A Compact Orthosis Compliance Monitoring Device Using Pressure Sensors and Accelerometers: Design and Proof-of-Concept Testing
Abstract
:1. Introduction
2. Materials and Methods
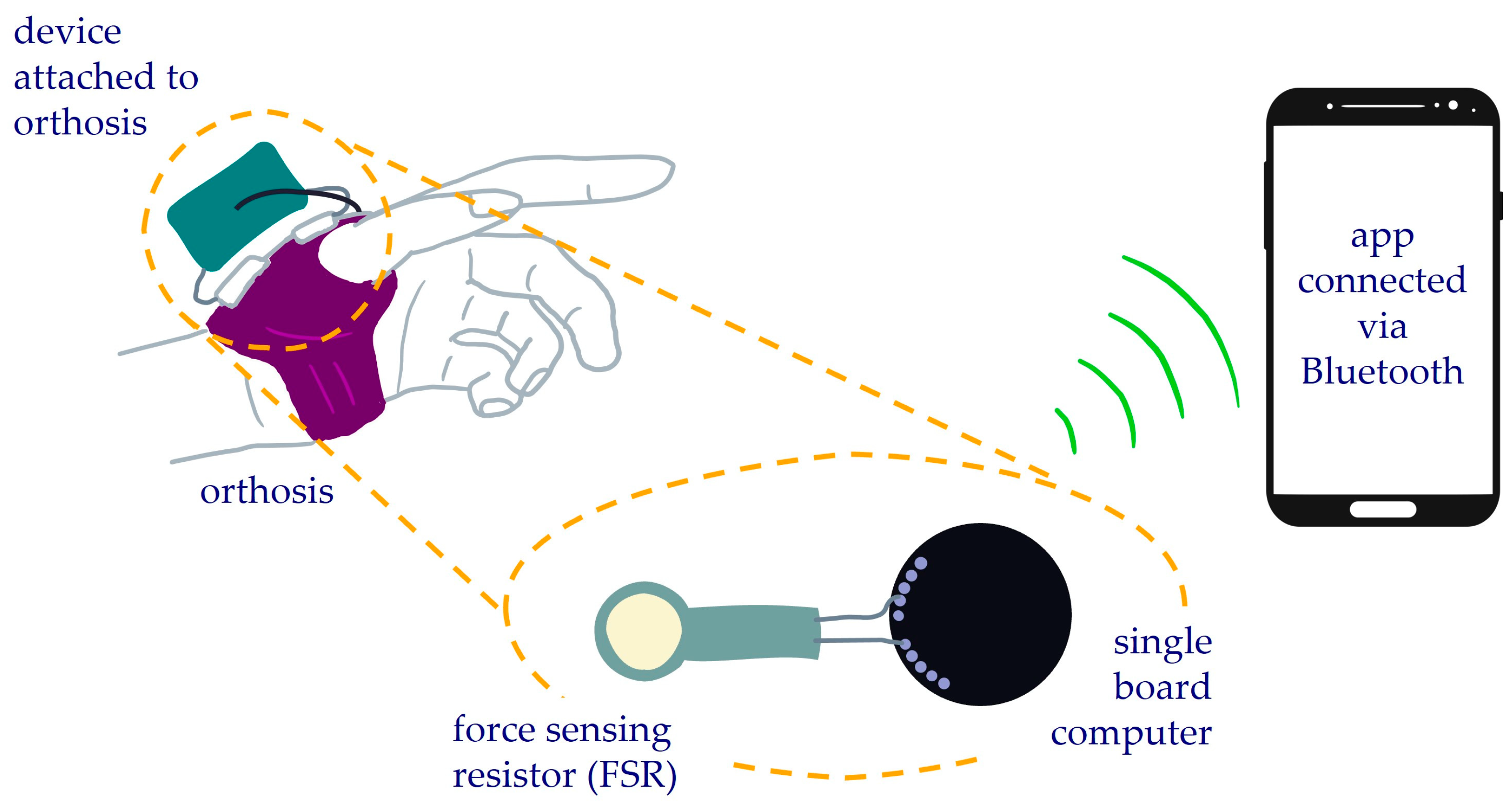
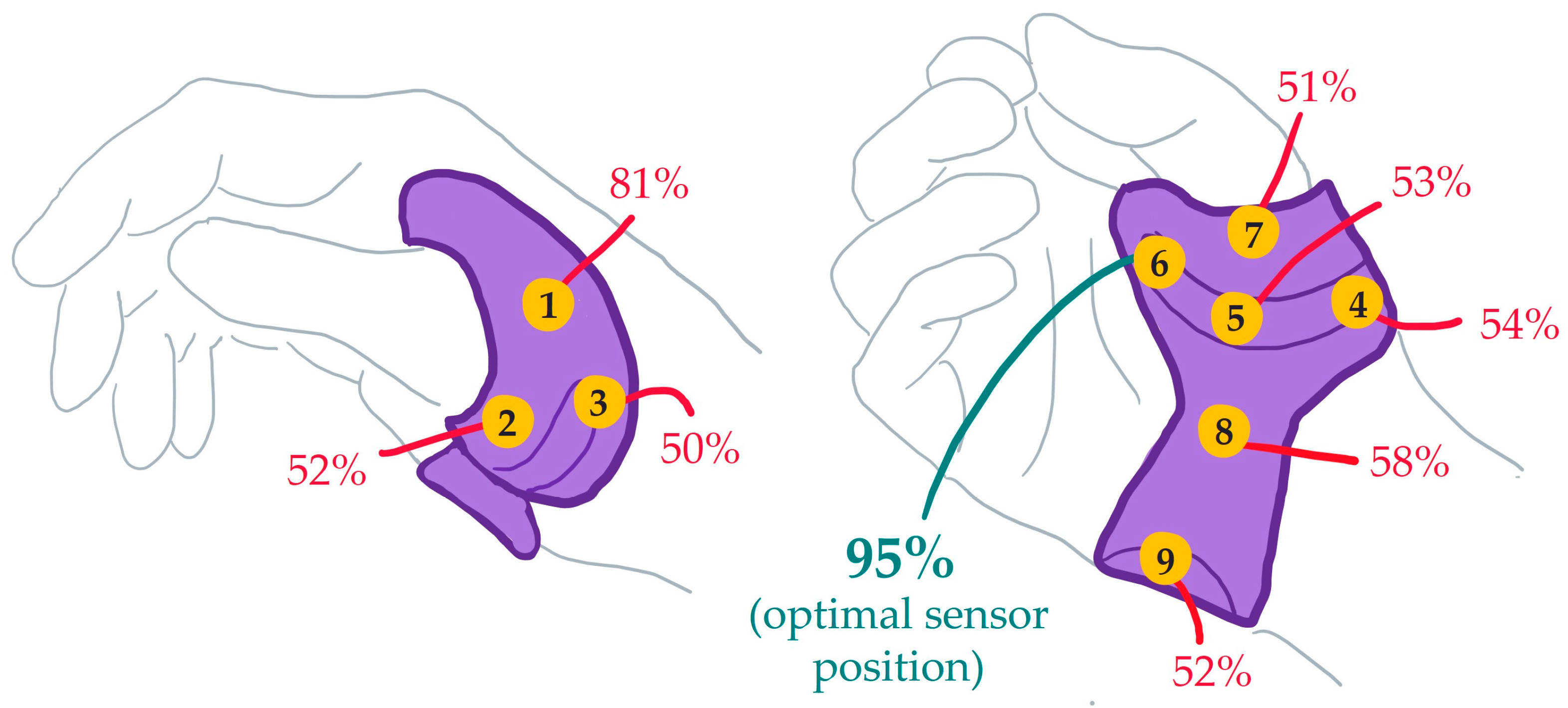
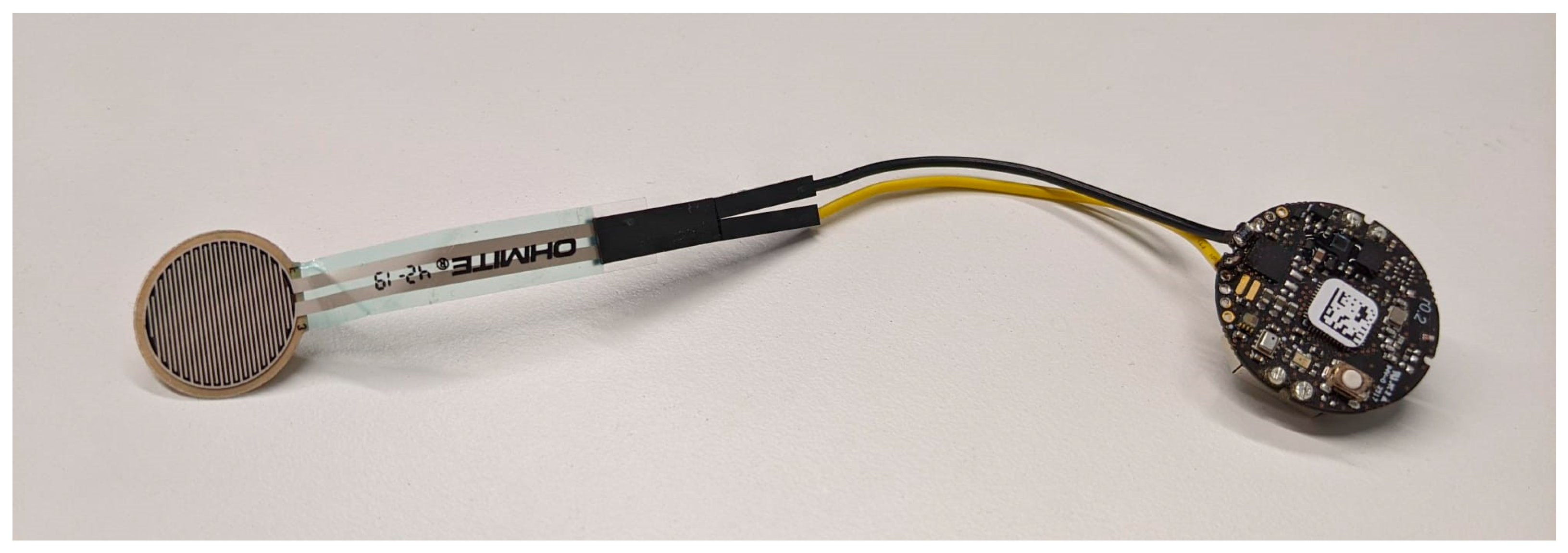
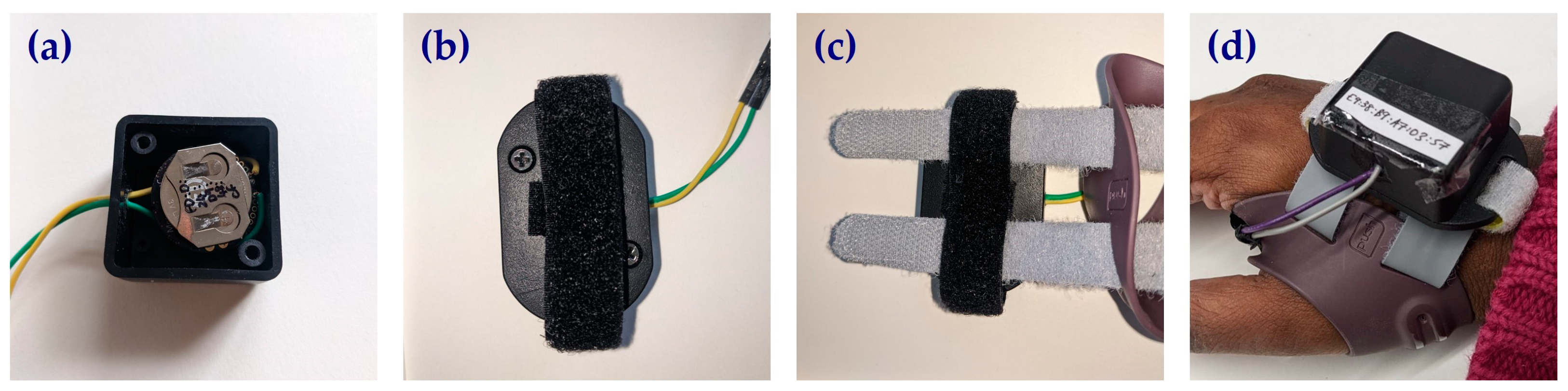
2.1. Compliance Monitoring Device
2.2. Electronics
2.3. Participants
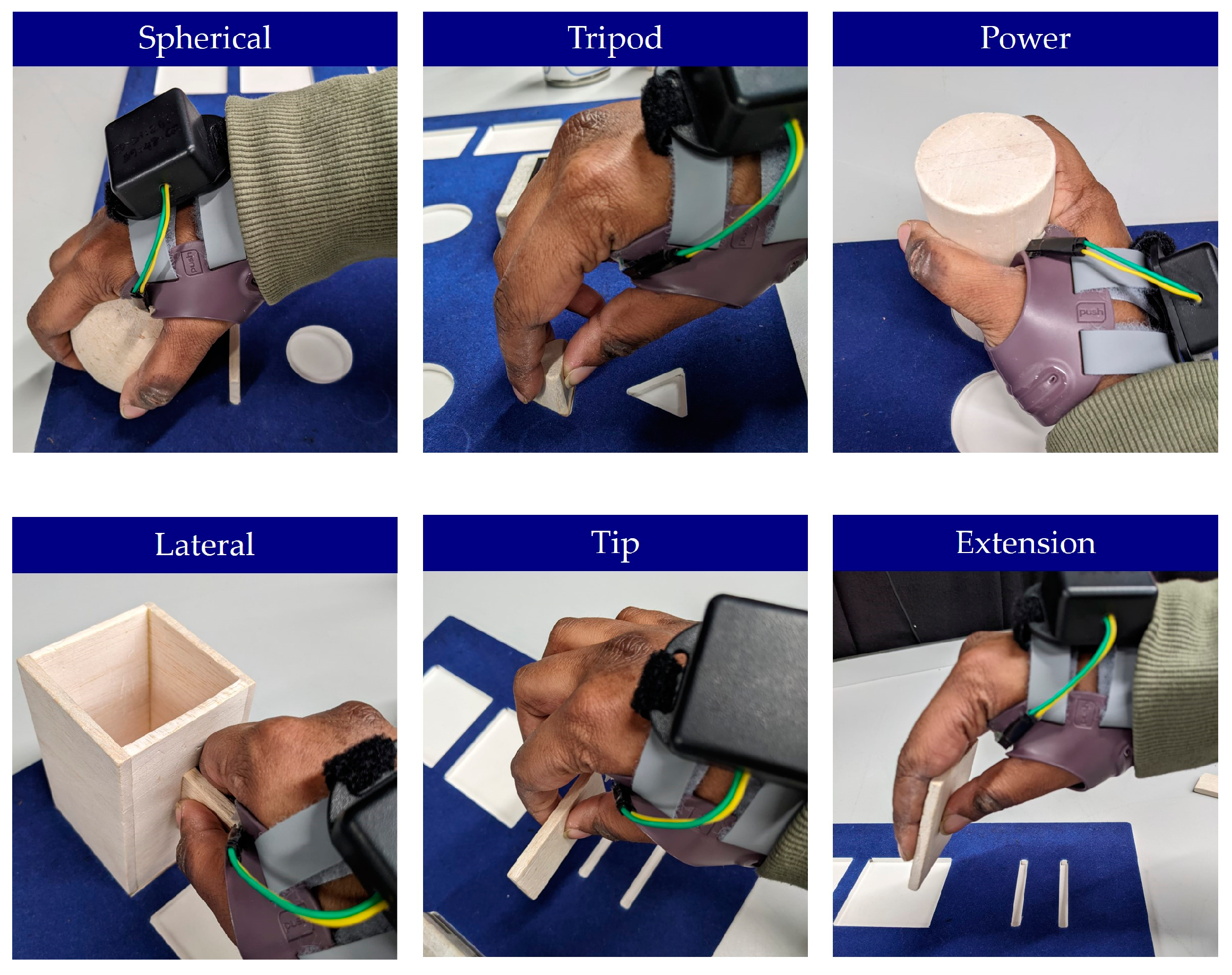
2.4. Experimental Protocol
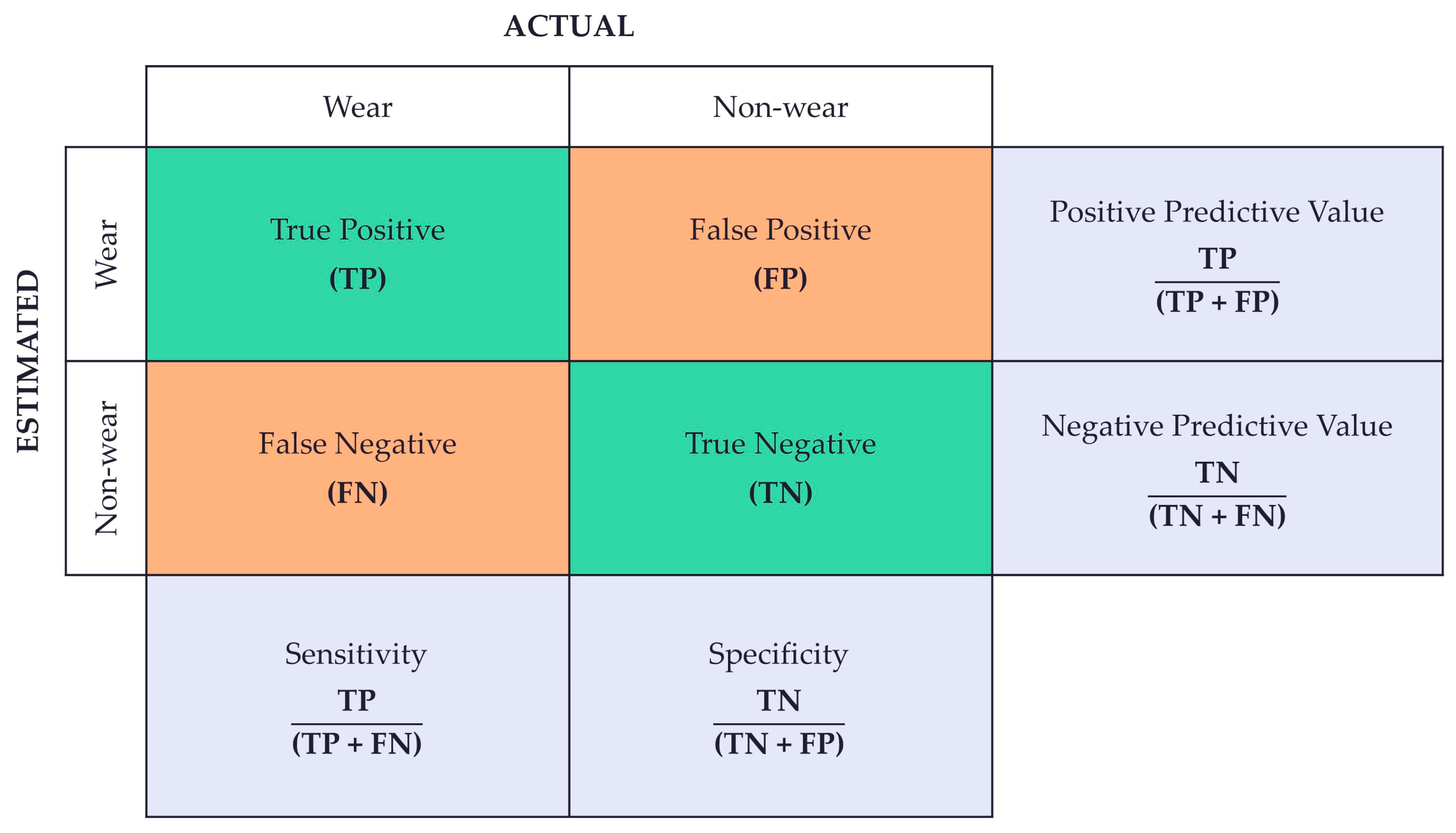
2.5. Data Analysis
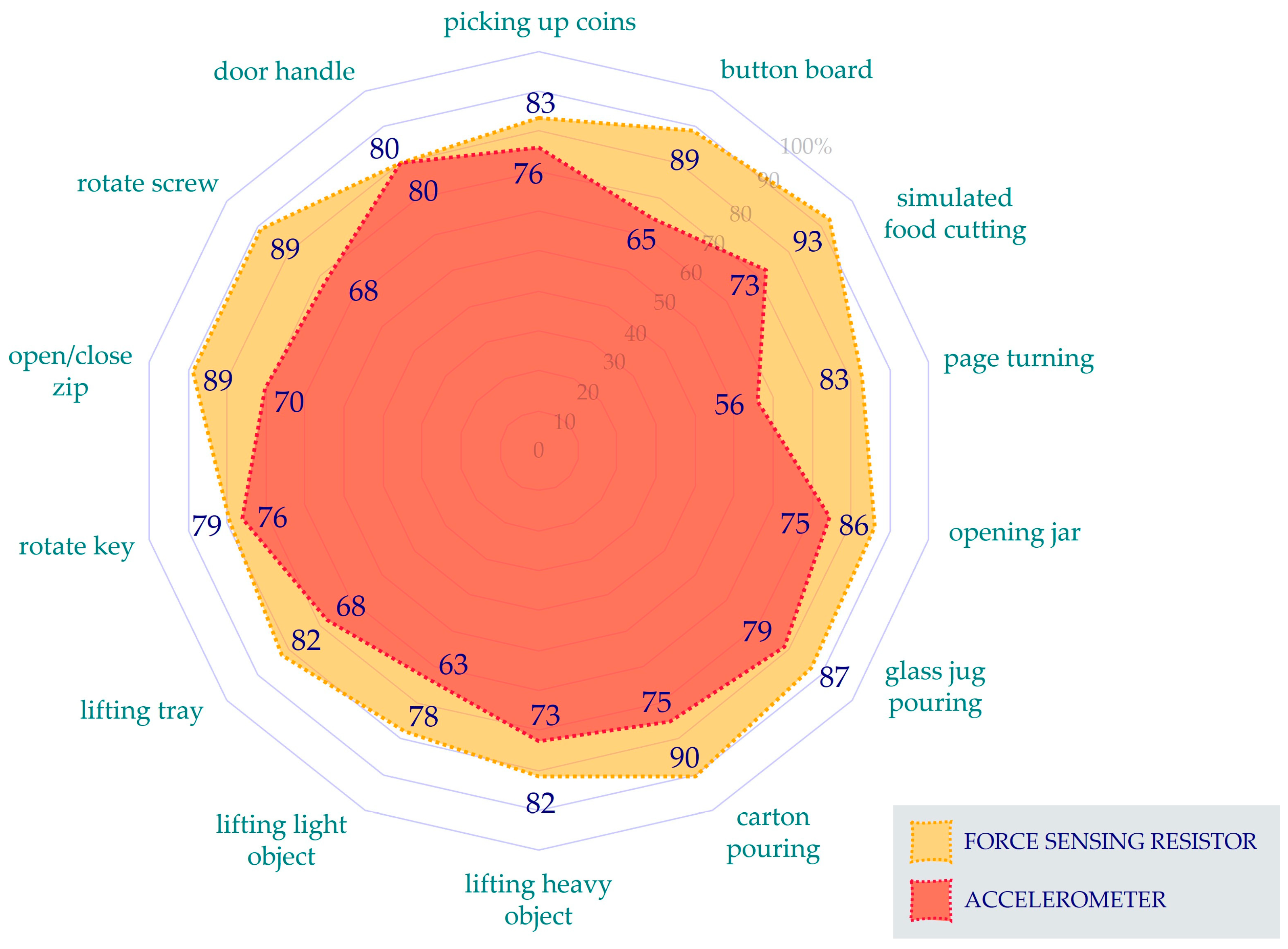
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Down, K.; Stead, E. Assistive Technology Workforce Development; Foundation for Assistive Technology: London, UK, 2007. [Google Scholar]
- Beaudreuil, J. Orthoses for Osteoarthritis: A Narrative Review. Ann. Phys. Rehabil. Med. 2017, 60, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.J.F.; Duarte, C.; Marques, A.; Cardoso, D.; Apóstolo, J.; da Silva, J.A.P.; Barbieri-Figueiredo, M. Effectiveness of Non-Pharmacological and Non-Surgical Interventions for Rheumatoid Arthritis: An Umbrella Review. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 1494–1531. [Google Scholar] [CrossRef] [PubMed]
- Packham, T.L.; Ball, P.D.; MacDermid, J.C.; Bain, J.R.; DalCin, A. A Scoping Review of Applications and Outcomes of Traction Orthoses and Constructs for the Management of Intra-Articular Fractures and Fracture Dislocations in the Hand. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2016, 29, 246–268. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.; Edelstein, J.; Zubrenic, E.; Tsao, L.; Pustina, K.; Berendsen, M.; Wafford, E. Systematic Review of Orthoses for Stroke-Induced Upper Extremity Deficits. Top. Stroke Rehabil. 2019, 26, 389–398. [Google Scholar] [CrossRef]
- Garbellini, S.; Robert, Y.; Randall, M.; Elliott, C.; Imms, C. Rationale for Prescription, and Effectiveness of, Upper Limb Orthotic Intervention for Children with Cerebral Palsy: A Systematic Review. Disabil. Rehabil. 2018, 40, 1361–1371. [Google Scholar] [CrossRef]
