A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare
Abstract
:1. Introduction
2. Techniques for Lactate Detection in the Medical Domain
3. Biometric Elements for Lactic Acid Electrochemical Sensors
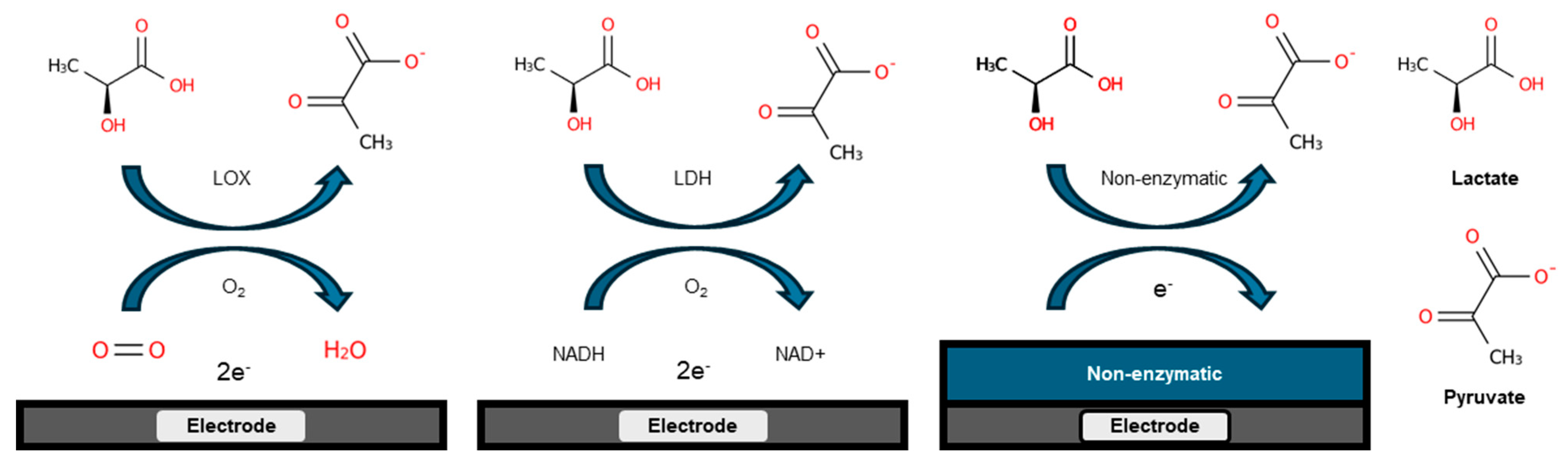
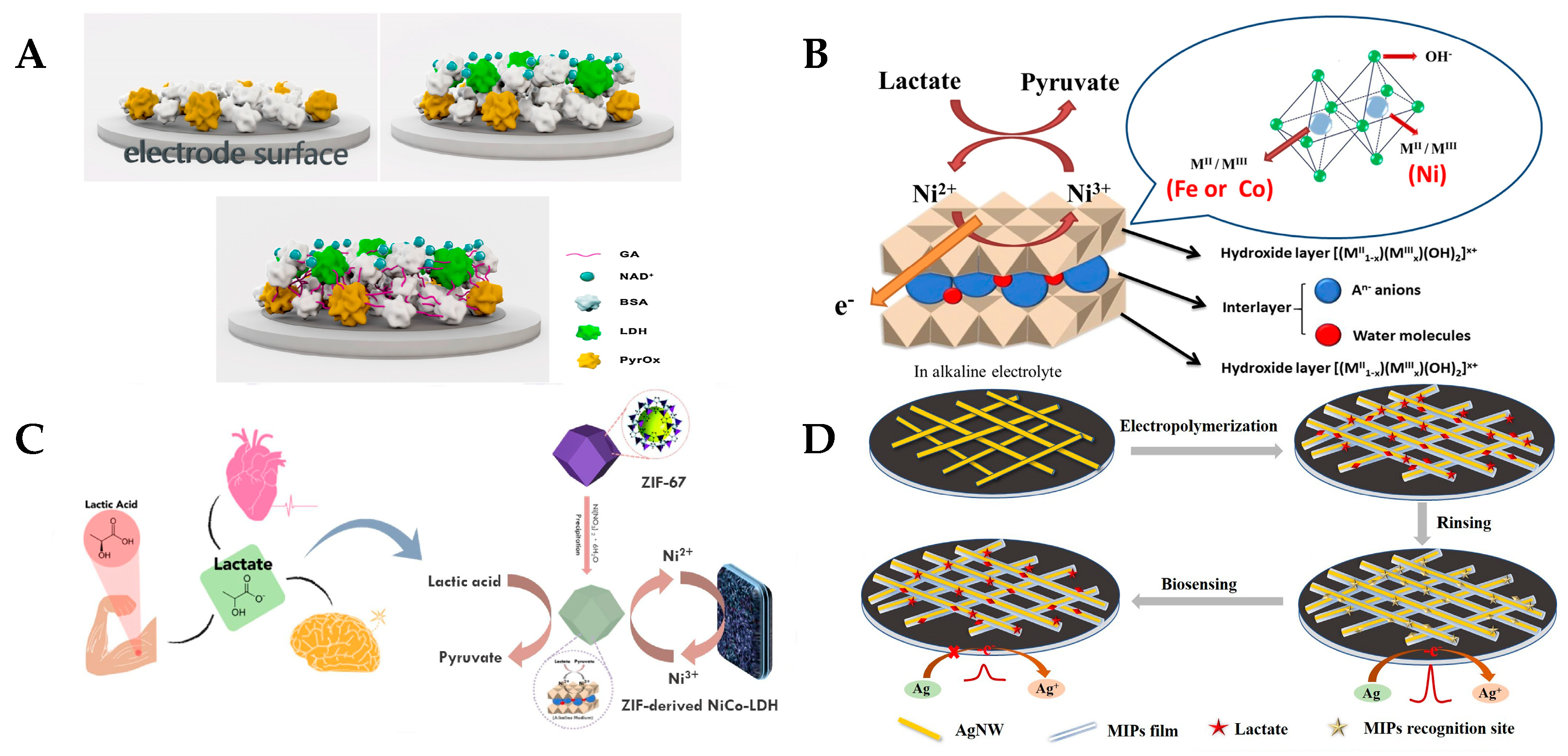
3.1. Enzyme-Based Lactate Electrochemical Biosensors
3.2. Enzyme-Free Lactate Electrochemical Biosensors
4. Electrochemical Lactate Sensors with Different Modified Materials
4.1. Lactate Electrochemical Biosensors Based on Nanomaterial Modifications
4.2. Membrane-Based Lactate Electrochemical Biosensors
4.3. Polymer-Based Lactate Electrochemical Biosensors
4.4. Hydrogel-Based Lactate Electrochemical Biosensors

5. Lactate Electrochemical Sensors with Different Detection Methods
5.1. Electrochemical Impedance Spectroscopy (EIS)
5.2. Differential Pulse Voltammetry (DPV)
5.3. Cyclic Voltammetry (CV)
5.4. Amperometry (AMP)
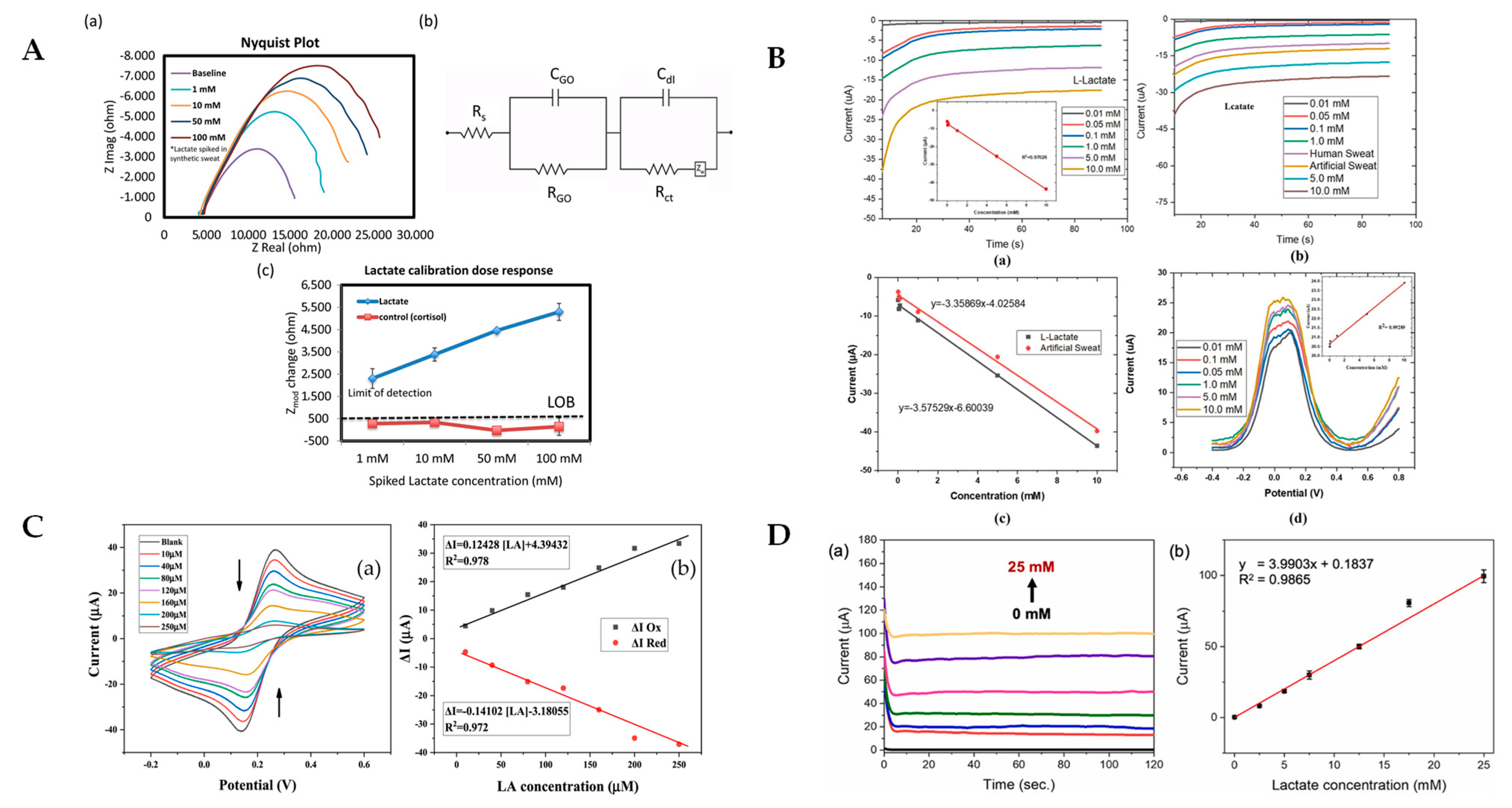
6. Implantable and Non-Implantable Miniaturized Lactic Acid Electrochemical Biosensors
6.1. Implantable Electrochemical Lactate Biosensors
6.2. Non-Implantable and Miniaturized Electrochemical Lactate Biosensors

7. Electrochemical Lactate Biosensors Based on Multiple Advanced Technologies
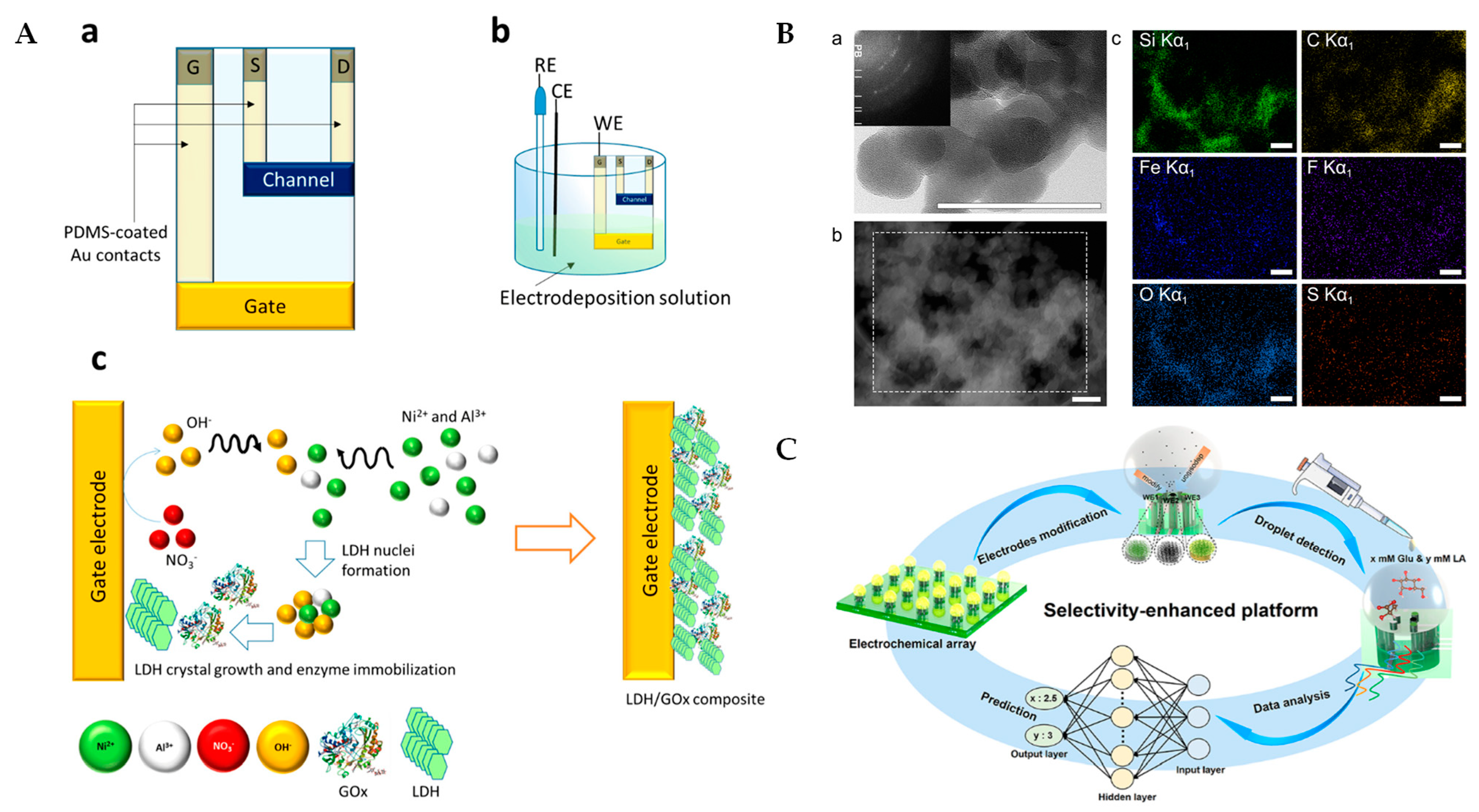
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Alternating Current |
| AgNWs | Silver Nanowires |
| AMP | Amperometry |
| AuNPs | Gold Nanoparticles |
| BSA | Bovine Serum Albumin |
| CS | Chitosan |
| CV | Cyclic Voltammetry |
| CZO | Copper-Doped Zinc Oxide |
| DOAJ | Directory Of Open-Access Journals |
| DPV | Differential Pulse Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FePtNPs | Iron–Platinum Nanoparticles |
| GA | Glutaraldehyde |
| GO | Graphene Oxide |
| G-PU-RGO-PB | Graphene–Polyurethane–Reduced Graphene Oxide–Prussian Blue Composite |
| HAADF-STEM | High-Angle Annular Dark-Field STEM |
| HNC/AgNPs | Hydrogels Nanocellulose/Silver Nanoparticles |
| HPLC | High-Performance Liquid Chromatography |
| HRP | Horseradish Peroxidase |
| ICU | Intensive Care Unit |
| LD | Linear Dichroism |
| LDH | Lactate Dehydrogenase |
| LOx | Lactate Oxidase |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MIPS | Molecularly Imprinted Polymers |
| MoFs | Metal–Organic Frameworks |
| MWCNT | Multi-Walled Carbon Nanotubes |
| NAD+ | Nicotinamide Adenine Dinucleotide (Oxidized Form) |
| NADH | Nicotinamide Adenine Dinucleotide (Reduced Form) |
| NDG | N-Doped Graphene |
| NiLDH | Nickel-Based Layered Double Hydroxide |
| PA | Porous Polyamide |
| PAM | Polyacrylamide |
| PANHS | 1-Pyrenebutyric Acid–N-Hydroxysuccinimide Ester |
| PANI | Polyaniline |
| PB | Prussian Blue |
| PMM | Poly [2-Methacryloyloxyethyl Phosphorylcholine-Co-N-Methacryloyloxyethyl Tyrosine Methylester] |
| PPy | Polypyrrole |
| PtNPs | Platinum Nanoparticles |
| PU | Polyurethane |
| PVA | Polyvinyl Alcohol |
| PyrOx | Pyruvate Oxidase |
| rGO | Reduced Graphene Oxide |
| SA | Sodium Alginate |
| SEM | Scanning Electron Microscopy |
| SilKNCT | Nitrogen-Doped Carbon Textile (Derived from Silk Fabric) |
| SPCE | Screen-Printed Carbon Electrode |
| SWCNTs | Single-Walled Carbon Nanotubes |
| TLA | Three Letter Acronyms |
| TTF | Tetrathiafulvalene |
References
- Laimoud, M.; Alanazi, M. The Clinical Significance of Blood Lactate Levels in Evaluation of Adult Patients with Veno-Arterial Extracorporeal Membrane Oxygenation. Egypt Heart J. 2020, 72, 74. [Google Scholar] [CrossRef] [PubMed]
- Pino, R.M.; Singh, J. Appropriate Clinical Use of Lactate Measurements. Anesthesiology 2021, 134, 637. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; Findikli, H.A. Prognostic Value of the Lactate/Albumin Ratio for Predicting Mortality in Patients with Pneumosepsis in Intensive Care Units. Medicine 2022, 101, e28748. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Byun, Y.-H.; Park, J.-S.; Lee, J.S.; Ryu, J.-M.; Choi, S.J. Lactic Acid Level as an Outcome Predictor in Pediatric Patients with Intussusception in the Emergency Department. BMC Pediatr. 2020, 20, 184. [Google Scholar] [CrossRef]
- Woodford, M.R.; Andreou, A.; Baba, M.; Van De Beek, I.; Malta, C.D.; Glykofridis, I.; Grimes, H.; Henske, E.P.; Iliopoulos, O.; Kurihara, M.; et al. Seventh BHD International Symposium: Recent Scientific and Clinical Advancement. Oncotarget 2022, 13, 173–181. [Google Scholar] [CrossRef]
- Liu, M.; Yang, M.; Wang, M.; Wang, H.; Cheng, J. A Flexible Dual-Analyte Electrochemical Biosensor for Salivary Glucose and Lactate Detection. Biosensors 2022, 12, 210. [Google Scholar] [CrossRef]
