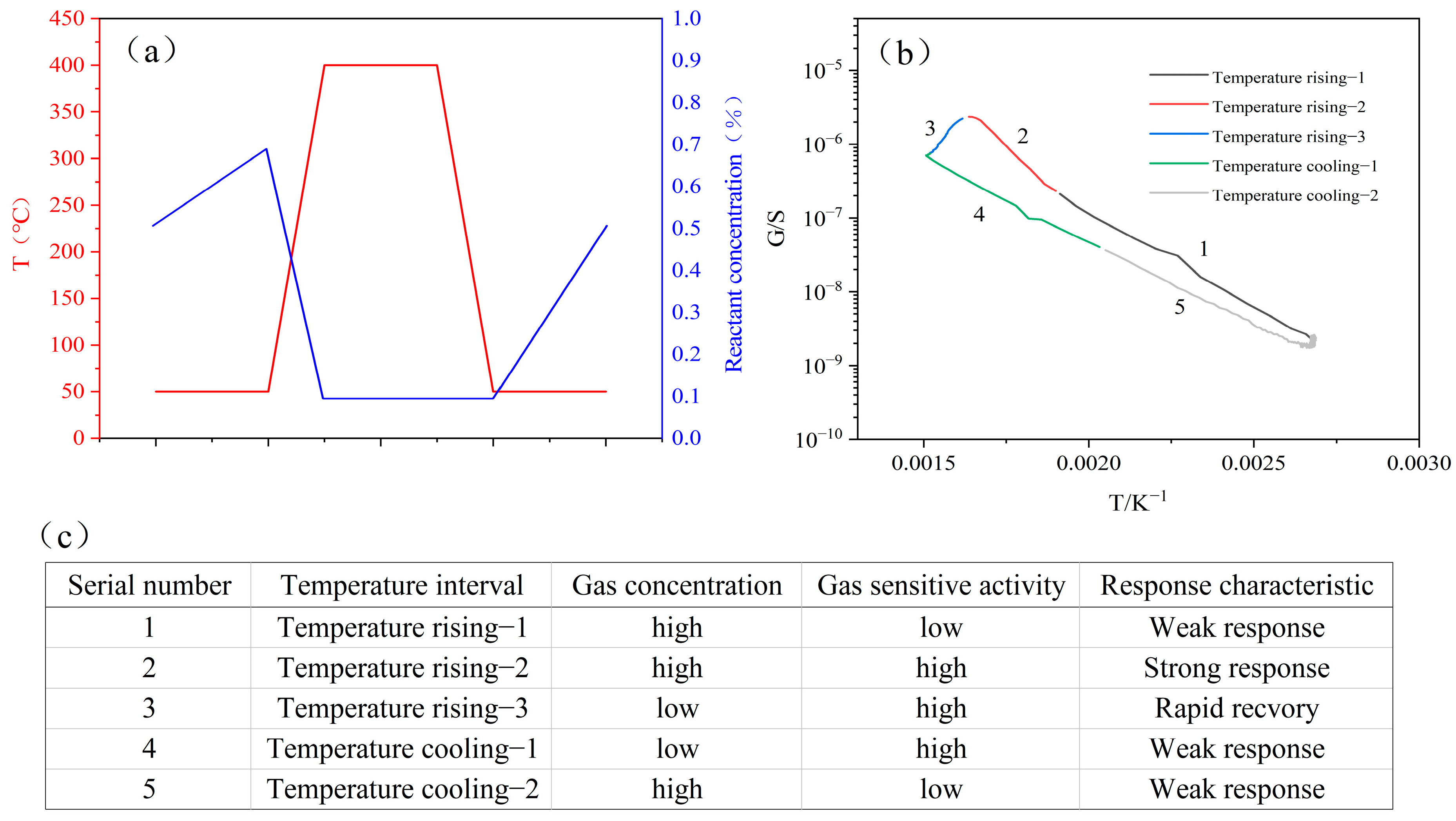
3.2. Definition of Characteristic Peak
3.3. Selectivity
3.4. Sensitivity and Detection Limit
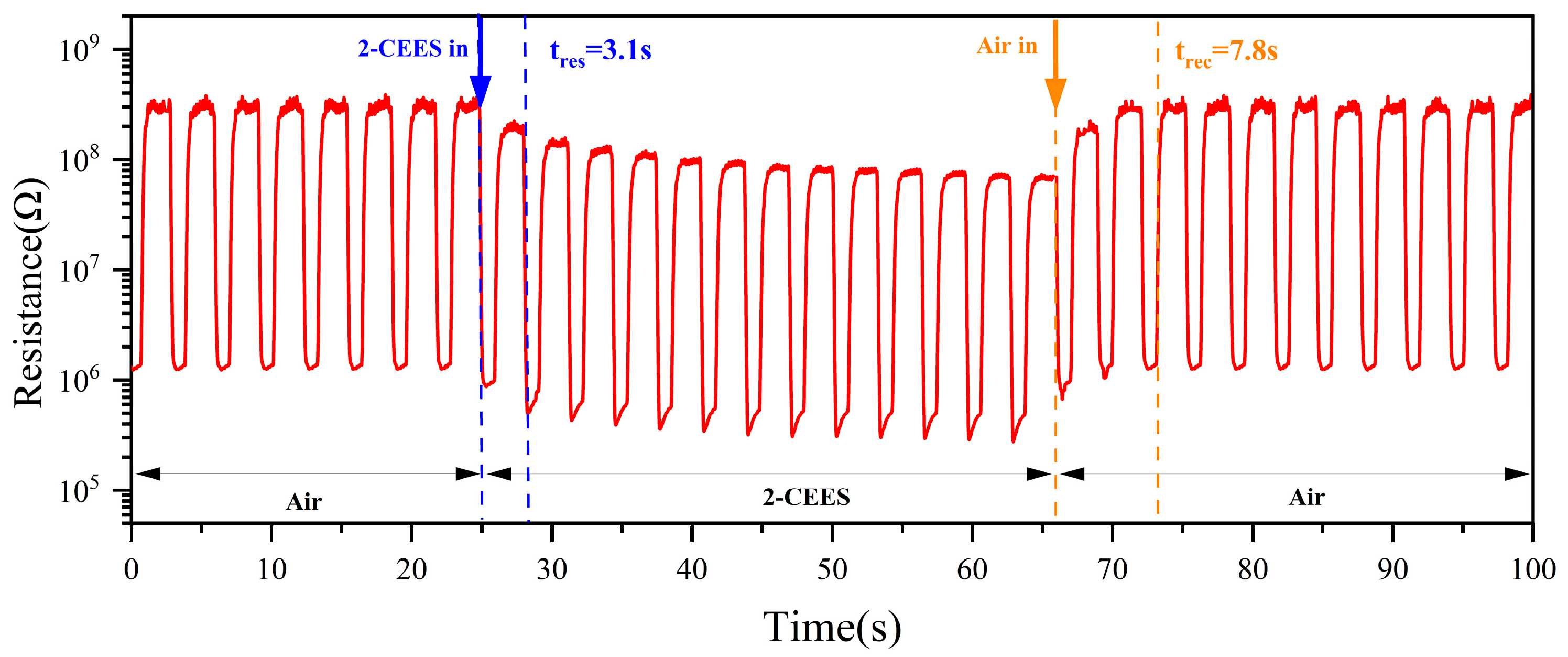
3.5. Response/Recovery Times
3.6. Anti-Interference
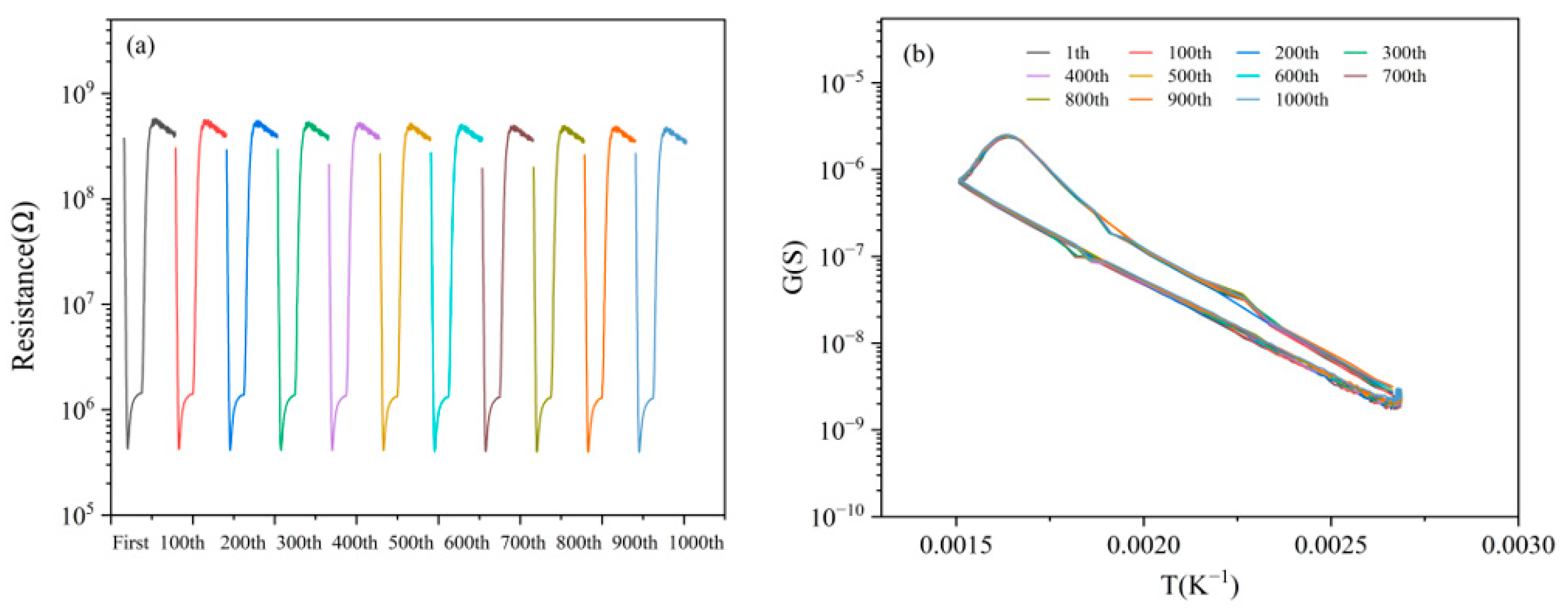
3.7. Stability
3.8. Highly Selective Sensing Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, C.; Stewart, M.K. Vesicant Agent Attack. In Ciottone’s Disaster Medicine; Elsevier: Amsterdam, The Netherlands, 2024; pp. 686–692. [Google Scholar]
- Xie, X.-J.; Si, R.-J.; Zheng, J.; Wei, K.; Zheng, X.-Y.; Chen, C.; Wang, C.-C. Synthesis of ZnO/NiO hollow spheres and their humidity sensing performance. J. Alloy. Compd. 2021, 879, 160487. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, Z.; Guo, T.; Li, H.; Xue, Q. Synthesis of nanowire bundle-like WO3-W18O49 heterostructures for highly sensitive NH3 sensor application. J. Hazard. Mater. 2018, 353, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, H.; Lin, Y.; Zhou, Y.; Wu, Y. Highly sensitive humidity sensor based on quartz crystal microbalance coated with ZnO colloid spheres. Sens. Actuators B Chem. 2013, 177, 1083–1088. [Google Scholar] [CrossRef]