- Freeman, J.; Nikjou, D.; Maloney, J.; Covington, S.; Pew, S.; Wie, C.; Strand, N.; Abd-Elsayed, A. The Role of Orthoses in Chronic Axial Spinal Conditions. Curr. Pain Headache Rep. 2024, 28, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.; Pauls, M. Shoulder Orthoses for the Prevention and Reduction of Hemiplegic Shoulder Pain and Subluxation: Systematic Review. Clin. Rehabil. 2017, 31, 444–453. [Google Scholar] [CrossRef]
- Howell, J. 12—Principles and Components of Upper Limb Orthoses. In Atlas of Orthoses and Assistive Devices, 5th ed.; Webster, J.B., Murphy, D.P., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 134–145.e1. [Google Scholar] [CrossRef]
- Edwards, J.; Fenwick, L.B. Chapter 14—Upper Limb Orthotics. In Clinician’s Guide to Assistive Technology; Olson, D.A., DeRuyter, F., Eds.; Mosby: Saint Louis, MI, USA, 2002; pp. 237–249. [Google Scholar] [CrossRef]
- Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Egan, M.Y.; Brousseau, L. Splinting for Osteoarthritis of the Carpometacarpal Joint: A Review of the Evidence. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 2007, 61, 70–78. [Google Scholar] [CrossRef]
- Adams, J.; Barratt, P.; Arden, N.K.; Barbosa Bouças, S.; Bradley, S.; Doherty, M.; Dutton, S.; Dziedzic, K.; Gooberman-Hill, R.; Hislop Lennie, K.; et al. The Osteoarthritis Thumb Therapy (OTTER) II Trial: A Study Protocol for a Three-Arm Multi-Centre Randomised Placebo Controlled Trial of the Clinical Effectiveness and Efficacy and Cost-Effectiveness of Splints for Symptomatic Thumb Base Osteoarthritis. BMJ Open 2019, 9, e028342. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Barratt, P.; Rombach, I.; Arden, N.; Barbosa Bouças, S.; Bradley, S.; Doherty, M.; Dutton, S.J.; Gooberman-Hill, R.; Hislop-Lennie, K.; et al. The Clinical and Cost Effectiveness of Splints for Thumb Base Osteoarthritis: A Randomized Controlled Clinical Trial. Rheumatology 2021, 60, 2862–2877. [Google Scholar] [CrossRef]
