Sensitive Electrochemical Determination of Vanillin Using a Bimetallic Hydroxide and Reduced Graphene Oxide Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of ZIF-67
2.3. Synthesis of NiCoZIF-67
2.4. Synthesis of GO
2.5. Synthesis of NiCo(OH)2.rGO
2.6. Characterization Techniques
2.7. Electrochemical Measurement
2.8. Preparation of the Electrode
3. Results and Discussion
3.1. Physical Characterization
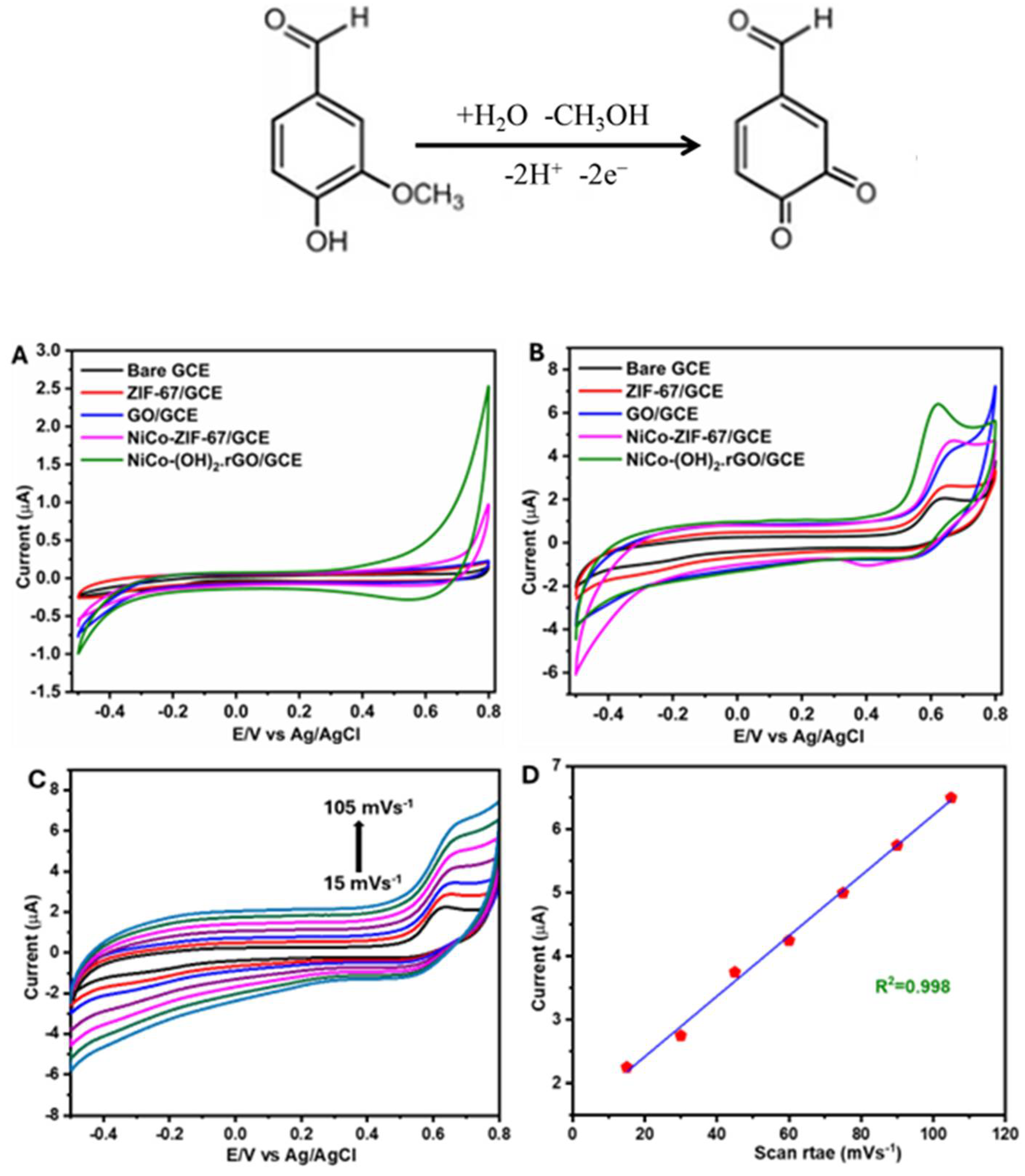
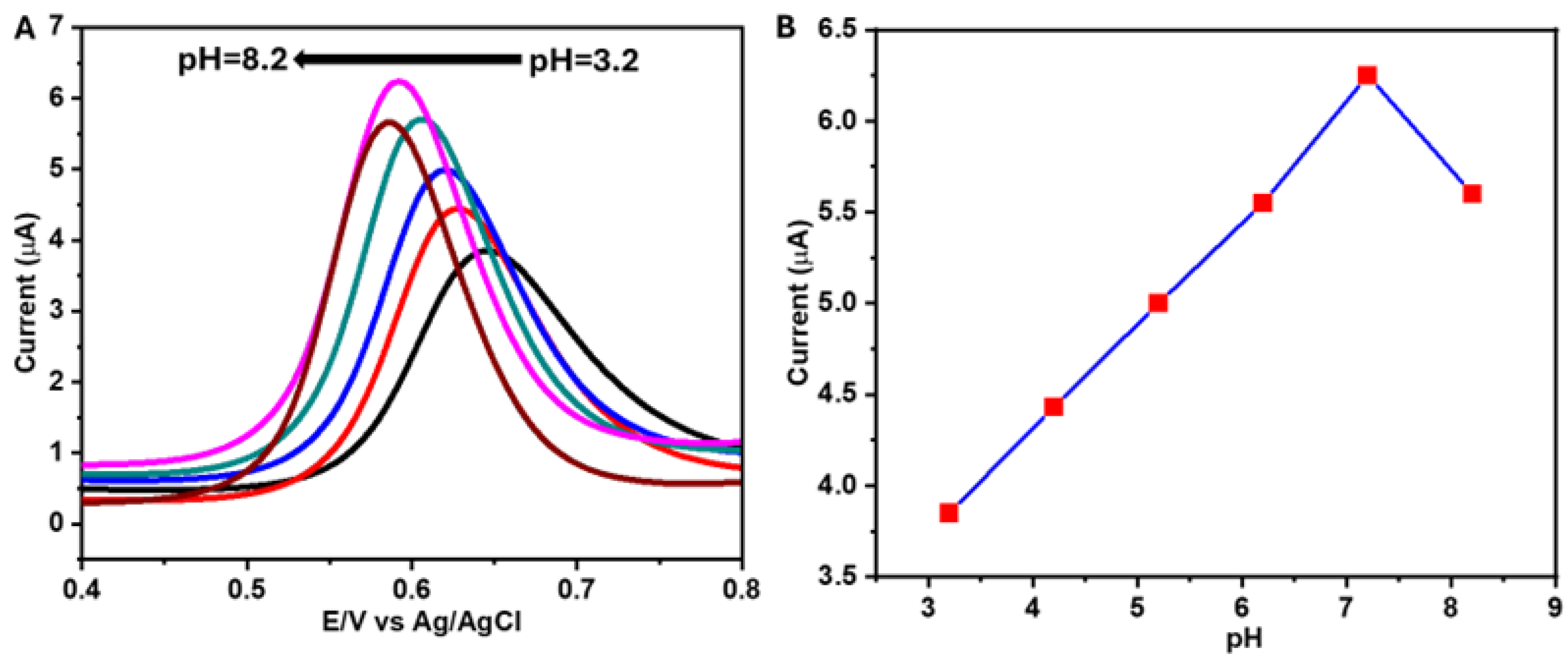
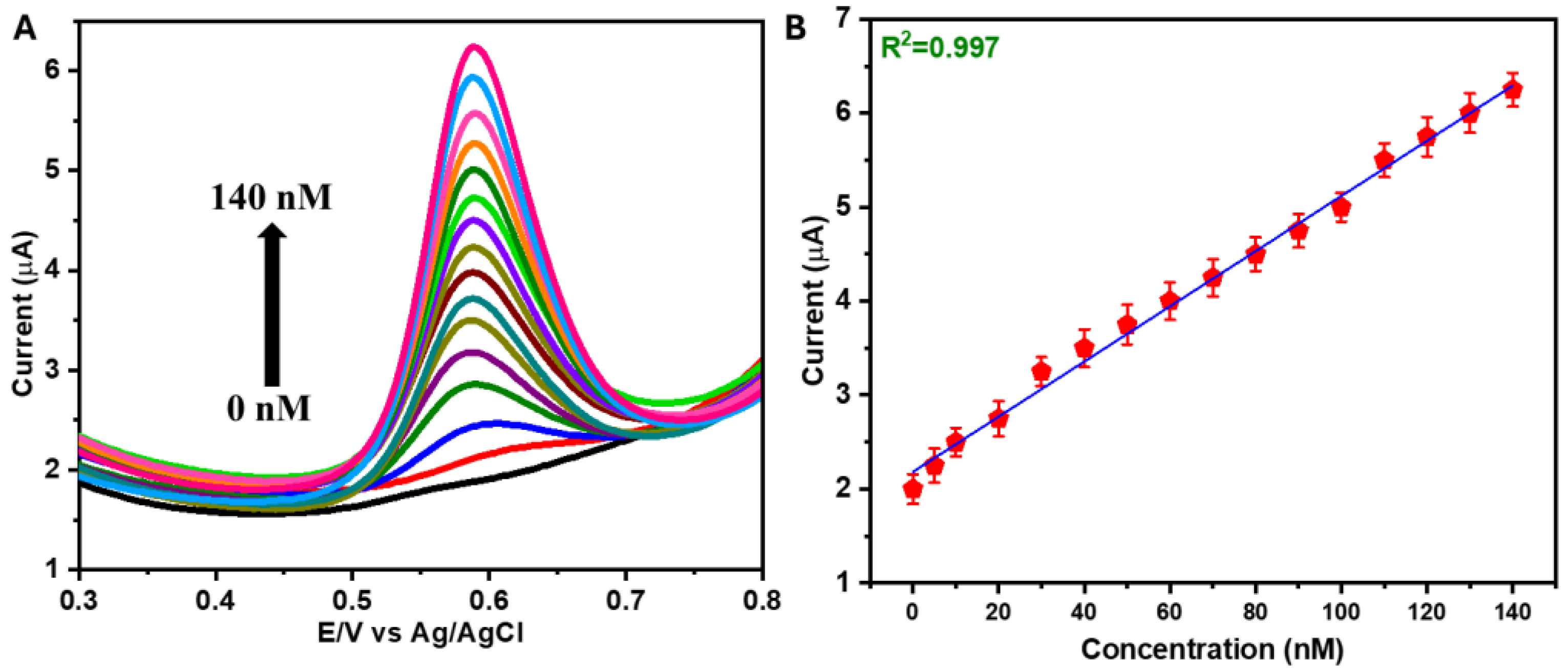
3.2. Electrochemical Studies
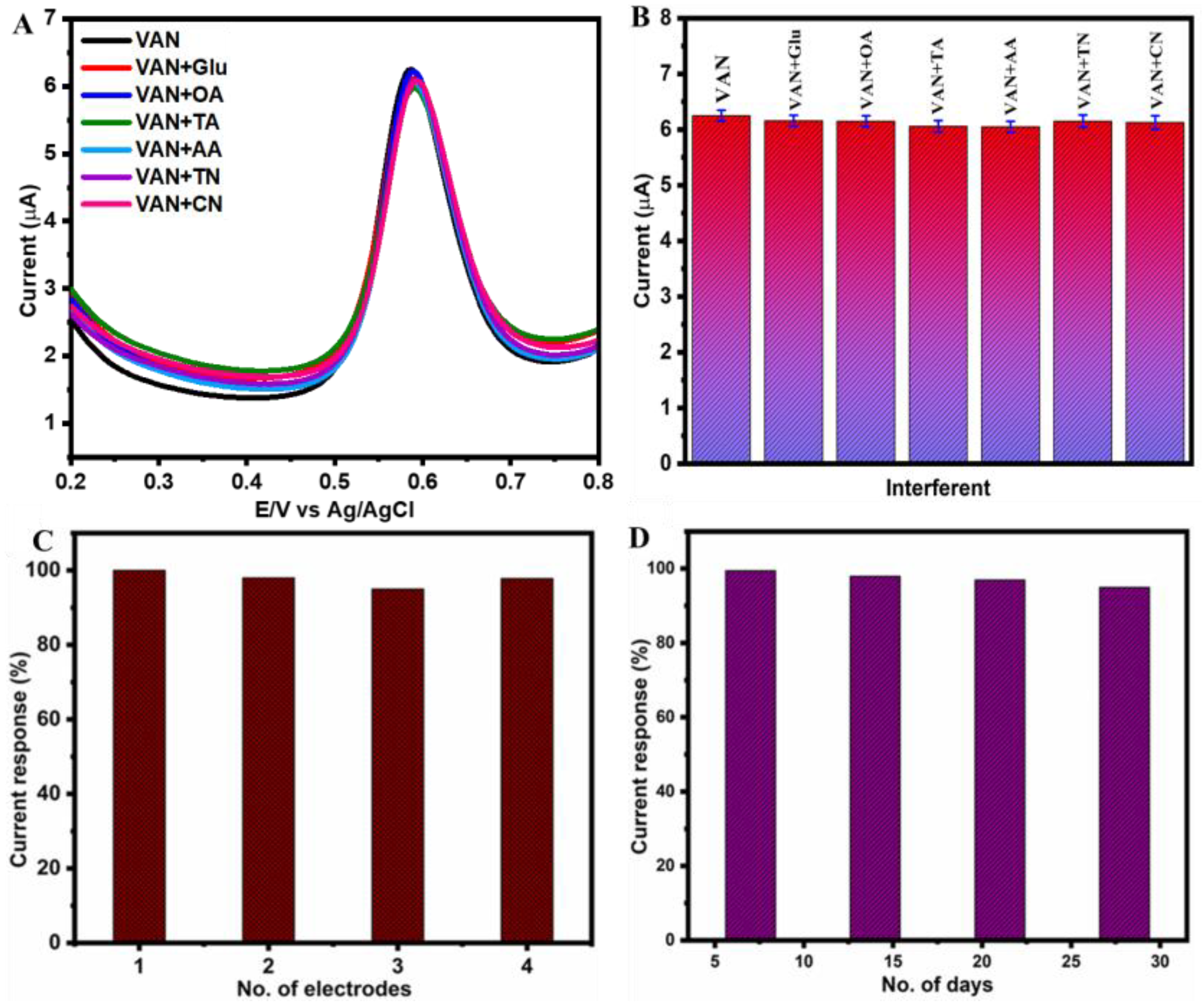
3.3. Selectivity, Reproducibility, and Stability
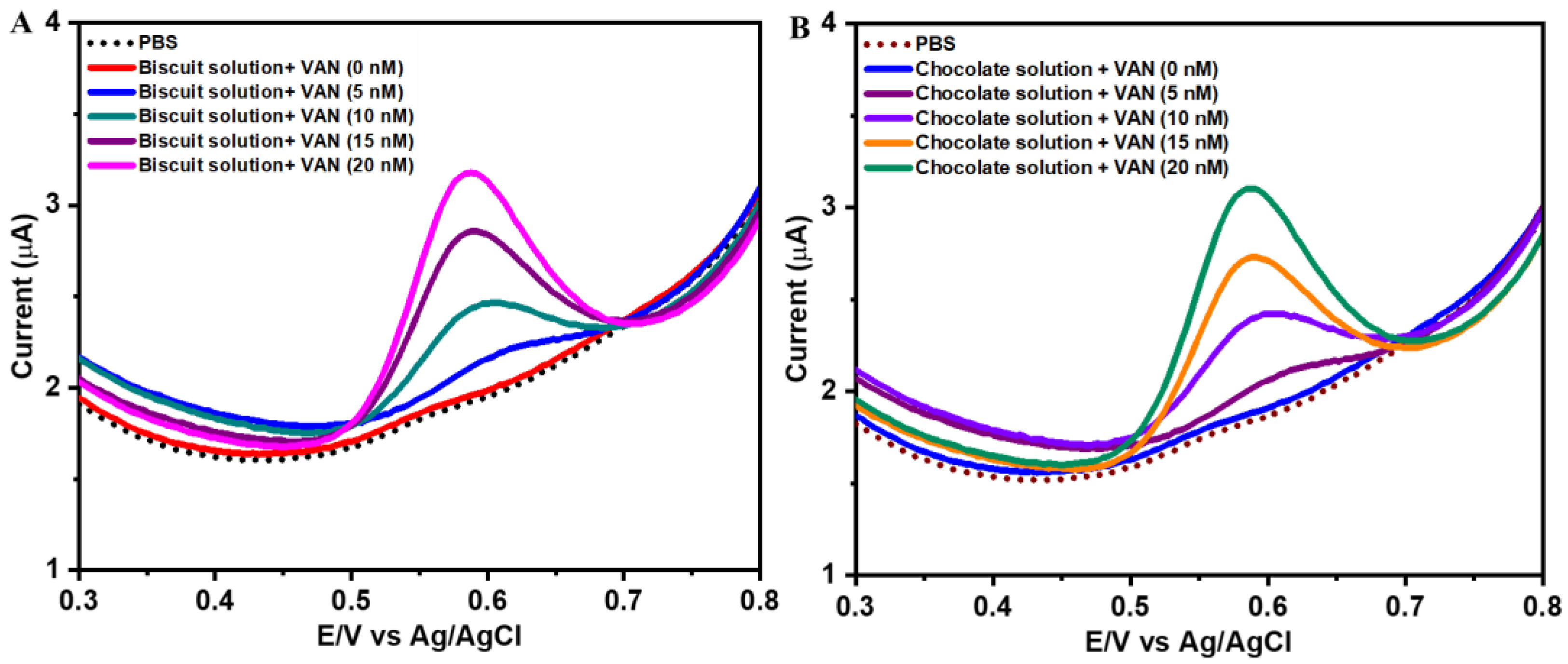
3.4. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziyatdinova, G.; Zhupanova, A.; Davletshin, R. Simultaneous Determination of Ferulic Acid and VAN in Vanilla Extracts Using Voltammetric Sensor Based on Electropolymerized Bromocresol Purple. Sensors 2022, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Calam, T.T.; Taskin, G. Optimization of voltammetric parameters for sensitive and simultaneous determination of ferulic acid and VAN using a glassy carbon electrode based on 2-aminonicotinic acid in the presence of surfactant media. Food Chem. 2024, 436, 137752. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Sriram, B.; Wang, S.F.; Stanley, M.M.; Lin, W.C.; Kogularasu, S.; Chang, G.P.; George, M. Eco-innovative electrochemical sensing for precise detection of VAN and sulfadiazine additives in confectioneries. Appl. Surf. Sci. Adv. 2024, 20, 100584. [Google Scholar] [CrossRef]
- Gupta, R.; Ali, H.; Verma, N. Detection of VAN in food products over cu-laser induced graphene nanocomposite using the combined electrochemistry and UV-visible spectroscopy principles. Microchem. J. 2024, 199, 109984. [Google Scholar] [CrossRef]