- Cai, Z.X.; Li, H.Y.; Ding, J.C.; Guo, X. Hierarchical flowerlike WO3 nanostructures assembled by porousnanoflakes for enhanced NO gas sensing. Sens. Actuators B Chem. 2017, 246, 225–234. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T.T. 1D metal oxide semiconductor materials for chemiresistive gas sensors: A review. Adv. Elect. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Balouria, V.; Kumar, A.; Samanta, S.; Singh, A.; Debnath, A.; Mahajan, A.; Bedi, R.; Aswal, D.; Gupta, S. Nano-crystalline Fe2O3 thin films for ppm level detection of H2S. Sens. Actuators B Chem. 2013, 181, 471–478. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Cao, S.; Yang, J.; Yan, C.; Zhang, L.; Huang, Q.; Zhao, J. High-performance metal-oxide gas sensors based on hierarchical core–shell ZnFe2O4 microspheres for detecting 2-chloroethyl ethyl sulfide. Anal. Methods 2023, 15, 3084–3091. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.; Balboa, A.; Giles, S. Visible Light-Induced Reactivity of Plasmonic Gold Nanoparticles Incorporated into TiO2 Matrix towards 2-Chloroethyl Ethyl Sulfide. Crystals 2021, 11, 659. [Google Scholar] [CrossRef]
- Yoo, R.; Oh, C.; Song, M.-J.; Cho, S.; Lee, W. Sensing Properties of ZnO Nanoparticles for Detection of 2-Chloroethyl Ethyl Sulfide as a Mustard Simulant. J. Nanosci. Nanotechnol. 2018, 18, 1232–1236. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen Gas Sensors Based on Semiconductor Oxide Nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Raut, B.; Godse, P.; Pawar, S.; Chougule, M.; Bandgar, D.; Patil, V. Novel method for fabrication of polyaniline–CdS sensor for H2S gas detection. Measurement 2012, 45, 94–100. [Google Scholar] [CrossRef]
