Development of an IMU-Based Post-Stroke Gait Data Acquisition and Analysis System for the Gait Assessment and Intervention Tool
Abstract
1. Introduction
- Development of an IMU-Based Gait Data Acquisition System: Proposed a wearable device based on inertial measurement units (IMUs) that can collect real-time gait data from stroke patients, overcoming the limitations of traditional gait assessment tools;
- Simple and Accurate Calibration Process: Designed a straightforward calibration method to mitigate drift errors in IMU data, ensuring the accuracy and reliability of the measurements;
- Application of Convolutional Neural Networks (CNNs): Utilized a CNN model for gait data analysis, enabling accurate prediction of gait parameters and providing real-time feedback;
- Analysis of Eight Stages of the Gait Cycle: Clearly defined the eight stages of the gait cycle and developed a scoring formula based on these stages, enhancing the objectivity of gait assessment;
- High Correlations with Physician Observations: Demonstrated significant correlations between G.A.I.T. scores calculated from IMU data and those assessed by physicians, validating the system’s effectiveness;
- Clinical Application Potential: Provided a tool for gait training in daily life, assisting healthcare teams in establishing patient-specific functional mobility benchmarks, thereby improving recovery outcomes for stroke patients;
- Advancement of Gait Assessment Technology: Promoted the application of gait assessment technology in clinical practice through small, lightweight wearable devices, offering new directions for future research and practice.