- Dehdashtian, N.; Sahidi, S.A.; Hasansarei, A.G.; Hosseini, S.; Ahmadi, M. Trace level monitoring of VAN in food products using a modified electrode amplified with NiO/Nitrogen doped carbon quantum dots nanocomposite. J. Food Meas. Charact. 2024, 18, 117–124. [Google Scholar] [CrossRef]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A food additive with multiple biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Liaqat, F.; Xu, L.; Khazi, M.I.; Ali, S.; Rahman, M.U.; Zhu, D. Extraction, purification, and applications of vanillin: A review of recent advances and challenges. Ind. Crops Prod. 2023, 204, 117372. [Google Scholar] [CrossRef]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Liu, J.; Li, G. Rapid and Sensitive Determination of VAN Based on a Glassy Carbon Electrode Modified with Cu2O-Electrochemically Reduced Graphene Oxide Nanocomposite Film. Sensors 2018, 18, 2762. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Antonova, T.; Davletshin, R. Voltammetric Sensor Based on the Poly(p-Aminobenzoic Acid) for the Simultaneous Quantification of Aromatic Aldehydes as Markers of Cognac and Brandy Quality. Sensors 2023, 23, 2348. [Google Scholar] [CrossRef]
- Firmansyah, D.; Khodizah, A.T.; Ferryansyah, M.A.; Divananda, I.M.; Juliawaty, L.D.; Mujahidin, D.; Yuliarto, B.; Ishikawa, N.; Santria, A. Spectroscopic and Computational Study of VAN-Dipyrrin Ligand that Capable of Colorimetric Sensor for Zinc and Copper Ions. J. Mol. Struct. 2024, 1313, 138718. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, S.C.; Saxena, N.; Chadda, N.; Singh, N.P.; Sinha, A.K. Microwave- and Ultrasound-Assisted Extraction of VAN and Its Quantification by High-Performance Liquid Chromatography in Vanilla Planifolia. J. Sep. Sci. 2006, 29, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Bai, X.; Wang, T.; Shi, Q.; Zhu, J.; Wang, B. One-Step Synthesis of Fluorescent Graphene Quantum Dots as an Effective Fluorescence Probe for VAN Detection. RSC Adv. 2021, 11, 9121–9129. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Feng, Y.; Kuang, G.; Cheng, Z. A Ratio Fluorescence Method Based on Dual Emissive Copper Nanoclusters for the Detection of VAN. J. Fluoresc. 2024, 5, 987–995. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, G.; Kokot, S. Simultaneous Spectrophotometric Determination of Maltol, Ethyl Maltol, VAN and Ethyl VAN in Foods by Multivariate Calibration and Artificial Neural Networks. Food Chem. 2005, 89, 465–473. [Google Scholar] [CrossRef]
- Ohashi, M.; Omae, H.; Hashida, M.; Sowa, Y.; Imai, S. Determination of VAN and Related Flavor Compounds in Cocoa Drink by Capillary Electrophoresis. J. Chromatogr. A 2007, 1138, 262–267. [Google Scholar] [CrossRef]
- Sostaric, T.; Boyce, M.C.; Spickett, E.E. Analysis of the Volatile Components in Vanilla Extracts and Flavorings by Solid-Phase Microextraction and Gas Chromatography. J. Agric. Food Chem. 2000, 48, 5802–5807. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, G.; Wei, X.; Ding, B.; Huang, C.; Xu, B. Determination of VAN and Ethyl-VAN in Milk Powder by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Food Anal. Methods 2016, 9, 3360–3366. [Google Scholar] [CrossRef]
- Remaud, G.S.; Akoka, S. A Review of Flavors Authentication by Position-Specific Isotope Analysis by Nuclear Magnetic Resonance Spectrometry: The Example of VAN. Flavour. Fragr. J. 2017, 32, 77–84. [Google Scholar] [CrossRef]
- Barho, F.B.; Gonzalez-Posada, F.; Milla, M.J.; Bomers, M.; Cerutti, L.; Tournié, E.; Taliercio, T. Highly Doped Semiconductor Plasmonic Nanoantenna Arrays for Polarization Selective Broadband Surface-Enhanced Infrared Absorption Spectroscopy of VAN. Nanophotonics 2017, 7, 507–516. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Alizadeh, M.; Karaman, O.; Vasseghian, Y.