- Meng, F.; Shi, X.; Yuan, Z.; Ji, H.; Qin, W.; Shen, Y.; Xing, C. Detection of four alcohol homologue gases by ZnO gas sensor in dynamic interval temperature modulation mode. Sens. Actuators B Chem. 2022, 350, 130867. [Google Scholar] [CrossRef]
- Qi, J.; Chen, K.; Xing, Y.; Fan, H.; Zhao, H.; Yang, J.; Li, L.; Yan, B.; Zhou, J.; Guo, L.; et al. Application of 3D hierarchical monoclinic-type structural Sb-doped WO3 towards NO2 gas detection at low temperature. Nanoscale 2018, 10, 7440–7450. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-G.; Park, J.-S.; Lee, H.-J. Pt-doped SnO2 thin film based micro gas sensors with high selectivity to toluene and HCHO. Sens. Actuators B Chem. 2017, 248, 1011–1016. [Google Scholar] [CrossRef]
- Lu, N.; Fan, S.; Zhao, Y. A selective methane gas sensor with printed catalytic films as active filters. Sens. Actuators B Chem. 2021, 347, 130603. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Yoon, J.-W.; Kim, T.-H.; Jeong, H.-M.; Lee, C.-S.; Kang, Y.C.; Lee, Y.-H. Ultra-selective detection of sub-ppm-level benzene using Pd-SnO2 yolk–shell micro-reactors with a catalytic Co3O4 overlayer for monitoring air quality. J. Mater. Chem. A 2017, 5, 1446–1454. [Google Scholar] [CrossRef]