- Rivett, L.; Rothberg, A.; Stewart, A.; Berkowitz, R. The Relationship between Quality of Life and Compliance to a Brace Protocol in Adolescents with Idiopathic Scoliosis: A Comparative Study. BMC Musculoskelet. Disord. 2009, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Yeomans, D.; Tolkien, Z.; Kreis, I.A.; Potter, S.; Gardiner, M.D.; Jain, A.; Henderson, J.; Blazeby, J.M. Methods for Assessment of Patient Adherence to Removable Orthoses Used after Surgery or Trauma to the Appendicular Skeleton: A Systematic Review. Trials 2020, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Houwen-van Opstal, S.L.S.; van den Elzen, Y.M.E.M.; Jansen, M.; Willemsen, M.A.A.P.; Cup, E.H.C.; De Groot, I.J.M. Facilitators and Barriers to Wearing Hand Orthoses by Adults with Duchenne Muscular Dystrophy: A Mixed Methods Study Design. J. Neuromuscul. Dis. 2020, 7, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Kerckhofs, E. Compliance of Patients Wearing an Orthotic Device or Orthopedic Shoes: A Systematic Review. J. Bodyw. Mov. Ther. 2015, 19, 759–770. [Google Scholar] [CrossRef]
- Thatipelli, S.; Arun, A.; Chung, P.; Etemadi, M.; Heller, J.A.; Kwiat, D.; Imamura-Ching, J.; Harrison, M.R.; Roy, S. Review of Existing Brace Adherence Monitoring Methods to Assess Adherence. J. Prosthet. Orthot. 2016, 28, 126–135. [Google Scholar] [CrossRef]
- Liavaag, S.; Brox, J.I.; Pripp, A.H.; Enger, M.; Soldal, L.A.; Svenningsen, S. Immobilization in External Rotation after Primary Shoulder Dislocation Did Not Reduce the Risk of Recurrence: A Randomized Controlled Trial. J. Bone Jt. Surg. Am. Vol. 2011, 93, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Silverio, L.M.; Cheung, E.V. Patient Adherence with Postoperative Restrictions after Rotator Cuff Repair. J. Shoulder Elb. Surg. 2014, 23, 508–513. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.S.; Ulrich, S.D.; Bonutti, P.M.; Smith, J.M.; Seyler, T.M.; Mont, M.A. Evaluation of Static Progressive Stretch for the Treatment of Wrist Stiffness. J. Hand Surg. Am. 2008, 33, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Grubhofer, F.; Gerber, C.; Meyer, D.C.; Wieser, K.; Ernstbrunner, L.; Catanzaro, S.; Bouaicha, S. Compliance with Wearing an Abduction Brace after Arthroscopic Rotator Cuff Repair: A Prospective, Sensor-Controlled Study. Prosthet. Orthot. Int. 2019, 43, 440–446. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J. Patients’ Beliefs about Prescribed Medicines and Their Role in Adherence to Treatment in Chronic Physical Illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef]