- Deulkar, P.; Singam, A.; Mudiganti, V.N.K.S.; Jain, A. Lactate Monitoring in Intensive Care: A Comprehensive Review of Its Utility and Interpretation. Cureus 2024, 16, e66356. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood Lactate Measurements and Analysis during Exercise: A Guide for Clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. [Google Scholar] [CrossRef]
- Chang, T.-C.; Lee, H.-T.; Pan, S.-C.; Cho, S.-H.; Cheng, C.; Ou, L.-H.; Lin, C.-I.; Lin, C.-S.; Wei, Y.-H. Metabolic Reprogramming in Response to Alterations of Mitochondrial DNA and Mitochondrial Dysfunction in Gastric Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 1857. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C.; Wei, Y.; Jin, J. Early Lactate Measurement Is Associated with Better Outcomes in Septic Patients with an Elevated Serum Lactate Level. Crit. Care 2019, 23, 351. [Google Scholar] [CrossRef]
- Campanella, B.; Lomonaco, T.; Benedetti, E.; Onor, M.; Nieri, R.; Bramanti, E. Validation and Application of a Derivatization-Free RP-HPLC-DAD Method for the Determination of Low Molecular Weight Salivary Metabolites. Int. J. Environ. Res. Public Health 2020, 17, 6158. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Veličković, J.; Palibrk, I.; Miličić, B.; Veličković, D.; Jovanović, B.; Rakić, G.; Petrović, M.; Bumbaširević, V. The Association of Early Postoperative Lactate Levels with Morbidity after Elective Major Abdominal Surgery. Bosn. J. Basic. Med. Sci. 2019, 19, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Grip, J.; Falkenström, T.; Promsin, P.; Wernerman, J.; Norberg, Å.; Rooyackers, O. Lactate Kinetics in ICU Patients Using a Bolus of 13C-Labeled Lactate. Crit. Care 2020, 24, 46. [Google Scholar] [CrossRef]
- Lactic Acidosis: Background, Etiology, Epidemiology. Available online: https://emedicine.medscape.com/article/167027-overview (accessed on 13 January 2025).
- Ikeda, T.; Kamohara, H.; Suda, S.; Nagura, T.; Tomino, M.; Sugi, M.; Wajima, Z. Comparative Evaluation of Endotoxin Activity Level and Various Biomarkers for Infection and Outcome of ICU-Admitted Patients. Biomedicines 2019, 7, 47. [Google Scholar] [CrossRef]
- Pan, H.-C.; Huang, T.-M.; Sun, C.-Y.; Chou, N.-K.; Tsao, C.-H.; Yeh, F.-Y.; Lai, T.-S.; Chen, Y.-M.; Wu, V.-C. Predialysis Serum Lactate Levels Could Predict Dialysis Withdrawal in Type 1 Cardiorenal Syndrome Patients. EClinicalMedicine 2022, 44, 101232. [Google Scholar] [CrossRef]
- Connolly, C.; Stättner, S.; Niederwieser, T.; Primavesi, F. Systematic Review on Peri-operative Lactate Measurements to Predict Outcomes in Patients Undergoing Liver Resection. J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 359–370. [Google Scholar] [CrossRef]
- Cai, H.; Wang, X.; Zhang, Z.; Chen, J.; Wang, F.; Wang, L.; Liu, J. Moderate L-Lactate Administration Suppresses Adipose Tissue Macrophage M1 Polarization to Alleviate Obesity-Associated Insulin Resistance. J. Biol. Chem. 2022, 298, 101768. [Google Scholar] [CrossRef]
- Han, X.; Edelson, D.P.; Snyder, A.; Pettit, N.; Sokol, S.; Barc, C.; Howell, M.D.; Churpek, M.M. Implications of Centers for Medicare & Medicaid Services Severe Sepsis and Septic Shock Early Management Bundle and Initial Lactate Measurement on the Management of Sepsis. Chest 2018, 154, 302–308. [Google Scholar] [CrossRef]
- López, R.; Pérez-Araos, R.; Baus, F.; Moscoso, C.; Salazar, Á.; Graf, J.; Montes, J.M.; Samtani, S. Outcomes of Sepsis and Septic Shock in Cancer Patients: Focus on Lactate. Front. Med. 2021, 8, 603275. [Google Scholar] [CrossRef]
- Dezman, Z.D.W.; Comer, A.C.; Smith, G.S.; Narayan, M.; Scalea, T.M.; Hirshon, J.M. Failure to Clear Elevated Lactate Predicts 24-Hour Mortality in Trauma Patients. J. Trauma Acute Care Surg. 2015, 79, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Mamandipoor, B.; Yeung, W.; Agha-Mir-Salim, L.; Stone, D.J.; Osmani, V.; Celi, L.A. Prediction of Blood Lactate Values in Critically Ill Patients: A Retrospective Multi-Center Cohort Study. J. Clin. Monit. Comput. 2022, 36, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Tortuous Path of Lactate Shuttle Discovery: From Cinders and Boards to the Lab and ICU. J. Sport. Health Sci. 2020, 9, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Hundshammer, C.; Braeuer, M.; Müller, C.A.; Hansen, A.E.; Schillmaier, M.; Düwel, S.; Feuerecker, B.; Glaser, S.J.; Haase, A.; Weichert, W.; et al. Simultaneous Characterization of Tumor Cellularity and the Warburg Effect with PET, MRI and Hyperpolarized 13C-MRSI. Theranostics 2018, 8, 4765–4780. [Google Scholar] [CrossRef]
- Vieira, I.H.; Petrova, M.; Moura, J.P.; Vieira, I.H.; Petrova, M.; Moura, J. Does the Same Hyperlactatemia Cut-Off in the Context of Acute Diseases Hold the Same Meaning in Diabetes Mellitus? Cureus 2022, 14, e25163. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Selvin, E.; Miller, E.R.; Brancati, F.L.; Young, J.H. Plasma Lactate and Diabetes Risk in 8,045 Participants of the Atherosclerosis Risk in Communities (ARIC) Study. Ann. Epidemiol. 2013, 23, 791–796.e4. [Google Scholar] [CrossRef]