2. Materials and Methods
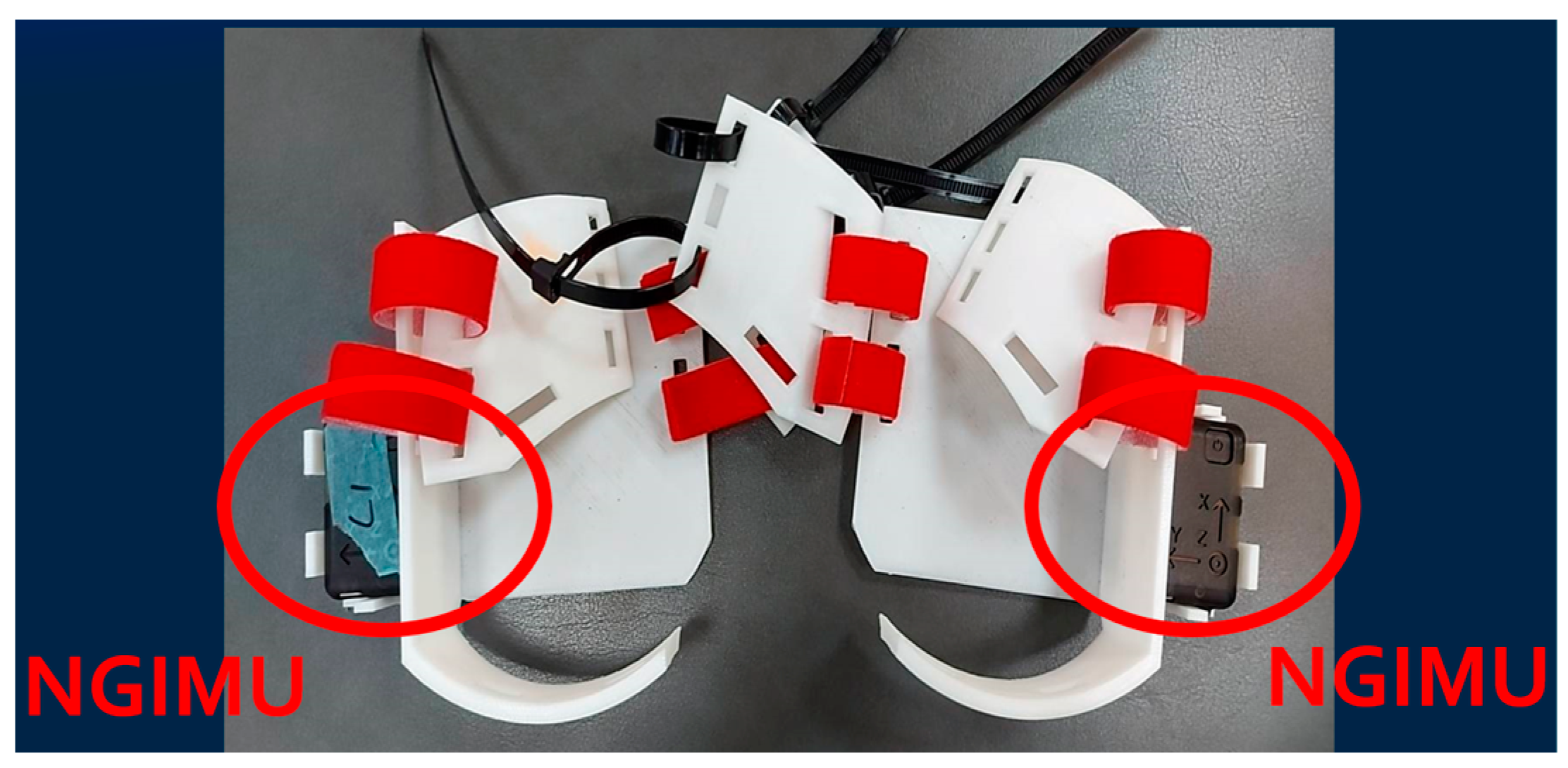
2.1. IMU Sensor
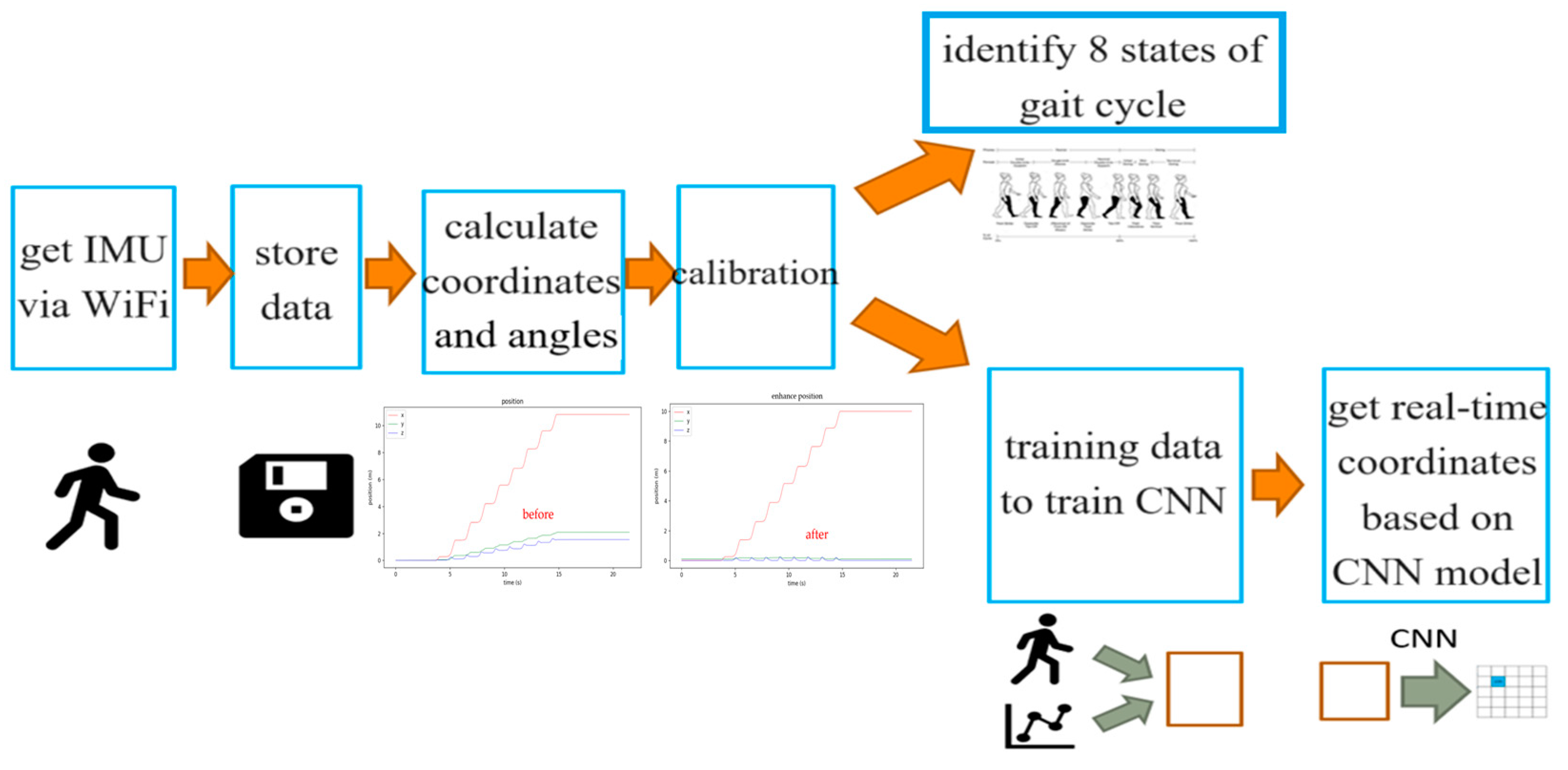
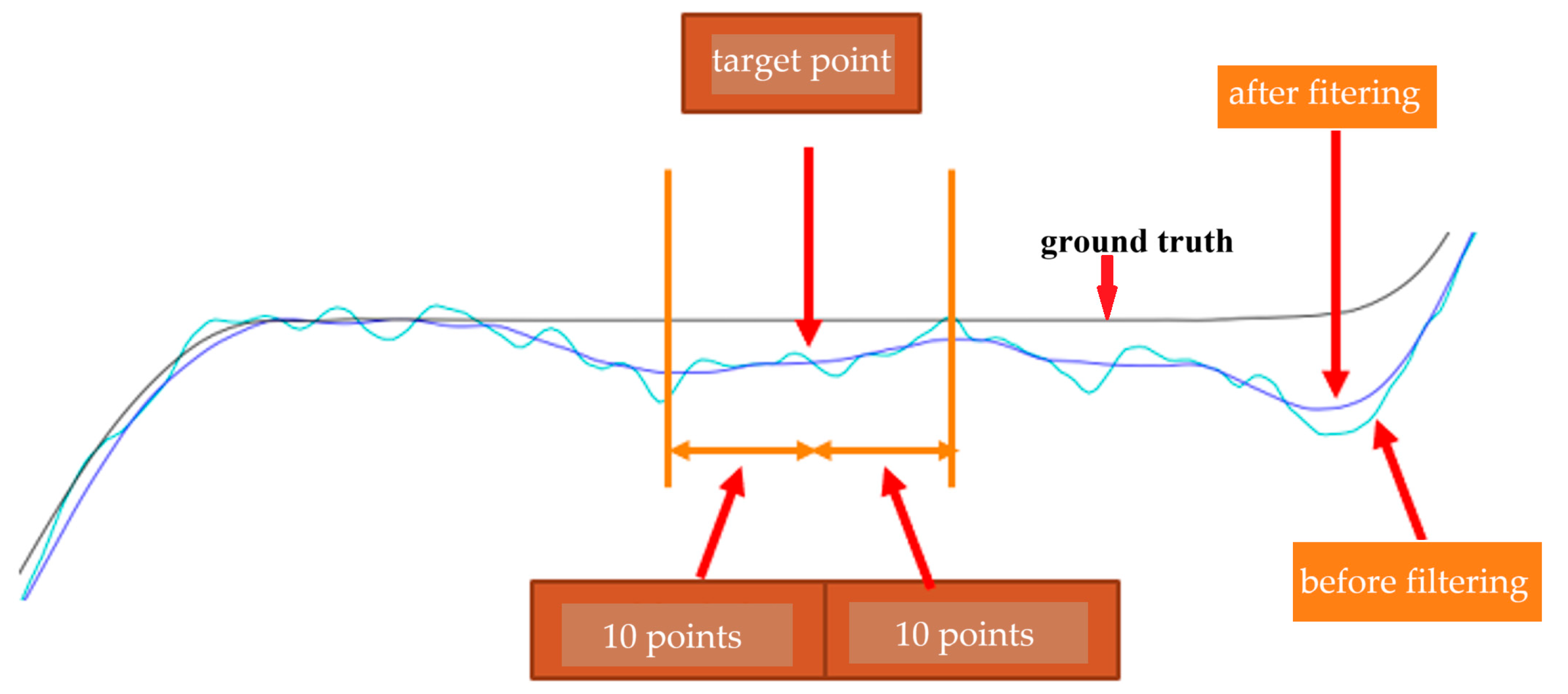
2.2. Data Analysis Procedure
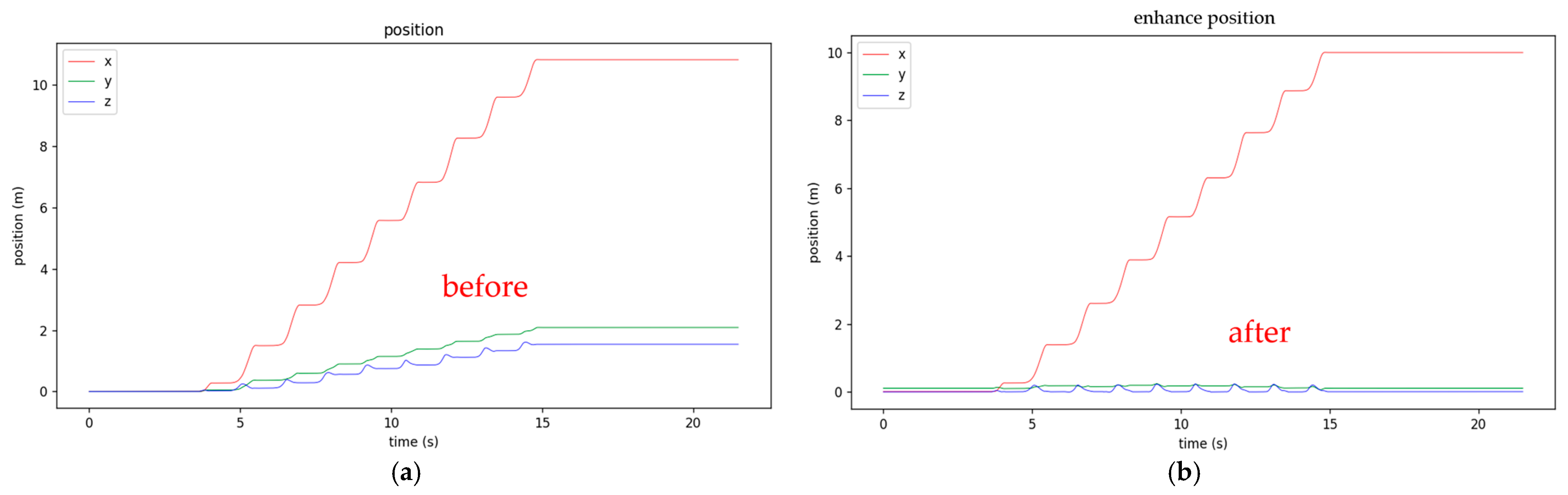
2.3. Three-Axis XYZ Distance Calibration
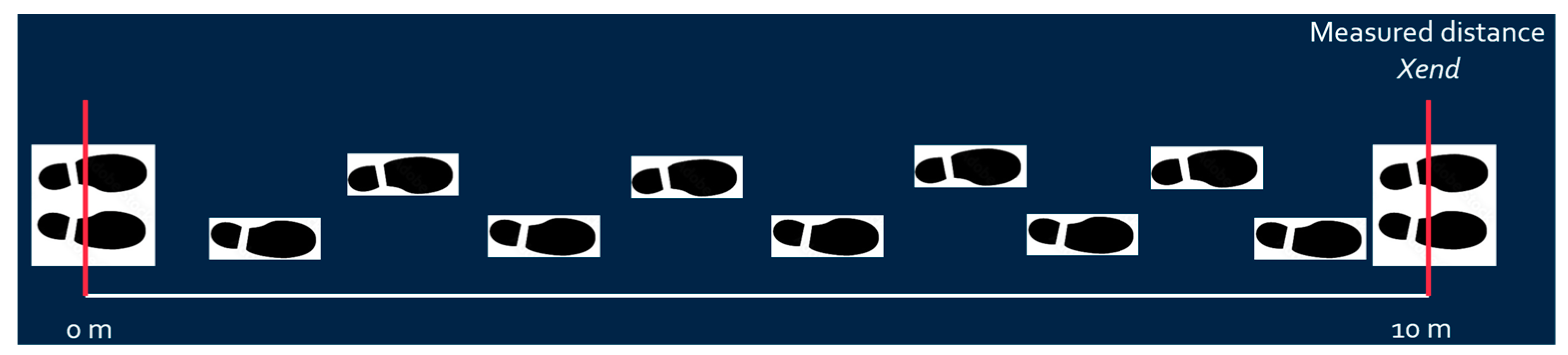
- First, fix the walking x-axis linear distance (e.g., ten meters for post-stroke patient to walk), mark the starting and ending lines, and ideally draw a straight line between the starting and ending lines. The subject should walk between the starting and ending points;
- X-axis distance calibration: The predetermined x-axis length value (e.g., 10 m) calibrates the calculated x distance based on Equation (1) as shown in Figure 6.where is the measured x-axis distance obtained from IMU, is the measured x-axis distance at the end point, and is the calibrated x-axis distance;