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo Dyes Using an Impressive Electrochemical Sensor Based on Carbon Paste Electrode Modified with ZIF-8/g-C3N4/Co and Ionic Liquid in Mouthwash and Toothpaste as Real Samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar]
- Remirez, C.A.; Thiruppathi, A.R.; Ozoemena, O.; Chen, A. Recent advances in graphene oxide-based electrochemical sensor. Can. J. Chem. 2024, 102, 667–681. [Google Scholar] [CrossRef]
- Gimadutdinova, L.; Ziyatdinova, G.; Davletshin, R. Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF. Sensors 2024, 24, 930. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Boateng, E.; Durairaj, S.; Chen, A. Electrochemical Sensing of VAN Based on Fluorine-Doped Reduced Graphene Oxide Decorated with Gold Nanoparticles. Foods 2022, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, P.; Alshawabkeh, R.; Rui, A.; Sun, N.X. A Comprehensive Review of Biomarker Sensors for a Breathalyzer Platform. Sensors 2024, 24, 7263. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Li, H.; Feng, S. Heterojunction of branched benzopyrazine-based polymers coating on graphene for electrochemical sensing of vanillin. Talanta 2024, 277, 126420. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; Zalm, J.V.D.; Chen, A. Graphene-Oxide-Based Electrochemical Sensors for the Sensitive Detection of Pharmaceutical Drug Naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef]
- Hira, S.A.; Nallal, M.; Park, K.H. Fabrication of PdAg Nanoparticle Infused Metal-Organic Framework for Electrochemical and Solution-Chemical Reduction and Detection of Toxic 4-Nitrophenol. Sens. Actuators B Chem. 2019, 298, 126861. [Google Scholar] [CrossRef]
- Zalm, J.V.D.; Quintal, J.; Hira, S.A.; Chen, S.; Chen, A. Recent trends in electrochemical catalyst design for hydrogen evolution, oxygen evolution, and overall water splitting. Electrochim. Acta. 2023, 439, 141715. [Google Scholar] [CrossRef]