- Liang, D.; Zhou, X.; Hong, X.; Feng, X.; Shan, P.; Xie, Q.; Xu, T.; Cai, M.; Zhou, J.; Wang, S.; et al. Association between Admission Lactate Levels and Mortality in Patients with Acute Coronary Syndrome: A Retrospective Cohort Study. Coron. Artery Dis. 2019, 30, 26–32. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Gao, P.; Hu, H. Lactylation in Cancer: Current Understanding and Challenges. Cancer Cell 2024, 42, 1803–1807. [Google Scholar] [CrossRef]
- Morrow, B.; Malkoc, A.; Gong, T.; Probst, D.; Lin, C.; Sen, A.; La Belle, J.T. Development of Electrochemical Methods to Enzymatically Detect Lactate and Glucose Using Imaginary Impedance for Enhanced Management of Glycemic Compromised Patients. Crit. Rev. Biomed. Eng. 2019, 47, 179–191. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Tridente, A.; Banks, C.E.; Dempsey-Hibbert, N.C. Evaluating the Possibility of Translating Technological Advances in Non-Invasive Continuous Lactate Monitoring into Critical Care. Sensors 2021, 21, 879. [Google Scholar] [CrossRef]
- Spehar-Délèze, A.-M.; Anastasova, S.; Vadgama, P. Monitoring of Lactate in Interstitial Fluid, Saliva and Sweat by Electrochemical Biosensor: The Uncertainties of Biological Interpretation. Chemosensors 2021, 9, 195. [Google Scholar] [CrossRef]
- Md Shakhih, M.F.; Rosslan, A.S.; Noor, A.M.; Ramanathan, S.; Lazim, A.M.; Wahab, A.A. Review-Enzymatic and Non-Enzymatic Electrochemical Sensor for Lactate Detection in Human Biofluids. J. Electrochem. Soc. 2021, 168, 067502. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; Sierra-Padilla, A.; Palacios-Santander, J.M.; Fernández-Alba, J.J.; Macías, C.G.; Cubillana-Aguilera, L. What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications. Biosensors 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Jannath, K.A.; Karim, M.M.; Saputra, H.A.; Seo, K.; Kim, K.B.; Shim, Y. A Review on the Recent Advancements in Nanomaterials for Nonenzymatic Lactate Sensing. Bull. Korean Chem. Soc. 2023, 44, 407–419. [Google Scholar] [CrossRef]
- Lafuente, J.-L.; González, S.; Aibar, C.; Rivera, D.; Avilés, E.; Beunza, J.-J. Continuous and Non-Invasive Lactate Monitoring Techniques in Critical Care Patients. Biosensors 2024, 14, 148. [Google Scholar] [CrossRef]
- Hegde, K.R.; Kovtun, S.; Varma, S.D. Inhibition of Glycolysis in the Retina by Oxidative Stress: Prevention by Pyruvate. Mol. Cell Biochem. 2010, 343, 101–105. [Google Scholar] [CrossRef]
- Zheng, X.; Han, H.; Liu, G.; Ma, Y.; Pan, R.; Sang, L.; Li, R.; Yang, L.; Marks, J.R.; Wang, W.; et al. LncRNA Wires up Hippo and Hedgehog Signaling to Reprogramme Glucose Metabolism. EMBO J. 2017, 36, 3325–3335. [Google Scholar] [CrossRef]
- Jin, N.; Bi, A.; Lan, X.; Xu, J.; Wang, X.; Liu, Y.; Wang, T.; Tang, S.; Zeng, H.; Chen, Z.; et al. Identification of Metabolic Vulnerabilities of Receptor Tyrosine Kinases-Driven Cancer. Nat. Commun. 2019, 10, 2701. [Google Scholar] [CrossRef]
- Vaňkátová, P.; Kubíčková, A.; Cigl, M.; Kalíková, K. Ultra-Performance Chromatographic Methods for Enantioseparation of Liquid Crystals Based on Lactic Acid. J. Supercrit. Fluids 2019, 146, 217–225. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N.Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of Bio-Electrochemical Sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Caliò, A.; Dardano, P.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.; Iuele, H.; De Stefano, L. Polymeric Microneedles Based Enzymatic Electrodes for Electrochemical Biosensing of Glucose and Lactic Acid. Sens. Actuators B Chem. 2016, 236, 343–349. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Tsao, P.-K.; Chen, K.-J.; Lin, Y.-C.; Aulia, S.; Chang, L.-Y.; Ho, K.-C.; Chang, C.; Mizuguchi, H.; Yeh, M.-H. Designing Bimetallic Ni-Based Layered Double Hydroxides for Enzyme-Free Electrochemical Lactate Biosensors. Sens. Actuators B Chem. 2021, 346, 130505. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Tsao, P.-K.; Rinawati, M.; Chen, K.-J.; Chen, K.-Y.; Chang, C.; Yeh, M.-H. Designing ZIF-67 Derived NiCo Layered Double Hydroxides with 3D Hierarchical Structure for Enzyme-Free Electrochemical Lactate Monitoring in Human Sweat. Chem. Eng. J. 2022, 427, 131687. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Huang, L.; Ren, X.; Wang, C.; Wang, Y. Wearable Electrochemical Biosensor Based on Molecularly Imprinted Ag Nanowires for Noninvasive Monitoring Lactate in Human Sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. Ratiometric Fluorescent Biosensors for Glucose and Lactate Using an Oxygen-Sensing Membrane. Biosensors 2021, 11, 208. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, C.; Zhang, Q.; Ye, Y.; Cai, Y.; Li, K.; Li, Y.; Huang, X.; Wang, Y. In-Situ Preparation of Lactate-Sensing Membrane for the Noninvasive and Wearable Analysis of Sweat. Biosens. Bioelectron. 2022, 210, 114303. [Google Scholar] [CrossRef]