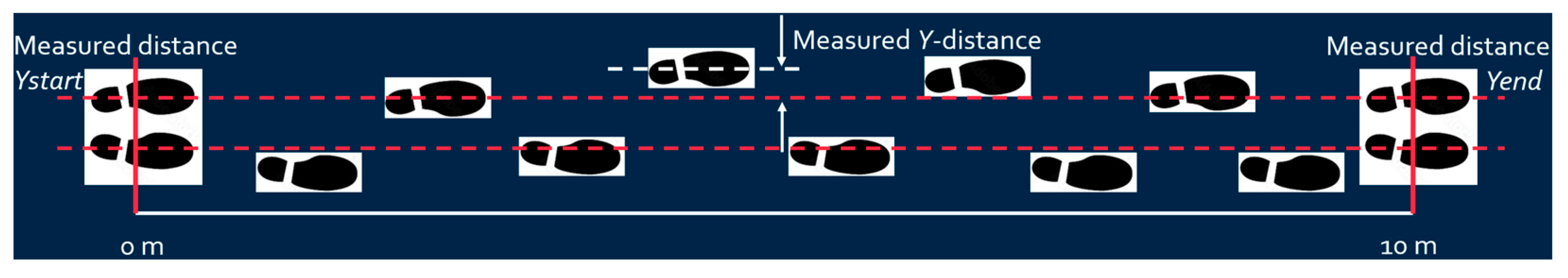
- Y-axis distance calibration: Set the Y distance to zero when both feet stand at the starting point. Assume the Y distance at the end of the walk is approximately the same as at the starting point (since walking along the x-axis line), and calibrate the Y-distance accordingly based on Equation (2) as shown in Figure 7.where is the measured Y-axis distance obtained from IMU, and are the measured Y-axis distances at the end and starting points, respectively, and is the calibrated Y-axis distance. Since the walking lane has starting and end lines, it is reasonable to assume the patient begins and finishes walking with both feet standing at the starting