- Hira, S.A.; Nagappan, S.; Annas, D.; Kumar, Y.A.; Park, K.H. NO2-Functionalized Metal–Organic Framework Incorporating Bimetallic Alloy Nanoparticles as a Sensor for Efficient Electrochemical Detection of Dopamine. Electrochem. Commun. 2021, 125, 107012. [Google Scholar] [CrossRef]
- Hira, S.A.; Nagappan, S.; Yusuf, M.; Chen, A.; Lee, J.; Park, K.H. L-Cysteine anchored Co-MOF derived cobalt-nitrogen-carbon hierarchical architecture as an efficient sensor for the electrochemical detection of catecholamine. Microchem. J. 2023, 190, 108748. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H.C. Hybrid OER Electrocatalyst Combining Mesoporous Hollow Spheres of N, P-Doped Carbon with Ultrafine Co2NiOx. ACS Appl. Nano Mater. 2022, 5, 11045–11058. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Y.C.; Wang, Y.; Cen, J.; Yang, H.; Chen, X.; Tong, X.; Su, D.; Dong, C.L.; Wang, S. Quinary Defect-Rich Ultrathin Bimetal Hydroxide Nanosheets for Water Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 50324–50332. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, F.; Zhang, S.J.; Fisher, A.; Zhou, Y.; Wang, Z.; Li, Y.; Xu, B.B.; Li, J.T.; Sun, S.G. Interfacial Interaction between FeOOH and Ni-Fe LDH to Modulate the Local Electronic Structure for Enhanced OER Electrocatalysis. ACS Catal. 2018, 8, 11342–11351. [Google Scholar] [CrossRef]
- Abner, S.; Chen, A. Nanostructured Cobalt/Copper catalysts for efficient electrochemical carbon dioxide reduction. Nanoscale 2024, 16, 12967–12981. [Google Scholar] [CrossRef]
- Gonkalves, G.M.; Guimaraes, R.R.; Nunes, C.V.; Duarte, A.; Brandao, B.B.N.; Toma, H.E.; Araki, K. Electrode materials based on α-NiCo(OH)2 and rGO for high performance energy storage devices. RSC Adv. 2016, 6, 102504–102512. [Google Scholar] [CrossRef]