- Chan, D.; Barsan, M.M.; Korpan, Y.; Brett, C.M.A. L-Lactate Selective Impedimetric Bienzymatic Biosensor Based on Lactate Dehydrogenase and Pyruvate Oxidase. Electrochim. Acta 2017, 231, 209–215. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Hallaj, R. Low Potential Detection of NADH Based on Fe3O4 Nanoparticles/Multiwalled Carbon Nanotubes Composite: Fabrication of Integrated Dehydrogenase-Based Lactate Biosensor. Biosens. Bioelectron. 2012, 33, 60–68. [Google Scholar] [CrossRef]
- Di Mauro, F.M.; Schoeffler, G.L. Point of Care Measurement of Lactate. Top. Companion Anim. Med. 2016, 31, 35–43. [Google Scholar] [CrossRef]
- Heo, S.G.; Yang, W.-S.; Kim, S.; Park, Y.M.; Park, K.-T.; Oh, S.J.; Seo, S.-J. Synthesis, Characterization and Non-Enzymatic Lactate Sensing Performance Investigation of Mesoporous Copper Oxide (CuO) Using Inverse Micelle Method. Appl. Surf. Sci. 2021, 555, 149638. [Google Scholar] [CrossRef]
- Han, J.; Shaohui, J. Fabrication of a Novel Sensor for Lactate Screening in Saliva Samples before and after Exercise in Athletes. Alex. Eng. J. 2024, 92, 171–175. [Google Scholar] [CrossRef]
- Mansouri Majd, S.; Salimi, A.; Astinchap, B. Label-Free Attomolar Detection of Lactate Based on Radio Frequency Sputtered of Nickel Oxide Thin Film Field Effect Transistor. Biosens. Bioelectron. 2017, 92, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Rattu, G.; Krishna, P.M. TiO2 Nanoparticles Reagent Based Nonenzymatic Label-Free Optical Sensor for the Rapid Detection of L-Lactate in Apple Juice. Sens. Actuator Rep. 2021, 3, 100067. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Fan, H.; Gong, Y.; Xu, Y.; Lv, Q.; Xu, Y.; Xiao, F.; Wang, S.; Wang, Z.; et al. In Vitro and in Vivo Detection of Lactate with Nanohybrid-Functionalized Pt Microelectrode Facilitating Assessment of Tumor Development. Biosens. Bioelectron. 2021, 191, 113474. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Maduraiveeran, G. Gold-Dispersed Hierarchical Flower-like Copper Oxide Microelectrodes for the Sensitive Detection of Glucose and Lactic Acid in Human Serum and Urine. Biomater. Sci. 2022, 10, 4538–4548. [Google Scholar] [CrossRef]
- Nien, Y.-H.; Kang, Z.-X.; Su, T.-Y.; Ho, C.-S.; Chou, J.-C.; Lai, C.-H.; Kuo, P.-Y.; Lai, T.-Y.; Dong, Z.-X.; Chen, Y.-Y.; et al. Investigation of Flexible Arrayed Lactate Biosensor Based on Copper Doped Zinc Oxide Films Modified by Iron–Platinum Nanoparticles. Polymers 2021, 13, 2062. [Google Scholar] [CrossRef]
- Wang, Z.; Gui, M.; Asif, M.; Yu, Y.; Dong, S.; Wang, H.; Wang, W.; Wang, F.; Xiao, F.; Liu, H. A Facile Modular Approach to the 2D Oriented Assembly MOF Electrode for Non-Enzymatic Sweat Biosensors. Nanoscale 2018, 10, 6629–6638. [Google Scholar] [CrossRef]
- Phamonpon, W.; Promphet, N.; Saengkiettiyut, K.; Boonyongmaneerat, Y.; Rattanawaleedirojn, P.; Hinestroza, J.P.; Rodthongkum, N. Novel Bioelectrode for Sweat Lactate Sensor Based on Platinum Nanoparticles/Reduced Graphene Oxide Modified Carbonized Silk Cocoon. Sens. Actuators B Chem. 2025, 423, 136717. [Google Scholar] [CrossRef]
- Dagar, K.; Pundir, C.S. An Improved Amperometric L-Lactate Biosensor Based on Covalent Immobilization of Microbial Lactate Oxidase onto Carboxylated Multiwalled Carbon Nanotubes/Copper Nanoparticles/Polyaniline Modified Pencil Graphite Electrode. Enzym. Microb. Tech. 2017, 96, 177–186. [Google Scholar] [CrossRef]
- Tian, L.; Cai, L.; Ding, Z.; Zhou, Y.; Zhang, Y.; Liu, Q.; Ge, X.; Yu, C. Sweat Lactate Biosensor Based on Lactate Oxidase Immobilized with Flower-like NiCO2O4 and Carbon Nanotubes. Microchem. J. 2024, 200, 110417. [Google Scholar] [CrossRef]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous Capillary-Flow Sensing of Glucose and Lactate in Sweat with an Electrochemical Sensor Based on Functionalized Graphene Oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martinez, M.J. Dual Range Lactate Oxidase-Based Screen Printed Amperometric Biosensor for Analysis of Lactate in Diversified Samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Neampet, S.; Ruecha, N.; Qin, J.; Wonsawat, W.; Chailapakul, O.; Rodthongkum, N. A Nanocomposite Prepared from Platinum Particles, Polyaniline and a Ti3C2 MXene for Amperometric Sensing of Hydrogen Peroxide and Lactate. Microchim. Acta 2019, 186, 752. [Google Scholar] [CrossRef]
- Pereira, T.C.; Stradiotto, N.R. Electrochemical Sensing of Lactate by Using an Electrode Modified with Molecularly Imprinted Polymers, Reduced Graphene Oxide and Gold Nanoparticles. Microchim. Acta 2019, 186, 764. [Google Scholar] [CrossRef]