and end line. The measured and are supposed to be the same, and the difference between these two values can be used to calibrate the measured Y-axis distance;
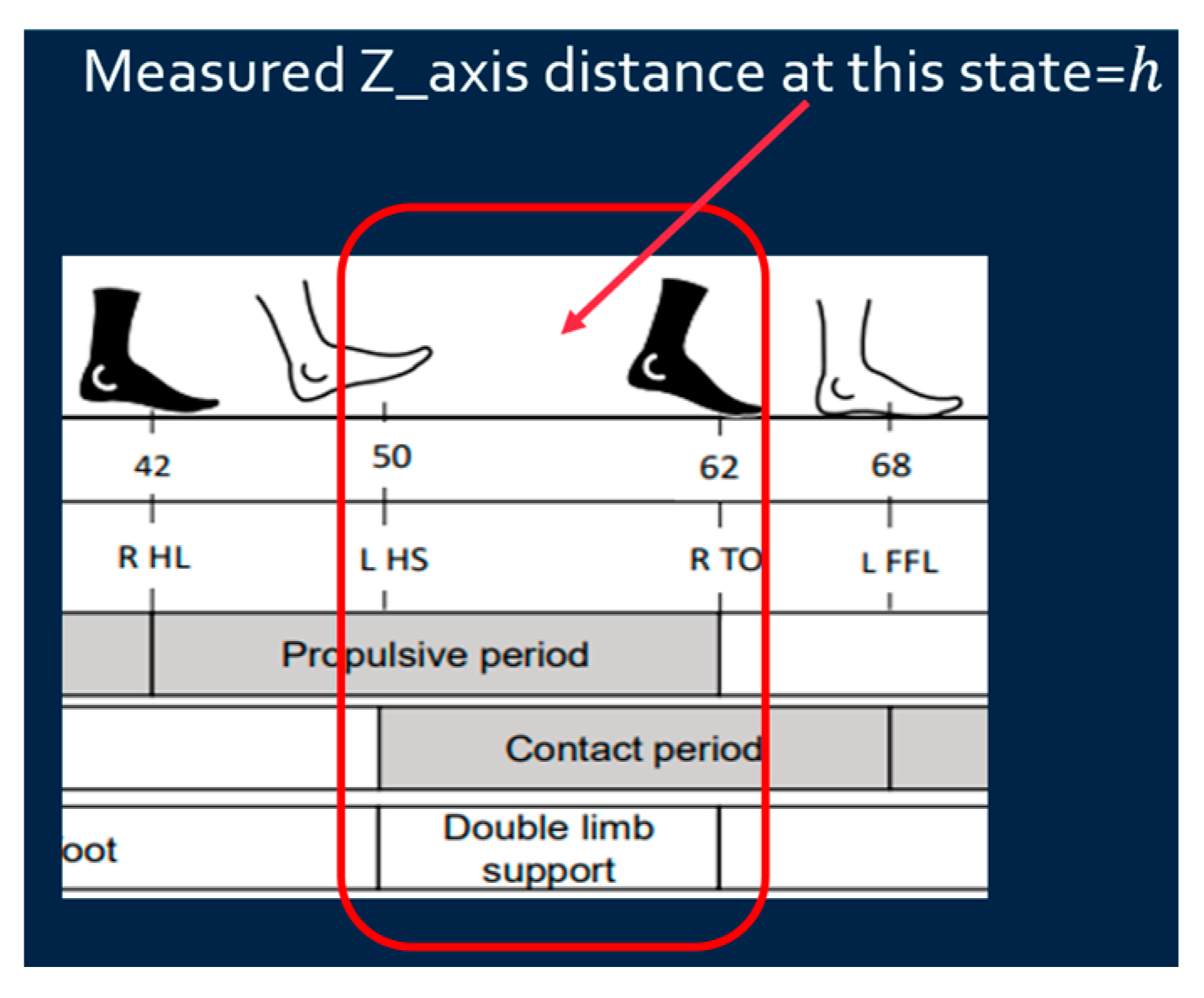
- Z-axis distance calibration: Since walking is on a flat floor, the Z-distance when the foot is fully on the ground (stationary) is regarded as zero to calibrate the z-distance based on Equation (3) as shown in Figure 8.where is the measured Z-axis distance obtained from IMU, is the measured Z-axis distance at double limb support state, and is the calibrated Z-axis distance. Since the Z-axis distance (foot height) is supposed to be zero at the double limb support state, the difference between the measured Z-axis distance at this state and zero can be used to calibrate the measured Z-axis distance.