- Cao, W.; Zhao, W.; Xiong, C.; Long, Q.; Chen, N.; Du, G. NiCo-MOF derived nanostructured NiCo-LDH@Ni(OH)2 heterogenous composite as electrode material for hybrid super capacitors. J. Energy Storage 2023, 64, 107213. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Dong, L.; Wang, J.; Wang, L.; Yan, W.; Jang, J. MOF-deviated zinc-nickel–cobalt ZIF-67 electrode material for high-performance symmetrical coin-shaped supercapacitors. J. Colloid Interface Sci. 2020, 574, 140–151. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Mümin, Y. Simultaneous Electrochemical Determination of Caffeine and Vanillin by Using Poly(Alizarin Red S) Modified Glassy Carbon Electrode. Food Anal. Methods 2017, 10, 31–40. [Google Scholar] [CrossRef]
- Huang, L.; Hou, K.; Jia, X.; Pan, H.; Du, M. Preparation of Novel Silver Nanoplates/Graphene Composite and Their Application in VAN Electrochemical Detection. Mater. Sci. Eng. C 2014, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Raril, C.; Manjunatha, J.G. A Simple Approach for the Electrochemical Determination of VAN at Ionic Surfactant Modified Graphene Paste Electrode. Microchem. J. 2020, 154, 104575. [Google Scholar] [CrossRef]
- Rajendran, K.; Nallal, M.; Ganesan, M.; Shanmugasundaram, M.; Hira, S.A.; Gopalakrishnan, G.; Murugan, S.; Aharon, G.; Park, K.H. Fabrication of dual functional 3D-CeVO4/MWNT hybrid nanocomposite as a high-performance electrode material for supercapacitor and L-Tryptophan detection. Electrochim. Acta 2023, 445, 142020. [Google Scholar] [CrossRef]
- Kalaiyarasi, J.; Meenakshi, S.; Pandian, K.; Gopinath, S.C.B. Simultaneous Voltammetric Determination of VAN and Guaiacol in Food Products on Defect Free Graphene Nanoflakes Modified Glassy Carbon Electrode. Microchim. Acta 2017, 184, 2131–2140. [Google Scholar] [CrossRef]