- Haynes, R.B.; Ackloo, E.; Sahota, N.; McDonald, H.P.; Yao, X. Interventions for Enhancing Medication Adherence. Cochrane Database Syst. Rev. 2008, CD000011. [Google Scholar] [CrossRef]
- Devanand, D.B.; Kedgley, A.E. Objective Methods of Monitoring Usage of Orthotic Devices for the Extremities: A Systematic Review. Sensors 2023, 23, 7420. [Google Scholar] [CrossRef]
- Haarman, C.J.W.; Hekman, E.E.G.; Rietman, J.S.; van der Kooij, H. Accurate Estimation of Upper Limb Orthosis Wear Time Using Miniature Temperature Loggers. J. Rehabil. Med. 2022, 54, 43. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Klein, A.; Kaveeshwar, S.; Jones, D.L.; Duvall, G.; Hovis, J.P.; Weir, T.B.; Enobun, B.; Hasan, S.A.; Henn, R.F.; et al. An Accurate Method of Measuring Shoulder Sling Compliance: A Validation Study. BMC Musculoskelet. Disord. 2021, 22, 524. [Google Scholar] [CrossRef] [PubMed]
- Grubhofer, F.; Ernstbrunner, L.; Gerber, C.; Hochreiter, B.; Schwihla, I.; Wieser, K.; Bouaicha, S. Effect of Abduction Brace Wearing Compliance on the Results of Arthroscopic Rotator Cuff Repair. JBJS Open Access 2022, 7, e21.00148. [Google Scholar] [CrossRef]
- Dinis, E.; Gonçalves, R.; Rodrigues, I.; Mendes, B.; Quintão, C.; Vigário, R.; Quaresma, C. Ortho-Monitorizer: A Portable Device to Monitor Pressure and Temperature During the Use of Upper Limb Orthoses. SN Comput. Sci. 2023, 4, 34. [Google Scholar] [CrossRef]
- Tenti, S.; Ferretti, F.; Gusinu, R.; Gallo, I.; Giannotti, S.; Pozza, A.; Fioravanti, A.; Coluccia, A. Impact of Thumb Osteoarthritis on Pain, Function, and Quality of Life: A Comparative Study between Erosive and Non-Erosive Hand Osteoarthritis. Clin. Rheumatol. 2020, 39, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Tecame, A.; Torre, G.; D’Adamio, S.; Maffulli, N.; Denaro, V. Small Joints Replacement for Hand Osteoarthritis: A Systematic Review. Br. Med. Bull. 2015, 116, 55–68. [Google Scholar] [CrossRef]
