Use of a Novel Passive E-Nose to Monitor Fermentable Prebiotic Fiber Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Prebiotic Supplement
Stool Collection
2.4. Electronic Nose
2.5. Procedures and Questionnaires
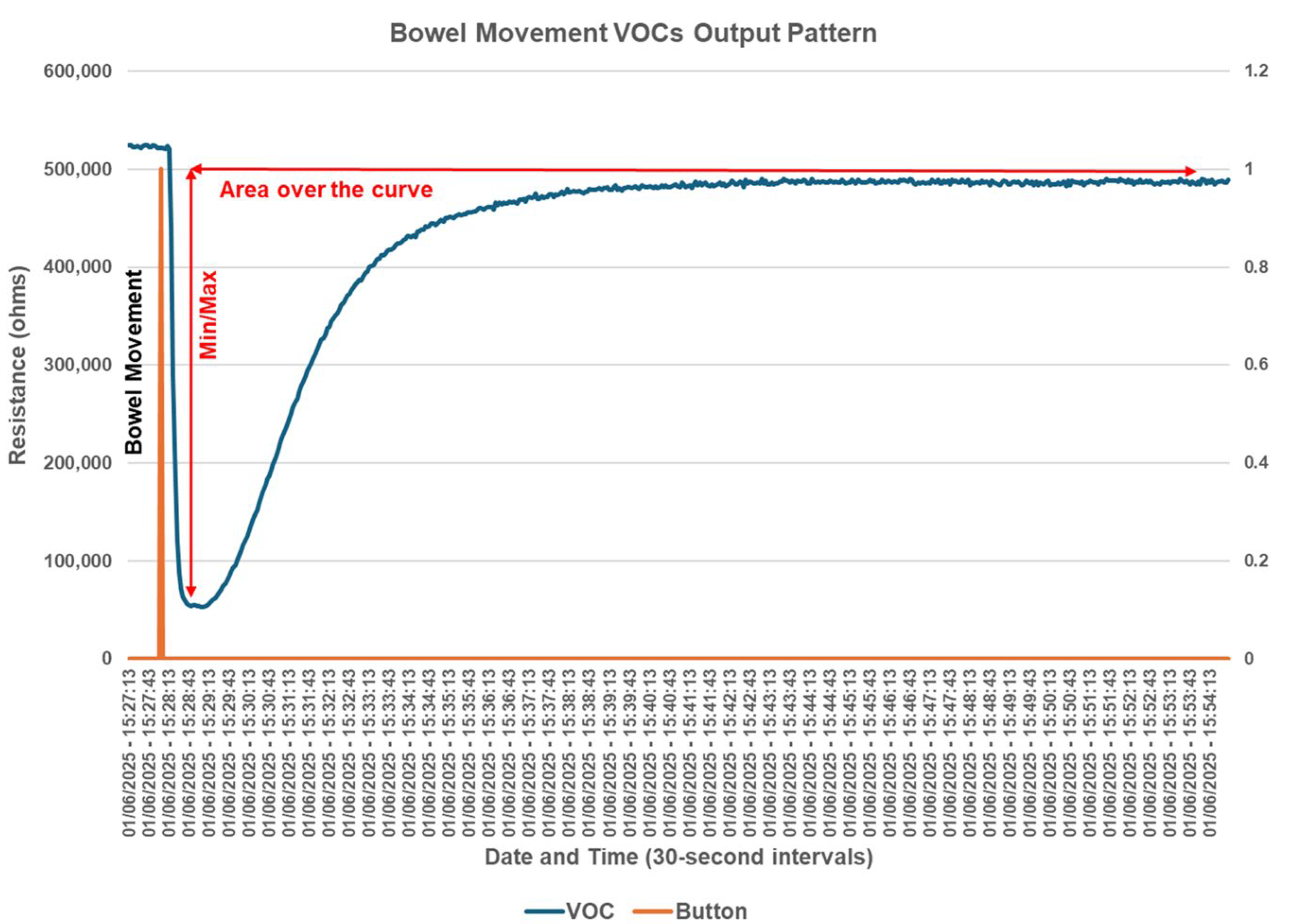
2.6. Electronic Nose Volatile Organic Compound Output Data Interpretation
2.7. DNA Extraction and Next-Generation Sequencing
2.8. Bioinformatics and Statistical Analyses
3. Results
3.1. Prebiotic Fiber Intake and Electronic Nose Performance
3.2. Prebiotic Intervention Effects on Gut Microbiota
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| E-Nose | Electronic nose |
| VOCs | Volatile organic compounds |
| SCFAs | Short-chain fatty acids |
| GLP-1 | Glucagon-like Peptide-1 |
| BMI | Body Mass Index |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PROMIS GI | Patient-Reported Outcomes Measurement Information System |
| CS1 CS2 | Common sequences 1 and 2 |
| EMP | Earth Microbiome Project |
| GMCF | Genomics and Microbiome Core Facility |
| ASVs | Amplicon sequence variants |
| Richness | Observed features |
| PERMANOVA | Permutational multivariate analysis of variance |
| PERMDISP | Permutational analysis of multivariate dispersions |
| NMDS | Non-metric multi-dimensional scaling |
| PICRUSt2 | Phylogenetic investigation of communities by reconstruction of unobserved states |
| BH | Benjamini–Hochberg |
| Boruta | Random forest algorithm |
| CIMCR | Rush Center for Integrated Microbiome and Chronobiology Research |
| NCBI | National Center for Biotechnology Information |
References
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Ionita-Mindrican, C.B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacsu, S.M.; Morosan, E.; Dumitrescu, D.E.; Rosca, A.C.; Draganescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Senés-Guerrero, C.; Gradilla-Hernández, M.S.; García-Gamboa, R.; García-Cayuela, T. Dietary Fiber and Gut Microbiota. In Science and Technology of Fibers in Food Systems; Welti-Chanes, J., Serna-Saldívar, S.O., Campanella, O., Tejada-Ortigoza, V., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 277–298. [Google Scholar]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, L.M.; Cantu-Jungles, T.M.; Chen, T.; Green, S.; Naqib, A.; Srichuwong, S.; Hamaker, B.R. Boosting the value of insoluble dietary fiber to increase gut fermentability through food processing. Food Funct. 2021, 12, 10658–10666. [Google Scholar] [CrossRef] [PubMed]
- Lemons, J.M.S. Too Much of a Good Thing: Imposing an Upper Limit to a High Fiber Diet. Gastroenterology 2024, 166, 250–251. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Prud’homme, D.; Blanchet, R.; Giroux, I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr. Metab. 2016, 13, 92. [Google Scholar] [CrossRef]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef]