- Durán, G.M.; Llorent-Martínez, E.J.; Contento, A.M.; Ríos, Á. Determination of VAN by Using Gold Nanoparticle-Modified Screen-Printed Carbon Electrode Modified with Graphene Quantum Dots and Nafion. Microchim. Acta 2018, 185, 204. [Google Scholar] [CrossRef]
- Silva, T.R.; Brondani, D.; Zapp, E.; CruzVieira, I. Electrochemical Sensor Based on Gold Nanoparticles Stabilized in Poly(Allylamine Hydrochloride) for Determination of VAN. Electroanalysis 2015, 27, 465–472. [Google Scholar] [CrossRef]
- Chethana, B.K.; Basavanna, S.; Naik, Y.A. Determination of VAN in Real Samples Using Lysine Modified Carbon Paste Electrode. J. Chem. Pharm. Res. 2012, 4(1), 538–545. [Google Scholar]
- Shang, L.; Zhao, F.; Zeng, B. Sensitive Voltammetric Determination of VAN with an AuPd Nanoparticles-Graphene Composite Modified Electrode. Food Chem. 2014, 151, 53–57. [Google Scholar] [CrossRef]
- Peng, J.; Hou, C.; Hu, X. A Graphene-Based Electrochemical Sensor for Sensitive Detection of VAN. Int. J. Electrochem. Sci. 2012, 7, 1724–1733. [Google Scholar] [CrossRef]
- Mei, Q.; Ding, Y.; Li, L.; Wang, A.; Duan, D.; Zhao, Y. Electrospun MoS2 Composite Carbon Nanofibers for Determination of VAN. J. Electroanal. Chem. 2019, 833, 297–303. [Google Scholar]
- Veeramani, V.; Madhu, R.; Chen, S.M.; Veerakumar, P.; Syu, J.J.; Liu, S. Bin Cajeput Tree Bark Derived Activated Carbon for the Practical Electrochemical Detection of VAN. New J. Chem. 2015, 39, 9109–9115. [Google Scholar] [CrossRef]
- Chen, L.; Chaisiwamongkhol, K.; Chen, Y.; Compton, R.G. Rapid Electrochemical Detection of VAN in Natural Vanilla. Electroanalysis 2019, 31, 1067–1074. [Google Scholar] [CrossRef]


| Electrode | Media | Method | Linear Rang (µM) | LOD (µM) | Ref. |
|---|---|---|---|---|---|
| Au-FrGO/GCE | 0.1 M PBS | DPV | 1–150 | 0.15 | [23] |
| Graphene Nanoflake/GCE | 0.1 M PBS | DPV | 0.01–53 | 0.012 | [44] |