- Haara, M.M.; Heliövaara, M.; Kröger, H.; Arokoski, J.P.A.; Manninen, P.; Kärkkäinen, A.; Knekt, P.; Impivaara, O.; Aromaa, A. Osteoarthritis in the Carpometacarpal Joint of the Thumb. Prevalence and Associations with Disability and Mortality. J. Bone Jt. Surg. Am. 2004, 86, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Riley, N.; Dean, B. Management of Osteoarthritis at the Base of the Thumb. Bone Jt. J. 2020, 102, 600–605. [Google Scholar] [CrossRef]
- Cooney, W.P., 3rd; Chao, E.Y. Biomechanical Analysis of Static Forces in the Thumb during Hand Function. J. Bone Jt. Surg. Am. 1977, 59, 27–36. [Google Scholar] [CrossRef]
- Kloppenburg, M.; van Beest, S.; Kroon, F.P.B. Thumb Base Osteoarthritis: A Hand Osteoarthritis Subset Requiring a Distinct Approach. Best Pract. Res. Clin. Rheumatol. 2017, 31, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Barbour, K.E.; Murphy, L.B.; Nelson, A.E.; Schwartz, T.A.; Helmick, C.G.; Allen, K.D.; Renner, J.B.; Baker, N.A.; Jordan, J.M. Lifetime Risk of Symptomatic Hand Osteoarthritis: The Johnston County Osteoarthritis Project. Arthritis Rheumatol. 2017, 69, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, V.H.; McGaha, J.L. Current Practice Patterns in Conservative Thumb CMC Joint Care: Survey Results. J. Hand Ther. 2014, 27, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Kroon, F.P.; Blanco, F.J.; Doherty, M.; Dziedzic, K.S.; Greibrokk, E.; Haugen, I.K.; Herrero-Beaumont, G.; Jonsson, H.; Kjeken, I.; et al. 2018 Update of the EULAR Recommendations for the Management of Hand Osteoarthritis. Ann. Rheum. Dis. 2019, 78, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Gardiner, M.D.; Burchette, D.; Khor, W.; Jansen, V.; McKenna, H. Evidence-Based Management of Thumb Base Osteoarthritis; British Society for Surgery of the Hand Evidence for Surgical Treatment (BEST): London, UK, 2023. [Google Scholar]