- Jeong, H.-M.; Jeong, S.-Y.; Kim, J.-H.; Kim, B.-Y.; Kim, J.-S.; Abdel-Hady, F.; Wazzan, A.A.; Al-Turaif, H.A.; Jang, H.W.; Lee, J.-H. Gas Selectivity Control in Co3O4 Sensor via Concurrent Tuning of Gas Reforming and Gas Filtering using Nanoscale Hetero-Overlayer of Catalytic Oxides. ACS Appl. Mater. Interfaces 2017, 9, 41397–41404. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.K.; Jeong, S.; Jo, Y.; Jo, Y.K.; Kang, Y.C.; Lee, J. Highly Selective Detection of Benzene and Discrimination of Volatile Aromatic Compounds Using Oxide Chemiresistors with Tunable Rh-TiO2 Catalytic Overlayers. Adv. Sci. 2021, 8, 2004078. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.K.; Jeong, S.-Y.; Kang, Y.C.; Lee, J.-H. Metal Oxide Gas Sensors with Au Nanocluster Catalytic Overlayer: Toward Tuning Gas Selectivity and Response Using a Novel Bilayer Sensor Design. ACS Appl. Mater. Interfaces 2019, 11, 32169–32177. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Moon, Y.K.; Wang, J.; Lee, J.-H. Exclusive detection of volatile aromatic hydrocarbons using bilayer oxide chemiresistors with catalytic overlayers. Nat. Commun. 2023, 14, 233. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Wang, Y.; Zhou, Y. Approaches for selectivity improvement of conductometric gas sensors: An overview. Sens. Diagn. 2024, 3, 336–353. [Google Scholar] [CrossRef]