| CoS nanorods/GCE | 0.025 M H2SO4 | DPV | 0.5–56 | 0.07 | [45] |
| AuNP-PAH/GCE | 0.2 M ABS | SWV | 0.9–15 | 0.05 | [46] |
| Lysine/CPE | 0.1 M PBS | DPV | 10–100 | 2.88 | [47] |
| AuPd–graphene/GCE | 0.1 M PBS | DPV | 10–40 | 0.02 | [48] |
| Gr/GCE | 0.1 M PBS | DPV | 0.6–48 | 0.05 | [49] |
| MoS2-CNF/GCE | 0.1 M PBS | CA | 0.3–135 | 0.15 | [50] |
| TBAC-900/GCE | 0.1 M PBS | DPV | 5–1150 | 0.68 | [51] |
| CNT-SPE | 0.05 M BRS | DPV | 2.5–750 | 1.03 | [52] |
| NiCo(OH)2.rGO/GCE | 0.1 M PBS | SWV | 0.005–0.14 | 0.006 µM | This Work |
| Sample | Added (nM) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Biscuit | 0 | ND | - | - |
| 5.0 | 5.01 | 100.2 | ||
| 10.0 | 10.03 | 100.3 | 3.12 | |
| 15.0 | 14.97 | 99.8 | ||
| 20.0 | 20.15 | 100.7 | ||
| Chocolate | 0 | ND | - | - |
| 5.0 | 5.03 | 100.6 | ||
| 10.0 | 9.99 | 99.9 | 3.17 | |
| 15.0 | 15.02 | 100.1 | ||
| 20.0 | 20.09 | 100.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hira, S.A.; Quintal, J.; Chen, A. Sensitive Electrochemical Determination of Vanillin Using a Bimetallic Hydroxide and Reduced Graphene Oxide Nanocomposite. Sensors 2025, 25, 1694. https://doi.org/10.3390/s25061694
Hira SA, Quintal J, Chen A. Sensitive Electrochemical Determination of Vanillin Using a Bimetallic Hydroxide and Reduced Graphene Oxide Nanocomposite. Sensors. 2025; 25(6):1694. https://doi.org/10.3390/s25061694
Chicago/Turabian StyleHira, Shamim Ahmed, Jonathan Quintal, and Aicheng Chen. 2025. "Sensitive Electrochemical Determination of Vanillin Using a Bimetallic Hydroxide and Reduced Graphene Oxide Nanocomposite" Sensors 25, no. 6: 1694. https://doi.org/10.3390/s25061694
APA StyleHira, S. A., Quintal, J., & Chen, A. (2025). Sensitive Electrochemical Determination of Vanillin Using a Bimetallic Hydroxide and Reduced Graphene Oxide Nanocomposite. Sensors, 25(6), 1694. https://doi.org/10.3390/s25061694