- Barbosa, R.; Gonçalves, R.; Blanco, G.E.O.; Saccardo, M.C.; Paiva, R.S.; Mastelaro, V.R.; Cruz, S.A.; Scuracchio, C.H. Plasma Treatment of Nafion Membranes: Understanding the Trade-off between Surface Modification and Electrochemical Degradation. J. Power Sources 2024, 610, 234741. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Chen, Q.; Zhao, H.; Gao, B.; Zhang, T. A Flexible Electrochemical Biosensor Based on Functionalized Poly(3,4-Ethylenedioxythiophene) Film to Detect Lactate in Sweat of the Human Body. J. Colloid. Interface Sci. 2022, 617, 454–462. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Cao, X.-Q.; Hua, Y.; Yu, C.-M. A Bifunctional Wearable Sensor Based on a Nanoporous Membrane for Simultaneous Detection of Sweat Lactate and Temperature. Anal. Chem. 2024, 96, 3087–3095. [Google Scholar] [CrossRef]
- Reza, M.S.; Seonu, S.; Abu Zahed, M.; Asaduzzaman, M.; Song, H.; Hoon Jeong, S.; Park, J.Y. Reduced Graphene Oxide-Functionalized Polymer Microneedle for Continuous and Wide-Range Monitoring of Lactate in Interstitial Fluid. Talanta 2024, 270, 125582. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qing, X.; Wang, Y.; Zhong, W.; Wang, W.; Chen, Y.; Liu, Q.; Li, M.; Wang, D. Fiber Organic Electrochemical Transistors Based on Multi-Walled Carbon Nanotube and Polypyrrole Composites for Noninvasive Lactate Sensing. Anal. Bioanal. Chem. 2020, 412, 7515–7524. [Google Scholar] [CrossRef]
- Mugo, S.M.; Robertson, S.V.; Lu, W. A Molecularly Imprinted Screen-Printed Carbon Electrode for Electrochemical Epinephrine, Lactate, and Cortisol Metabolites Detection in Human Sweat. Anal. Chim. Acta 2023, 1278, 341714. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Shi, Y.; Sun, Y.; Okeke, S.I.; Yang, L.; Zhang, W.; Zhang, Z.; Shi, Y.; Xiao, L. Recent Implementations of Hydrogel-Based Microbial Electrochemical Technologies (METs) in Sensing Applications. Sensors 2023, 23, 641. [Google Scholar] [CrossRef]
- Phumma, R.; Phamonpon, W.; Rodthongkum, N.; Ummartyotin, S. Fabrication of Silver Nanoparticle Loaded into Nanocellulose Derived from Hemp and Poly(Vinyl Alcohol)-Based Composite as an Electrode for Electrochemical Sensors for Lactate Determination. ACS Omega 2024, 9, 10371–10379. [Google Scholar] [CrossRef] [PubMed]
- Mukundan, G.; Ravipati, M.; Badhulika, S. Bimetallic Fe/Co-MOF Dispersed in a PVA/Chitosan Multi-Matrix Hydrogel as a Flexible Sensor for the Detection of Lactic Acid in Sweat Samples. Microchim. Acta 2024, 191, 614. [Google Scholar] [CrossRef] [PubMed]
- Somchob, B.; Promphet, N.; Rodthongkum, N.; Hoven, V.P. Zwitterionic Hydrogel for Preserving Stability and Activity of Oxidase Enzyme for Electrochemical Biosensor. Talanta 2024, 270, 125510. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible Graphene Oxide) Sensor for Electrochemical Monitoring Lactate in Low-Volume Passive Perspired Human Sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Briones, M.; Casero, E.; Petit-Domínguez, M.D.; Ruiz, M.A.; Parra-Alfambra, A.M.; Pariente, F.; Lorenzo, E.; Vázquez, L. Diamond Nanoparticles Based Biosensors for Efficient Glucose and Lactate Determination. Biosens. Bioelectron. 2015, 68, 521–528. [Google Scholar] [CrossRef]
- Nishiyama, K.; Mizukami, R.; Kuki, S.; Ishida, A.; Chida, J.; Kido, H.; Maeki, M.; Tani, H.; Tokeshi, M. Electrochemical Enzyme-Based Blood ATP and Lactate Sensor for a Rapid and Straightforward Evaluation of Illness Severity. Biosens. Bioelectron. 2022, 198, 113832. [Google Scholar] [CrossRef]
- Booth, M.A.; Gowers, S.A.N.; Hersey, M.; Samper, I.C.; Park, S.; Anikeeva, P.; Hashemi, P.; Stevens, M.M.; Boutelle, M.G. Fiber-Based Electrochemical Biosensors for Monitoring pH and Transient Neurometabolic Lactate. Anal. Chem. 2021, 93, 6646–6655. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Piskin, E.; Cetinkaya, A.; Eryaman, Z.; Karadurmus, L.; Unal, M.A.; Sezgintürk, M.K.; Hizal, J.; Ozkan, S.A. Development of Ultra-Sensitive and Selective Molecularly Imprinted Polymer-Based Electrochemical Sensor for L-Lactate Detection. Microchem. J. 2024, 204, 111163. [Google Scholar] [CrossRef]
- Khan, A.; Winder, M.; Hossain, G. Modified Graphene-Based Nanocomposite Material for Smart Textile Biosensor to Detect Lactate from Human Sweat. Biosens. Bioelectron. X 2022, 10, 100103. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 Magnetic Nanoparticles/Reduced Graphene Oxide Nanosheets as a Novel Electrochemical and Bioeletrochemical Sensing Platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Ben Moussa, F.; Achi, F.; Meskher, H.; Henni, A.; Belkhalfa, H. Green One-Step Reduction Approach to Prepare rGO@AgNPs Coupled with Molecularly Imprinted Polymer for Selective Electrochemical Detection of Lactic Acid as a Cancer Biomarker. Mater. Chem. Phys. 2022, 289, 126456. [Google Scholar] [CrossRef]