- Marotta, N.; Demeco, A.; Marinaro, C.; Moggio, L.; Pino, I.; Barletta, M.; Petraroli, A.; Ammendolia, A. Comparative Effectiveness of Orthoses for Thumb Osteoarthritis: A Systematic Review and Network Meta-Analysis. Arch. Phys. Med. Rehabil. 2021, 102, 502–509. [Google Scholar] [CrossRef]
- Duong, V.; Bennell, K.L.; Deveza, L.A.; Eyles, J.P.; Hodges, P.W.; Holden, M.A.; Hunter, D.J.; Jongs, R.; Knapp, D.; Mei, Y.; et al. Attitudes, Beliefs and Common Practices of Hand Therapists for Base of Thumb Osteoarthritis in Australia (The ABC Thumb Study). Hand Ther. 2017, 23, 19–27. [Google Scholar] [CrossRef]
- Grüschke, J.S.; Reinders-Messelink, H.A.; van der Vegt, A.E.; van der Sluis, C.K. User Perspectives on Orthoses for Thumb Carpometacarpal Osteoarthritis. J. Hand Ther. 2019, 32, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Jha, B.; Couzens, G.; Lisle, D.; Ross, M. The Effectiveness of the Push BraceTM Orthosis and Corticosteroid Injection for Managing First Carpometacarpal Joint Osteoarthritis: A Factorial Randomised Controlled Trial Protocol. Hand Ther. 2015, 20, 39–48. [Google Scholar] [CrossRef]
- Light, C.M.; Chappell, P.H.; Kyberd, P.J. Establishing a Standardized Clinical Assessment Tool of Pathologic and Prosthetic Hand Function: Normative Data, Reliability, and Validity. Arch. Phys. Med. Rehabil. 2002, 83, 776–783. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Griffiths, B.; Silver, N.; Granat, M.H.; Lebel, E. Measuring Foot Abduction Brace Wear Time Using a Single 3-Axis Accelerometer. Sensors 2022, 22, 2433. [Google Scholar] [CrossRef]
- Bus, S.A.; Waaijman, R.; Nollet, F. New Monitoring Technology to Objectively Assess Adherence to Prescribed Footwear and Assistive Devices during Ambulatory Activity. Arch. Phys. Med. Rehabil. 2012, 93, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- van Netten, J.J.; Dijkstra, P.U.; Geertzen, J.H.B.; Postema, K. What Influences a Patient’s Decision to Use Custom-Made Orthopaedic Shoes? BMC Musculoskelet. Disord. 2012, 13, 92. [Google Scholar] [CrossRef]