2.4. Determining the Eight States of the Gait Cycle
- Initial Contact (Heel Strike): The movement begins with the heel of the primary test foot touching the ground (start of first double support);
- Loading Response: The end of the first double support with the heel of the secondary test foot at its highest point under the condition that the X-distance of the secondary test foot is almost not changing;
- Mid-stance: The secondary test foot reaches the body’s center point (the secondary test foot position surpasses the primary test foot);
- Terminal Stance: The heel of the secondary test foot touches the ground (start of the second double support);
- Pre-swing: The end of the second double support phase with the heel of the primary test foot at its highest point under the condition that the X distance of the primary test foot is almost not changing;
- Initial Swing (Toe-off): The highest point of the primary test foot (maximum knee flexion);
- Mid-swing: The primary test foot is parallel to the floor;
- Terminal Swing: The heel of the primary test foot touches the ground.
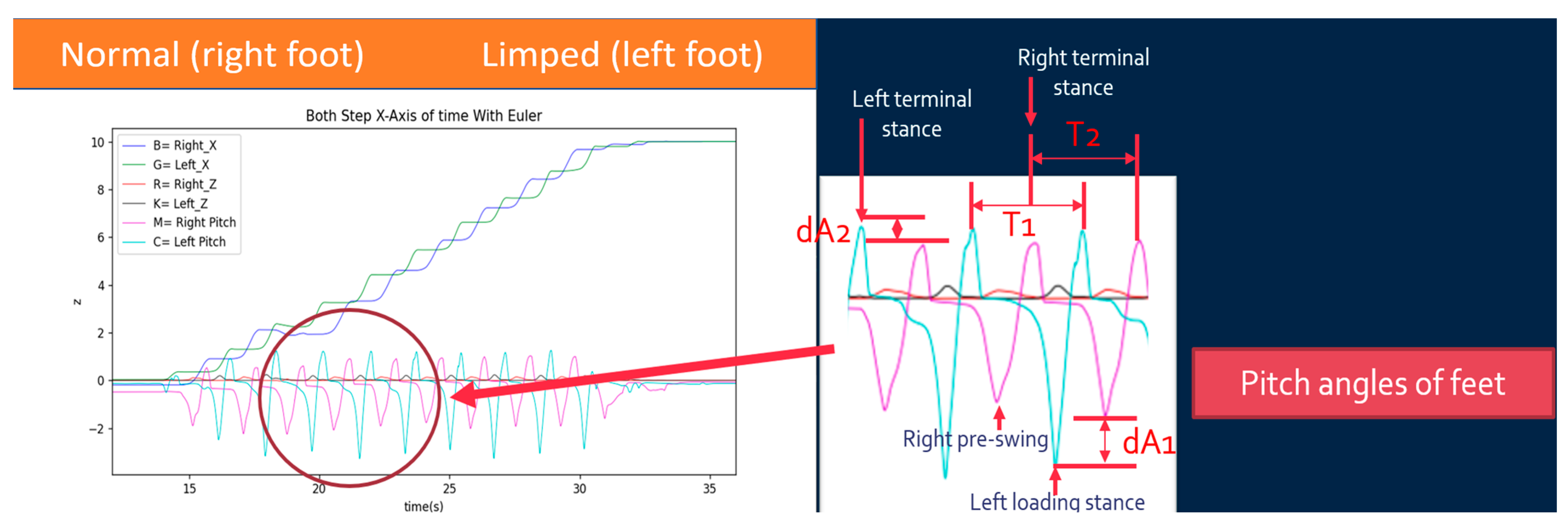
- Initial Contact: The maximum point of the right pitch angle;
- Loading Response: The minimum point of the left pitch angle;
- Mid-stance: The left x-axis position surpasses the right x-axis position;
- Terminal Stance: The maximum point of the left pitch angle;
- Pre-swing: The minimum point of the right pitch angle;
- Initial Swing: The maximum point of the right Z-axis height;
- Mid-swing: The right pitch angle returns to zero;
- Terminal Swing: The maximum point of the right pitch angle.