- Sainz, R.; del Pozo, M.; Vázquez, L.; Vilas-Varela, M.; Castro-Esteban, J.; Blanco, E.; Petit-Domínguez, M.D.; Quintana, C.; Casero, E. Lactate Biosensing Based on Covalent Immobilization of Lactate Oxidase onto Chevron-like Graphene Nanoribbons via Diazotization-Coupling Reaction. Anal. Chim. Acta 2022, 1208, 339851. [Google Scholar] [CrossRef]
- Xu, Y.; De La Paz, E.; Paul, A.; Mahato, K.; Sempionatto, J.R.; Tostado, N.; Lee, M.; Hota, G.; Lin, M.; Uppal, A.; et al. In-Ear Integrated Sensor Array for the Continuous Monitoring of Brain Activity and of Lactate in Sweat. Nat. Biomed. Eng. 2023, 7, 1307–1320. [Google Scholar] [CrossRef]
- Phamonpon, W.; Promphet, N.; Ummartyotin, S.; Rodthongkum, N. A Dual-Lactate Sensor for Milk Spoilage Based on Modified Recycled UHT Milk Carton Cellulose Surface. J. Clean. Prod. 2022, 363, 132519. [Google Scholar] [CrossRef]
- Li, J.; Bo, X. Laser-Enabled Flexible Electrochemical Sensor on Finger for Fast Food Security Detection. J. Hazard. Mater. 2022, 423, 127014. [Google Scholar] [CrossRef]
- Ozoglu, O.; Uzunoglu, A.; Unal, M.A.; Gumustas, M.; Ozkan, S.A.; Korukluoglu, M.; Gunes Altuntas, E. Electrochemical Detection of Lactate Produced by Foodborne Presumptive Lactic Acid Bacteria. J. Biosci. Bioeng. 2023, 135, 313–320. [Google Scholar] [CrossRef]
- Wali, R.; Moulaee, K.; Qasymeh, M.; Maalej, R.; Neri, G. Atomic Layer Deposition of NiO-Coated CNT Nanocomposites: Tailoring Electrochemical Properties for Salivary Lactate Detection. J. Electroanal. Chem. 2024, 971, 118594. [Google Scholar] [CrossRef]
- He, Q. An Engineered Lactate Oxidase Based Electrochemical Sensor for Continuous Detection of Biomarker Lactic Acid in Human Sweat and Serum. Heliyon 2024, 10, e34301. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.; Saliba, W.; Khatib, M.; Zheng, Y.; Pieters, C.; Oved, H.; Silberman, E.; Zohar, O.; Hu, Z.; Kloper, V.; et al. Biodegradable, Biocompatible, and Implantable Multifunctional Sensing Platform for Cardiac Monitoring. ACS Sens. 2024, 9, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Aghavali, R.; Hosseini-Toudeshki, H.; Brown, C.; Zhang, F.; et al. An Integrated Wearable Microneedle Array for the Continuous Monitoring of Multiple Biomarkers in Interstitial Fluid. Nat. Biomed. Eng. 2022, 6, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Molinero-Fernandez, Á.; Wei, Q.; Xuan, X.; Konradsson-Geuken, Å.; Cuartero, M.; Crespo, G.A. Intradermal Lactate Monitoring Based on a Microneedle Sensor Patch for Enhanced In Vivo Accuracy. ACS Sens. 2024, 9, 3115–3125. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-Based Detection of Ketone Bodies along with Glucose and Lactate: Toward Real-Time Continuous Interstitial Fluid Monitoring of Diabetic Ketosis and Ketoacidosis. Anal. Chem. 2020, 92, 2291–2300. [Google Scholar] [CrossRef]
- Chien, M.-N.; Fan, S.-H.; Huang, C.-H.; Wu, C.-C.; Huang, J.-T. Continuous Lactate Monitoring System Based on Percutaneous Microneedle Array. Sensors 2022, 22, 1468. [Google Scholar] [CrossRef]
- Wang, Y.; Ausri, I.R.; Wang, Z.; Derry, C.; Tang, X.S. Towards a Transdermal Membrane Biosensor for the Detection of Lactate in Body Fluids. Sens. Actuators B Chem. 2020, 308, 127645. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, Q.; Wang, Q.; Qiu, H.; Li, H.; Xu, T. Fully Integrated Microneedle Biosensor Array for Wearable Multiplexed Fitness Biomarkers Monitoring. Biosens. Bioelectron. 2024, 265, 116697. [Google Scholar] [CrossRef]
- Dai, Y.; Nolan, J.; Madsen, E.; Fratus, M.; Lee, J.; Zhang, J.; Lim, J.; Hong, S.; Alam, M.A.; Linnes, J.C.; et al. Wearable Sensor Patch with Hydrogel Microneedles for In Situ Analysis of Interstitial Fluid. ACS Appl. Mater. Inter. 2023, 15, 56760–56773. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated Textile Sensor Patch for Real-Time and Multiplex Sweat Analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef] [PubMed]
- Rabost-Garcia, G.; Colmena, V.; Aguilar-Torán, J.; Vieyra Galí, J.; Punter-Villagrasa, J.; Casals-Terré, J.; Miribel-Catala, P.; Muñoz, X.; Cadefau, J.; Padullés, J.; et al. Non-Invasive Multiparametric Approach To Determine Sweat–Blood Lactate Bioequivalence. Acs Sens. 2023, 8, 1536–1541. [Google Scholar] [CrossRef]