- Lee, A.P.; Reedy, B.J. Temperature modulation in semiconductor gas sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Vergara, A.; Martinelli, E.; Llobet, E.; Giannini, F.; D’amico, A.; Di Natale, C. An alternative global feature extraction of temperature modulated micro-hotplate gas sensors array using an energy vector approach ☆. Sens. Actuators B Chem. 2007, 124, 352–359. [Google Scholar] [CrossRef]
- Shaffer, R.E.; Rose-Pehrsson, S.L. Improved Probabilistic Neural Network Algorithm for Chemical Sensor Array Pattern Recognition. Anal. Chem. 1999, 71, 4263–4271. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Wu, J.; Shen, J.; Chen, L.; Zhang, J.; Mao, Y.; Cheng, H.; Zhang, M.; Ma, Q.; et al. Ultrathin WO3 Nanosheets/Pd with Strong Metal–Support Interactions for Highly Sensitive and Selective Detection of Mustard-Gas Simulants. ACS Sens. 2024, 9, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.; Lee, D.; Cho, S.; Lee, W. Doping effect on the sensing properties of ZnO nanoparticles for detection of 2-chloroethyl ethylsulfide as a mustard simulant. Sens. Actuators B Chem. 2018, 254, 1242–1248. [Google Scholar] [CrossRef]
- Patil, L.A.; Deo, V.V.; Shinde, M.D.; Bari, A.R.; Patil, D.M.; Kaushik, M.P. Improved 2-CEES sensing performance of spray pyrolized Ru-CdSnO3 nanostructured thin films. Sens. Actuators B Chem. 2014, 191, 130–136. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, T.; Li, B.; Gao, R.; Zhang, X.; Cheng, X.; Huo, L.; Major, Z.; Xu, Y. Crosslinked WO3 nanonet for rapid detection of sulfur mustard gas simulant: Mechanism insights and sensing application. Sens. Actuators B Chem. 2023, 385, 133704. [Google Scholar] [CrossRef]
- Jang, B.W.L.; Spivey, J.J. Catalytic hydrodesulfurization and hydrodechlorination of chloroethyl ethyl sulfide. Catal. Today 2000, 55, 3–10. [Google Scholar] [CrossRef]




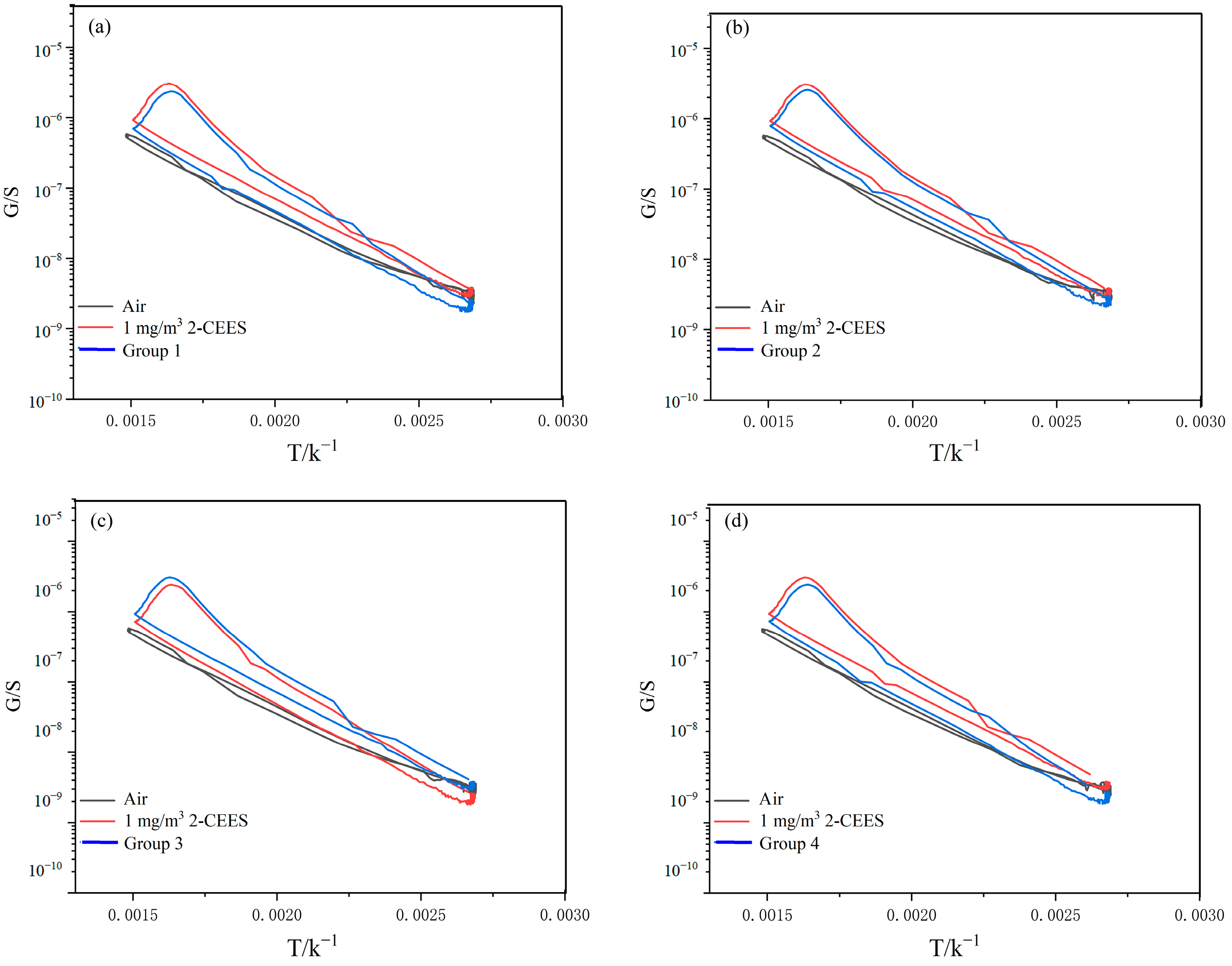
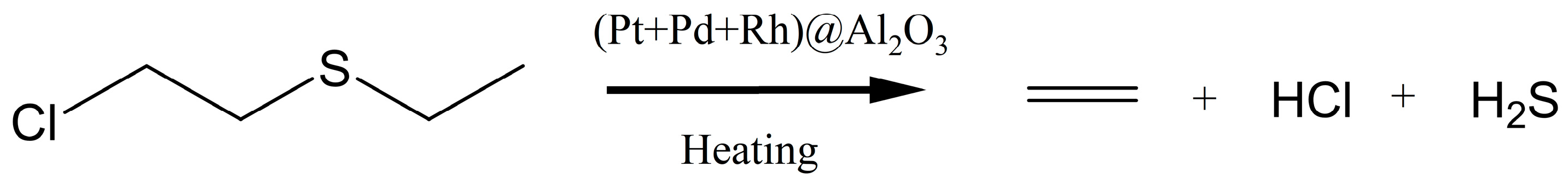
| Group Number | Gas Composition | |||
|---|---|---|---|---|
| 1 mg/m3 | 5 mg/m3 | |||
| 1 | 2-CEES | carbon monoxide | nitric oxide | nitrogen dioxide |
| 2 | 2-CEES | methane | ethene | toluene |
| 3 | 2-CEES | hydrogen chloride | chlorine | ammonia |
| 4 | 2-CEES | sulfur dioxide | dichloroethane | Isopropyl alcohol |
| Basic Metal Oxide Semiconductor | Test Concentration | Tem. (°C) | Sel. () | Sen. (Ra-Rg/Rg) | DL. | R/r T. (s) | AI. | Sta. (days) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| (Pt + Rh)@WO3/ (Pt + Pd + Rh)@Al2O3 | 182 ppb | 373 | >12 | 15.3 | 9 ppb | 3.1/7.8 | 12 gases | - | |
| WO3 NSs/Pd | 700 ppb | 260 | >8 | 7.5 | 15 ppb | 9/92 | - | 30 | [27] |
| Al-doped ZnO NPs | 20 ppm | 500 | >15 | 954.2 | - | -/ >250 | - | - | [28] |
| ZnO NPs | 1 ppm | 250 | >4.4 | 15 | 0.4 ppm | - | - | - | [10] |
| Ru loaded CdSnO3 | 4 ppm | 350 | >8.59 | 62.12 | - | 5/185 | - | - | [29] |