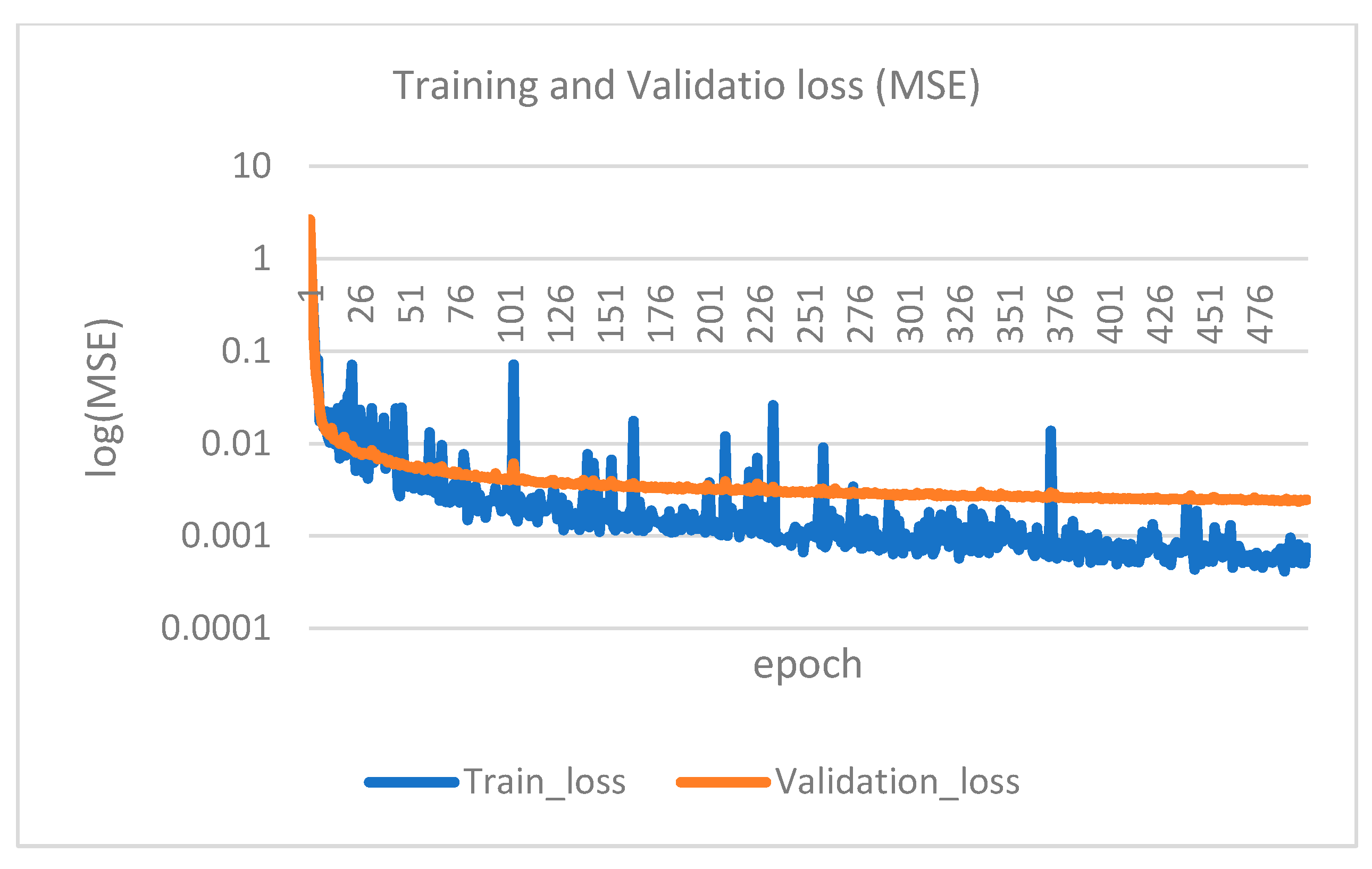
2.5. Convolutional Neural Network (CNN)
2.6. Gait Assessment and Intervention Tool (G.A.I.T.)
- Maximum pitch angle of left foot step i:
- Minimum pitch angle of left foot step i:
- Maximum pitch angle of right foot step i:
- Minimum pitch angle of right foot step i:
3. Results
3.1. XYZ Distance Calibration
3.2. Eight States of Gait Cycle
3.3. Convolutional Neural Network (CNN)
3.4. G.A.I.T. Score
4. Discussion
- Gait Cycle Time: The time between two consecutive landings of the same foot;
- One Step Move Time: The time between alternating landings of the left and right feet;
- Gait Cycle Length: The step length between two consecutive landings of the same foot;
- One Step Move Length: The distance between alternating landings of the left and right feet;
- Angle At Stop: The foot angle when a single foot stops during a step.