- Healey, G.; Brough, L.; Murphy, R.; Hedderley, D.; Butts, C.; Coad, J. Validity and Reproducibility of a Habitual Dietary Fibre Intake Short Food Frequency Questionnaire. Nutrients 2016, 8, 558. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hall, D.A.; Voigt, R.M.; Cantu-Jungles, T.M.; Hamaker, B.; Engen, P.A.; Shaikh, M.; Raeisi, S.; Green, S.J.; Naqib, A.; Forsyth, C.B.; et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat. Commun. 2023, 14, 926. [Google Scholar] [CrossRef]
- Kosinski, L.R.; Brill, J.; Shahab, O.; Lieberman, D.; Baum, C.; Kanute, A.; Metcalfe, L.; Singh, S.; Keshavarzian, A.; Villanueva, M.; et al. S294 Measurement of Fecal Volatile Organic Compounds Using a Passive Electronic Nose: Home-Based Test. Am. J. Gastroenterol. 2023, 118, S217–S218. [Google Scholar] [CrossRef]
- Kristal, A.R.; Kolar, A.S.; Fisher, J.L.; Plascak, J.J.; Stumbo, P.J.; Weiss, R.; Paskett, E.D. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J. Acad. Nutr. Diet. 2014, 114, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, B.M.; Hays, R.D.; Bolus, R.; Melmed, G.Y.; Chang, L.; Whitman, C.; Khanna, P.P.; Paz, S.H.; Hays, T.; Reise, S. Development of the NIH patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales. Am. J. Gastroenterol. 2014, 109, 1804–1814. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shaikh, M.; Engen, P.A.; Preuss, F.; Naqib, A.; Palmen, B.A.; Green, S.J.; Zhang, L.; Bogin, Z.R.; Lawrence, K.; et al. Evidence that the loss of colonic anti-microbial peptides may promote dysbiotic Gram-negative inflammaging-associated bacteria in aging mice. Front. Aging 2024, 5, 1352299. [Google Scholar] [CrossRef]
- Naqib, A.; Poggi, S.; Wang, W.; Hyde, M.; Kunstman, K.; Green, S.J. Making and Sequencing Heavily Multiplexed, High-Throughput 16S Ribosomal RNA Gene Amplicon Libraries Using a Flexible, Two-Stage PCR Protocol. Methods Mol. Biol. 2018, 1783, 149–169. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Gihring, T.M.; Green, S.J.; Schadt, C.W. Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ. Microbiol. 2012, 14, 285–290. [Google Scholar] [CrossRef]
- Kelly, B.J.; Gross, R.; Bittinger, K.; Sherrill-Mix, S.; Lewis, J.D.; Collman, R.G.; Bushman, F.D.; Li, H. Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics 2015, 31, 2461–2468. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.I.; Kwak, S.H.; Lee, Y.; Choi, J.H.; Cho, Y.M.; Om, A.S. A Controlled, Randomized, Double-blind Trial to Evaluate the Effect of Vegetables and Whole Grain Powder That Is Rich in Dietary Fibers on Bowel Functions and Defecation in Constipated Young Adults. J. Cancer Prev. 2015, 20, 64–69. [Google Scholar] [CrossRef]
- Solah, V.A.; Kerr, D.A.; Hunt, W.J.; Johnson, S.K.; Boushey, C.J.; Delp, E.J.; Meng, X.; Gahler, R.J.; James, A.P.; Mukhtar, A.S.; et al. Erratum: Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals. Nutrients 2017, 9, 149. [Google Scholar] [CrossRef]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Hjorth, M.F.; et al. Fasting Glucose State Determines Metabolic Response to Supplementation with Insoluble Cereal Fibre: A Secondary Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2385. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Yao, Y.; Suo, T.; Andersson, R.; Cao, Y.; Wang, C.; Lu, J.; Chui, E. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst. Rev. 2017, 1, CD003430. [Google Scholar] [CrossRef]