- Mengarda, P.; Dias, F.A.L.; Peixoto, J.V.C.; Osiecki, R.; Bergamini, M.F.; Marcolino-Junior, L.H. Determination of Lactate Levels in Biological Fluids Using a Disposable Ion-Selective Potentiometric Sensor Based on Polypyrrole Films. Sens. Actuators B Chem. 2019, 296, 126663. [Google Scholar] [CrossRef]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless Wearable Electrochemical Sensing Platform with Zero-Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, J.; Liu, J.; Li, Y. Wearable, Nanofiber-Based Microfluidic Systems with Integrated Electrochemical and Colorimetric Sensing Arrays for Multiplex Sweat Analysis. Chem. Eng. J. 2023, 454, 140248. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Leong, C.L.; Burnish, R.A.; Hassan, S.; Zhang, Y.; Clough, G.F.; Boutelle, M.G.; Voegeli, D.; Niu, X. Monitoring Biomolecule Concentrations in Tissue Using a Wearable Droplet Microfluidic-Based Sensor. Nat. Commun. 2019, 10, 2741. [Google Scholar] [CrossRef]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Arcangeli, D.; Possanzini, L.; Tonelli, D.; Fraboni, B.; Scavetta, E. Layered Double Hydroxide-Modified Organic Electrochemical Transistor for Glucose and Lactate Biosensing. Sensors 2020, 20, 3453. [Google Scholar] [CrossRef]
- Komkova, M.A.; Eliseev, A.A.; Poyarkov, A.A.; Daboss, E.V.; Evdokimov, P.V.; Eliseev, A.A.; Karyakin, A.A. Simultaneous Monitoring of Sweat Lactate Content and Sweat Secretion Rate by Wearable Remote Biosensors. Biosens. Bioelectron. 2022, 202, 113970. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, Z.; Lu, W.; Fang, T.; Ren, Y.; Yin, S.; Xuan, Q.; Li, D.; Xi, J.J.; Yao, B. Assessment of Drug Susceptibility for Patient-Derived Tumor Models through Lactate Biosensing and Machine Learning. ACS Sens. 2023, 8, 803–810. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, J.; Liu, C.; Xu, T.; Zhang, X. Machine Learning with Neural Networks to Enhance Selectivity of Nonenzymatic Electrochemical Biosensors in Multianalyte Mixtures. ACS Appl. Mater. Inter. 2022, 14, 52684–52690. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, H.; Zhao, H.; Fei, T.; Liu, S.; Chen, Q.; Gao, B.; Zhang, T. A Dual-Functional Polyaniline Film-Based Flexible Electrochemical Sensor for the Detection of pH and Lactate in Sweat of the Human Body. Talanta 2022, 242, 123289. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.; Vaughan, E.; Thompson, M.; O’ Riordan, A.; Galvin, P.; Iacopino, D.; Rodrigues Teixeira, S. Electrochemical Sensor for Enzymatic Lactate Detection Based on Laser-Scribed Graphitic Carbon Modified with Platinum, Chitosan and Lactate Oxidase. Talanta 2022, 246, 123492. [Google Scholar] [CrossRef] [PubMed]
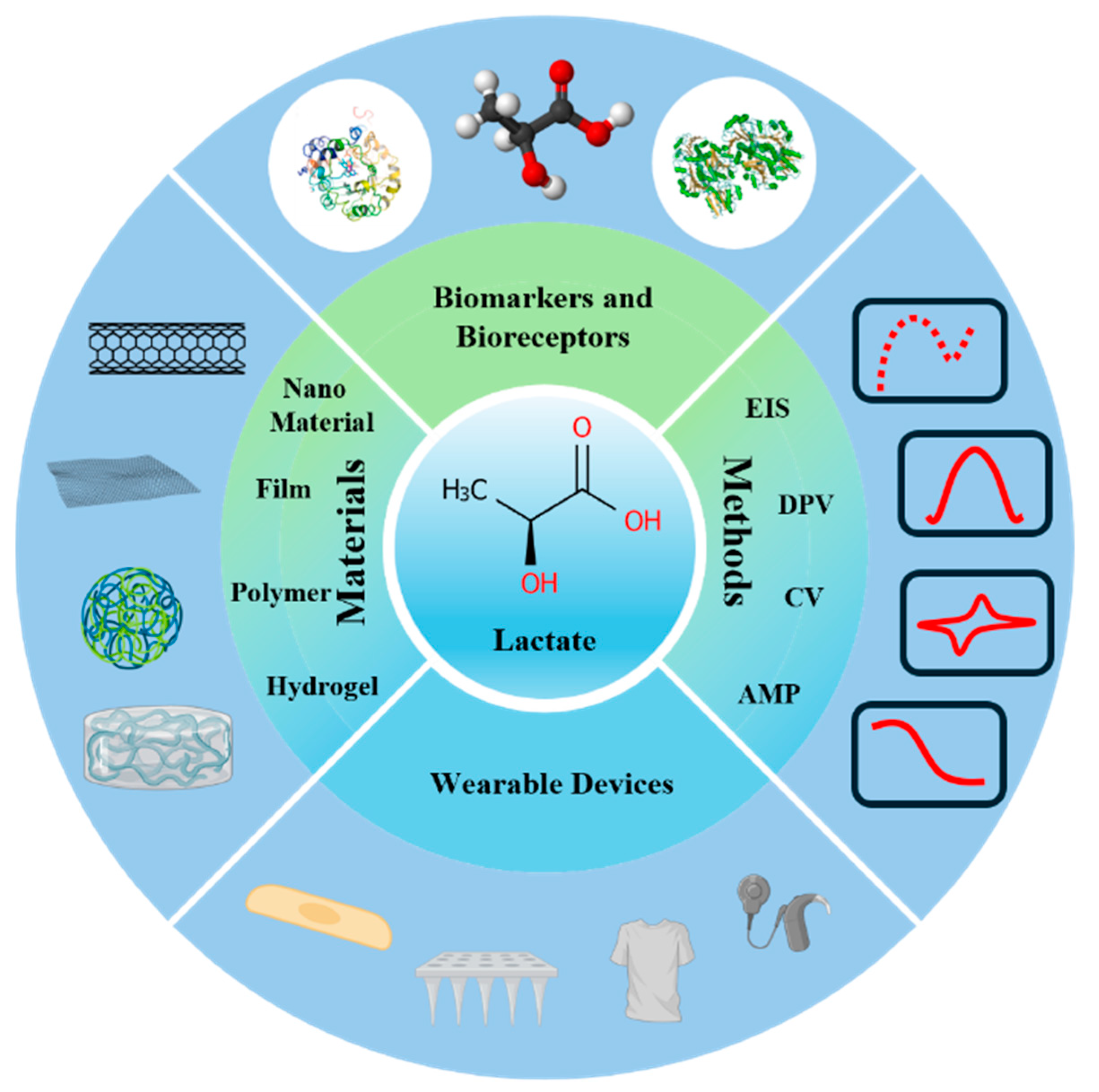
| Physiological State/Condition | Concentration Range (mM) | Description | Ref. |
|---|---|---|---|
| Normal Metabolism (Arterial) | 0.5–1.5 | Baseline arterial lactate levels in resting healthy individuals. | [2] |
| Mild Activity/Stress State | 2–4 | Elevated during moderate exercise or mild metabolic stress. | [7] |
| Intense Activity/Vigorous Exercise | 15–20 | Muscle lactate accumulates locally, higher than blood levels. | [8] |
| Tumor Microenvironment | 10–30 | Tumor lactate levels elevated, promoting progression and resistance. | [29] |
| ICU Patients (Arterial) | ≥2 | Diagnostic for septic shock; correlates with severity. | [12] |
| Critically Ill Patients (Lactic Acidosis) | ≥4 | Elevated lactate in shock or severe metabolic derangements. | [15] |
| Trauma Patients | ≥4 | Indicates inadequate perfusion, associated with high mortality. | [22] |
| Critical Threshold for Lactate (Emergency) | ≥8 | Indicates critical state; high 30-day mortality risk. | [23] |
| Theme | Year | Main Contributions | Limitations | Ref. |
|---|---|---|---|---|
| Electrochemical lactate detection | 2019 | Reviewed enzyme electrochemical methods and explored applications in critical care. | Discussion of non-enzymatic sensors is lacking. | [30] |
| Translational lactate sensing | 2021 | Explored the status of lactate biosensors for non-invasive real-time monitoring in the medical field. | Lacks in-depth analysis of core detection methods for lactate sensors. | [31] |
| Multi-fluid lactate sensing | 2021 | An overview of electrochemical sensors for the detection of lactate in a wide range of human fluids. | Lack of in-depth exploration of materials chemistry. | [32] |
| Enzymatic vs. non-enzymatic sensors | 2021 | Reviewed enzymatic and non-enzymatic sensors, focusing on their modifying materials and immobilization techniques. | A description of the medical field needs to be added. | [33] |
| Real-life lactate monitoring | 2022 | Sensor applications in the agri-food and clinical fields were reviewed, respectively. | Attention to innovative materials and emerging technologies needs to be increased. | [34] |
| Nanomaterial advancements | 2023 | Focused on non-enzymatic lactate sensors constructed from various nanomaterials. | Lack of discussion on specific feasibility for clinical and real-life applications. | [35] |
| Non-invasive lactate monitoring | 2024 | Described lactate sensors (based on sweat and interstitial fluids) for non-invasive detection in critical care. | No focus on cross-application of sensors with emerging technologies. | [36] |