- Morgenstein, A.; Davis, R.; Talwalkar, V.; Iwinski, H., Jr.; Walker, J.; Milbrandt, T.A. A Randomized Clinical Trial Comparing Reported and Measured Wear Rates in Clubfoot Bracing Using a Novel Pressure Sensor. J. Pediatr. Orthop. 2015, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; Chatwin, K.E.; Foden, P.; Hasan, A.N.; Sange, C.; Rajbhandari, S.M.; Reddy, P.N.; Vileikyte, L.; Bowling, F.L.; Boulton, A.J.M. Innovative Intelligent Insole System Reduces Diabetic Foot Ulcer Recurrence at Plantar Sites: A Prospective, Randomised, Proof-of-Concept Study. Lancet Digit. Health 2019, 1, e308–e318. [Google Scholar] [CrossRef] [PubMed]
- Dobele, S.; Deininger, C.; Sandmann, G.H.; Schmitt, A.; Freude, T.; Stockle, U.; Lucke, M. New Method for Monitoring Partial Weight Bearing (PWB) of Outpatients with an Independent Insole Sensor System. Acta Chir. Orthop. Traumatol. Cech. 2016, 83, 88–93. [Google Scholar] [PubMed]
- Lajevardi-Khosh, A.; Stuart, A.; Ackerman, M.; Rothberg, D.; Kubiak, E.; Petelenz, T. Characterization of Compliance to Weight-Bearing Protocols and Patient Weight-Bearing Behavior during the Recovery Period in Lower Extremity Fractures: A Pilot Study. Curr. Orthop. Pract. 2019, 30, 395–402. [Google Scholar] [CrossRef]
- Najafi, B.; Ron, E.; Enriquez, A.; Marin, I.; Razjouyan, J. Smarter Sole Survival: Will Neuropathic Patients at High Risk for Ulceration Use a Smart Insole-Based Foot Protection System? J. Diabetes Sci. Technol. 2017, 11, 702–713. [Google Scholar] [CrossRef]
- Wong, M.S.; Wu, H.D.; Beygi, B.H.; Zhang, Q.; Lin, Y.; Chan, W.S.; Lou, E. Compliance Study of Hip Protector Users for Prevention of Fragility Fracture: A Pilot Randomized Trial. Prosthet. Orthot. Int. 2022, 46, E392–E397. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Ducos, M.; Yang, P.; Jos, D.; Frings-Meuthen, P.; Bruggemann, G.-P.; Bloch, W.; Rittweger, J. The HEPHAISTOS Study: Compliance and Adherence with a Novel Orthotic Device for Calf Muscle Unloading. J. Musculoskelet. Neuronal Interact. 2013, 13, 487–495. [Google Scholar]