- Quagliani, D.; Felt-Gunderson, P. Closing America’s Fiber Intake Gap: Communication Strategies from a Food and Fiber Summit. Am. J. Lifestyle Med. 2017, 11, 80–85. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture’s Agricultural Research Service (USDA ARS). What We Eat in America: Nutrient Intakes from Food by Gender and Age; Nutrient Intake Data Report 2009–2010; USDA ARS: Beltsville, MD, USA, 2012.
- Wiss, D.A.; Criscitelli, K.; Gold, M.; Avena, N. Preclinical evidence for the addiction potential of highly palatable foods: Current developments related to maternal influence. Appetite 2017, 115, 19–27. [Google Scholar] [CrossRef]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). The Nutrition Facts Label—What’s In It For You? 2024. Available online: https://www.fda.gov/food/nutrition-education-resources-materials/nutrition-facts-label (accessed on 5 March 2024).
- Rose, D.J.; DeMeo, M.T.; Keshavarzian, A.; Hamaker, B.R. Influence of dietary fiber on inflammatory bowel disease and colon cancer: Importance of fermentation pattern. Nutr. Rev. 2007, 65, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Wu, Y.; Jiang, W.; Chen, X.; Zeng, M.; Yang, J.; Su, Y.; Hu, N.; Yang, Z. Target discrimination, concentration prediction, and status judgment of electronic nose system based on large-scale measurement and multi-task deep learning. Sens. Actuat. B Chem. 2022, 351, 130915. [Google Scholar] [CrossRef]
- Xu, G.; Xing, H.; Xue, Z.; Lu, D.; Fan, J.; Fan, J.; Shum, P.P.; Cong, L. Recent advances and perspective of photonic bound states in the continuum. Ultrafast Sci. 2023, 3, 0033. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef]
- Li, Z.; Suslick, K.S. The optoelectronic nose. Acc. Chem. Res. 2020, 54, 950–960. [Google Scholar] [CrossRef]
- Bosch, S.; de Menezes, R.X.; Pees, S.; Wintjens, D.J.; Seinen, M.; Bouma, G.; Kuyvenhoven, J.; Stokkers, P.C.F.; de Meij, T.G.J.; de Boer, N.K.H. Electronic Nose Sensor Drift Affects Diagnostic Reliability and Accuracy of Disease-Specific Algorithms. Sensors 2022, 22, 9246. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Wang, X.-F.; Xu, Y.; Mekhail, T.; Beukemann, M.C.; Na, J.; Kemling, J.W.; Suslick, K.S.; Sasidhar, M. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J. Thorac. Oncol. 2012, 7, 137–142. [Google Scholar] [CrossRef]
- Lu, X.; Suslick, K.S.; Li, Z. Nanoparticle Optical Sensor Arrays: Gas Sensing and Biomedical Diagnosis. Anal. Sens. 2023, 3, e202200050. [Google Scholar] [CrossRef]



| Characteristics | n = 14 | n = 12 + | n = 11 * |
|---|---|---|---|
| Age (mean ± SEM, Min/Max range) | 39.29 ± 3.79 (22–58) | 38.42 ± 4.12 (22–58) | 37.09 ± 3.92 (22–57) |