| Crosslinked WO3 nanonet | 50 ppm | 217 | >11 | 57 | 300 ppb | 1/- | - | 90 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, S.; You, L.; Xu, Y.; Si, R.; Zhang, S. Improving the Selectivity of Metal Oxide Semiconductor Sensors for Mustard Gas Simulant 2-Chloroethyl Ethyl Sulfide by Combining the Laminated Structure and Temperature Dynamic Modulation. Sensors 2025, 25, 525. https://doi.org/10.3390/s25020525
Liu Y, Zhao S, You L, Xu Y, Si R, Zhang S. Improving the Selectivity of Metal Oxide Semiconductor Sensors for Mustard Gas Simulant 2-Chloroethyl Ethyl Sulfide by Combining the Laminated Structure and Temperature Dynamic Modulation. Sensors. 2025; 25(2):525. https://doi.org/10.3390/s25020525
Chicago/Turabian StyleLiu, Yadong, Siyue Zhao, Lijuan You, Yong Xu, Renjun Si, and Shunping Zhang. 2025. "Improving the Selectivity of Metal Oxide Semiconductor Sensors for Mustard Gas Simulant 2-Chloroethyl Ethyl Sulfide by Combining the Laminated Structure and Temperature Dynamic Modulation" Sensors 25, no. 2: 525. https://doi.org/10.3390/s25020525
APA StyleLiu, Y., Zhao, S., You, L., Xu, Y., Si, R., & Zhang, S. (2025). Improving the Selectivity of Metal Oxide Semiconductor Sensors for Mustard Gas Simulant 2-Chloroethyl Ethyl Sulfide by Combining the Laminated Structure and Temperature Dynamic Modulation. Sensors, 25(2), 525. https://doi.org/10.3390/s25020525