- Telfer, S.; Munguia, J.; Pallari, J.; Dalgarno, K.; Steultjens, M.; Woodburn, J. Personalized Foot Orthoses with Embedded Temperature Sensing: Proof of Concept and Relationship with Activity. Med. Eng. Phys. 2014, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Bonanno, D.R. Objective Measurement of Adherence to Wearing Foot Orthoses Using an Embedded Temperature Sensor. Med. Eng. Phys. 2021, 88, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Miller Renfrew, L.; Rafferty, D.; Lord, A.; Hunter, R.; Paul, L. Determining Validity of the PALite and ODFS PACE Activity Logger for Measuring Step Count in Healthy Adults. Gait Posture 2020, 80, 315–317. [Google Scholar] [CrossRef]
- Barakat, A.; Manga, A.; Sheikh, A.; McWilliams, R.; Rowlands, A.V.; Singh, H. Feasibility of Using a GENEActiv Accelerometer with Triaxial Acceleration and Temperature Sensors to Monitor Adherence to Shoulder Sling Wear Following Surgery. Sensors 2024, 24, 880. [Google Scholar] [CrossRef] [PubMed]
- Ambar, R.; Mhd Poad, H.; Mohd Ali, A.; Ahmad, M.; Abdul Jamil, M.M. Multisensor Arm Rehabilitation Monitoring Device. In Proceedings of the 2012 International Conference on Biomedical Engineering (ICoBE), Penang, Malaysia, 27–28 February 2012. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devanand, D.B.; Gardiner, M.D.; Kedgley, A.E. A Compact Orthosis Compliance Monitoring Device Using Pressure Sensors and Accelerometers: Design and Proof-of-Concept Testing. Sensors 2025, 25, 1352. https://doi.org/10.3390/s25051352
Devanand DB, Gardiner MD, Kedgley AE. A Compact Orthosis Compliance Monitoring Device Using Pressure Sensors and Accelerometers: Design and Proof-of-Concept Testing. Sensors. 2025; 25(5):1352. https://doi.org/10.3390/s25051352
Chicago/Turabian StyleDevanand, Devi Baruni, Matthew D. Gardiner, and Angela E. Kedgley. 2025. "A Compact Orthosis Compliance Monitoring Device Using Pressure Sensors and Accelerometers: Design and Proof-of-Concept Testing" Sensors 25, no. 5: 1352. https://doi.org/10.3390/s25051352
APA StyleDevanand, D. B., Gardiner, M. D., & Kedgley, A. E. (2025). A Compact Orthosis Compliance Monitoring Device Using Pressure Sensors and Accelerometers: Design and Proof-of-Concept Testing. Sensors, 25(5), 1352. https://doi.org/10.3390/s25051352