| BMI (mean ± SEM) | 26.73 ± 0.87 | 26.36 ± 0.92 | 25.98 ± 0.96 |
| Sex | |||
| Female (n,%) | 12 (85.7%) | 11 (91.7%) | 10 (90.9%) |
| Male (n,%) | 2 (14.3%) | 1 (8.3%) | 1 (9.1%) |
| Race | |||
| Asian (n,%) | 2 (14.3%) | 2 (16.7%) | 2 (18.2%) |
| Caucasian (n,%) | 12 (85.7%) | 10 (83.3%) | 9 (81.8%) |
| Ethnicity | |||
| Not Hispanic or Latino (n,%) | 6 (42.9%) | 5 (41.7%) | 5 (45.5%) |
| Hispanic or Latino (n,%) | 8 (57.1%) | 7 (58.3%) | 6 (54.5%) |
| Parameter | Technical Data |
|---|---|
| Package Dimensions | 8-Pin LGA with metal 3.0 × 3.0 × 0.93 mm3 |
| Operation Range (Full Accuracy) | Pressure: 300–1100 hPa Humidity: 0–100% Temperature: −40–85 °C |
| Supply Voltage VDDIO | 1.2–3.6 V |
| Supply Voltage VDD | 1.71–3.6 V |
| Interface | I2C and SPI |
| Average Current Consumption (1 Hz data refresh rate) Average Current Consumption in Sleep Mode | 2.1 µA at 1 Hz Humidity and Temperature 3.1 µA at 1 Hz Pressure and Temperature 3.7 µA at 1 Hz Humidity, Pressure and Temperature 0.09–12 mA for p/h/t/gas, depending on operation mode |
| Gas Sensor | |
| Response Time (T 33–63%) | <1 s (for new sensors) |
| Sensor–to–Sensor Deviation | ±15% ± 15 |
| Power Consumption | <0.1 mA in Ultra-Low Power Mode |
| Output Data Processing | Direct Output of Index of Air Quality (IAQ) |
| Humidity Sensor | |
| Response Time (T 0–63%) | 8 s |
| Accuracy Tolerance | ±3% Relative Humidity |
| Hysteresis | ≤1.5% Relative Humidity |
| Pressure Sensor | |
| RMS Noise | 0.12 Pa (equiv. to 1.7 cm) |
| Sensitivity Error | ±0.25% (equiv. to 1 m at 400 m height change) |
| Temperature Coefficient Offset | ±1.3 Pa/K (equiv. to ±10.9 cm at 1 °C temperature change) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosinski, L.; Engen, P.A.; Swanson, B.; Villanueva, M.; Shaikh, M.; Green, S.J.; Naqib, A.; Hamaker, B.; Cantu-Jungles, T.M.; Keshavarzian, A. Use of a Novel Passive E-Nose to Monitor Fermentable Prebiotic Fiber Consumption. Sensors 2025, 25, 797. https://doi.org/10.3390/s25030797
Kosinski L, Engen PA, Swanson B, Villanueva M, Shaikh M, Green SJ, Naqib A, Hamaker B, Cantu-Jungles TM, Keshavarzian A. Use of a Novel Passive E-Nose to Monitor Fermentable Prebiotic Fiber Consumption. Sensors. 2025; 25(3):797. https://doi.org/10.3390/s25030797
Chicago/Turabian StyleKosinski, Lawrence, Phillip A. Engen, Barbara Swanson, Michelle Villanueva, Maliha Shaikh, Stefan J. Green, Ankur Naqib, Bruce Hamaker, Thaisa M. Cantu-Jungles, and Ali Keshavarzian. 2025. "Use of a Novel Passive E-Nose to Monitor Fermentable Prebiotic Fiber Consumption" Sensors 25, no. 3: 797. https://doi.org/10.3390/s25030797
APA StyleKosinski, L., Engen, P. A., Swanson, B., Villanueva, M., Shaikh, M., Green, S. J., Naqib, A., Hamaker, B., Cantu-Jungles, T. M., & Keshavarzian, A. (2025). Use of a Novel Passive E-Nose to Monitor Fermentable Prebiotic Fiber Consumption. Sensors, 25(3), 797. https://doi.org/10.3390/s25030797