| Material | Examples | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| AuNPs | Nanomaterials | High surface area, high conductivity, excellent catalytic activity | Cost of synthesis | [66] |
| CNTs | Nanomaterials | Excellent electrocatalytic performance, enhanced electron transfer | Stability issues, complex fabrication | [62] |
| Graphene | Nanomaterials | High surface area, good electrical conductivity | Stability issues, high cost, easily oxidized | [63] |
| MOFs | Nanomaterials | High surface area, tunable structure | Synthesis complexity, complex fabrication | [64] |
| MXenes | Nanomaterials | High conductivity, large surface area | Complex synthesis | [65] |
| PC nanoporous membranes | Membrane Materials | Selective permeability, enhanced sensitivity | May not block all interfering substances | [69] |
| Nafion | Membrane Materials | Selective permeability, protects enzyme activity | Limited permeability for small molecules, low conductivity | [70] |
| PPy | Polymer Materials | Good conductivity, enhanced electrochemical activity | Mechanical strength may degrade | [71] |
| PANI | Polymer Materials | Good conductivity, tunable properties | Cost of synthesis | [72] |
| PVA | Polymer Materials | Good biocompatibility, enhanced enzyme stability | Mechanical stability issues | [74] |
| PAM hydrogels | Hydrogel Materials | High water content, biocompatible | Loss of water content, may deform | [76] |
| SA hydrogels | Hydrogel Materials | High biocompatibility, tunable structure | Easily degradable, weak conductivity | [73] |
| Electrode | Targets | Methods | Detection Range | LOD | Sensitivity | Imp.1 | Ref. |
|---|---|---|---|---|---|---|---|
| PC/LOx/CS | Lac, Temp | AMP | 10 µM–35 mM | 144 nM | 82.4 nA mM−1 | N | [69] |
| MIPs-AgNWs | Lac | DPV | 1 µM–100 mM | 220 nM | 4.5 µA dacade−1 | N | [46] |
| PEDOT:PSS/CS/LOx | Lac, Glu, Alc | AMP | 500 µM–5 mM | 250 nM | 150 nA mM−1 cm−2 | I | [100] |
| Hydrogel/LOx | Lac | AMP | 0 mM–15 mM | 350 nM | 9 µA mM−1 mm−2 | N | [105] |
| AuE/rGO-AgNPs/MIP | Lac | CV | 10 µM–250 µM | 726 nM | 0.12428 µA mM−1 −0.14102 µA mM−1 | O | [86] |
| PB/GO/Au/LOx | Lac | AMP | 1 µM–222 µM 222 µM–25 mM | 800 nM | 40.6 µA mM−1 cm−2 1.9 µA mM−1 cm−2 | N | [48] |
| Rgo/PtNPs/LOx | Lac | AMP | 0 mM–10 mM | 2.04 µM | 43.96 µA mM−1 cm−2 | I | [70] |
| PEDOT:PSS/TTF/CS/Ldh | Lac, pH | AMP | 140 µM–13.32 mM | 5 µM | 1.1 µA dacade−1 | I | [94] |
| LOx-GO-ZnO | Lac | AMP | 15 µM–1.25 mM | 9 µM | 3.308 µA mM−1 | N | [53] |
| LOx/BzA/GNR | Lac | CV | 34 µM–280 µM | 11 µM | 5.5 µA mM−1 | O | [87] |
| PVC/CHI- LOx/PB/C-MN | Lac | AMP | 250 µM–35 mM | 14.8 µM | −8.04 nA mM−1 | I | [96] |
| LDH-NAD+/PyrOx/SPE | Lac | EIS | 10 µM–250 µM | 17 µM | 62.7 Ω cm2 mM−1 | O | [49] |
| LOx/PmPD/IrO2/PBE | Lac, pH | AMP | 0 mM–3 mM | 19 ± 7 µM | 2.63 ± 0.66 nA mM−1 | I | [80] |
| LOx/PB enzyme-nanozyme | Lac | AMP | 20 µM–100 mM | 20 µM | 4.4 ± 0.5 µA mM−1 cm−2 | N | [109] |
| SWCNT/NiCo2O4/HRP/LOx | Lac | AMP | 100 µM–30 mM | 39.9 µM | 98 nA mM−1 | N | [62] |
| PtNPs/rGO/LOx | Lac | AMP | 0 mM–25 mM | 70 µM | 0.87 µA mM−1 cm−2 | N | [60] |
| LOx/CS/PEDOT | Lac | DPV | 250 µM–1 mM 1 mM–40 mM | 83 µM | 43.42 µA mM−1 cm−2 0.32 µA mM−1 cm−2 | N | [68] |
| PANI | Lac, pH | AMP | 250 µM–10 mM 10 mM–60 mM | 83 µM | 18.62 nA mM−1 4.25 nA mM−1 | N | [112] |
| LOx/CS/Pt | Lac | AMP | 200 µM–3 mM | 110 µM | 35.8 µA mM−1 cm−2 | O | [113] |
| SilkNCT/PtNPs/LOx | Lac, Glu, AA, UA, Na+, K+ | AMP | 5 mM–35 mM | 500 µM | 174 nA mM−1 | N | [102] |
| PA/GO/PANHS/LOx | Lac | EIS | 1.3 mM–113.4 mM | 1 mM | N/A | N | [77] |
| NDG/LOx/PMM70 | Lac, Glu | AMP | 0 mM–25 mM | 6.5 mM | 443.2 nA mM−1 | O | [76] |
| Hydrogel MNs/GA/BSA/LOx | Lac, Glu | AMP | 100 µM–12 mM | N/A | 3 ± 0.4 nA mM−1 | I | [101] |
| LOx/PB/Au/PS | Lac, ATP | AMP | 1 µM–10 mM | N/A | 101 nA mM−1 | O | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Yang, L.; Wen, J.; Ma, Y.; Dai, G.; Mo, F.; Wang, J. A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare. Sensors 2025, 25, 1045. https://doi.org/10.3390/s25041045
Ding Y, Yang L, Wen J, Ma Y, Dai G, Mo F, Wang J. A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare. Sensors. 2025; 25(4):1045. https://doi.org/10.3390/s25041045
Chicago/Turabian StyleDing, Yifeng, Liuhong Yang, Jing Wen, Yuhang Ma, Ge Dai, Fengfeng Mo, and Jiafeng Wang. 2025. "A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare" Sensors 25, no. 4: 1045. https://doi.org/10.3390/s25041045
APA StyleDing, Y., Yang, L., Wen, J., Ma, Y., Dai, G., Mo, F., & Wang, J. (2025). A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare. Sensors, 25(4), 1045. https://doi.org/10.3390/s25041045