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Alali, S.I.I.I.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-stoke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 22, 650024. [Google Scholar]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [PubMed]
- Chan, C. Golden Recovery Period. Available online: https://www.strokefund.org.hk/en/golden-recovery-period/?form=MG0AV3 (accessed on 3 March 2025).
- Ofran, Y.; Karniel, N.; Tsenter, J.; Schwartz, I.; Protnoy, S. Functional Gait Measurement Prediction by Spatiotemporal and Gait Symmetry in Individuals Post Stroke. J. Dev. Phys. Disabil. 2019, 31, 611–622. [Google Scholar]
- Nadeau, S.; Betschart, M.; Bethoux, F. Gait Analysis for Poststroke Rehabilitation: The Relevance of Biomechanical Analysis and the Impact of Gait Speed. Phys. Med. Rehabil. Clin. 2013, 24, 265–276. [Google Scholar]
- Hsu, A.L.; Tang, P.F.; Jan, M.H. Analysis of Impairments Influencing Gait Velocity and Asymmetry of Hemiplegic Patients after Mild to Moderate Stroke. Arch. Phys. Med. Rehabil. 2003, 84, 1185–1193. [Google Scholar]
- Kim, C.M.; Eng, J.J. The Relationship of Lower-extremity Muscle Torque to Locomotor Performance in People with Stroke. Phys. Ther. 2003, 83, 49–57. [Google Scholar] [PubMed]
- Nadeau, S.; Arsenault, A.B.; Gravel, D.; Bourbonnais, D. Analysis of the Clinical Factors Determining Natural and Maximal Gait Speeds in Adults with A Stroke1. Am. J. Phys. Med. Rehabil. 1999, 78, 123–130. [Google Scholar] [CrossRef]
- Eng, J.J.; Chu, K.S.; Dawson, A.S.; Kim, C.M. Hepburn KE. Functional Walk Tests in Individuals with Stroke: Relation to Perceived Exertion and Myocardial Exertion. Stroke 2002, 33, 756–761. [Google Scholar]
- Daly, J.; Nethery, J.; McCabe, J.; Brenner, I.; Rogers, J.; Gansen, J.; Butler, K.; Burdsall, R.; Roenigk, K.; Holcomb, J. Development and Testing of the Gait Assessment and Intervention Tool (GAIT): A Measure of Coordinated Gait Components. J. Neurosci. Methods 2009, 178, 334–339. [Google Scholar]
- Toro, B.; Nester, C.; Farren, P. A Review of Observational Gait Assessment in Clinical Practice. Physiother. Theory Pract. 2003, 19, 137–149. [Google Scholar]
- Wallmann, H.W. Introduction to Observational Gait Analysis. Home Health Care Manag. Pract. 2009, 22, 66–68. [Google Scholar]
- Ferrarello, F.; Bianchi, V.A.M.; Baccini, M.; Rubbieri, G.; Mossello, E.; Cavallini, M.C.; Marchionni, N.; Di Bari, M. Tools for Observational Gait Analysis in Patients with Stroke: A systematic review. Phys. Ther. 2013, 93, 1673–1685. [Google Scholar] [PubMed]
- Turani, N.; Kemiksizoglu, A.; Karatas, M.; Özker, R. Assessment of Hemiplegic Gait using the Wisconsin Gait Scale. Scand. J. Caring Sci. 2004, 18, 103–108. [Google Scholar] [PubMed]
- Nishi, Y.; Iluno, K.; Takamura, Y.; Minamikawa, Y.; Moriloa, S. Modeling the Heterogeneity of Post-Stroke Gait Control in Free-Living Environments Using a Personalized Causal Network. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 3522–3530. [Google Scholar]
- Faisal, A.I.; Deen, M.J. Systematic Development of a Simple Human Gait Index. IEEE Rev. Biomed. Eng. 2024, 17, 229–242. [Google Scholar]
- Faisal, A.I.; Majumder, S.; Scott, R.; Mondal, T.; Cowan, D.; Deen, M.J. A Simple, Low-cost Multi-sensor-Based Smart Wearable Knee Monitoring System. IEEE Sens. J. 2021, 21, 8253–8266. [Google Scholar]
- Lencioni, T.; Carpinella, I.; Rabuffetti, M.; Marzegan, A.; Ferrarin, M. Human Kinematic, Kinetic and EMG Data During Different Walking and Stair Ascending and Descending Tasks. Sci. Data 2019, 6, 309. [Google Scholar]
- Nadeau, S.; Duclos, C.; Bouyer, L.; Richards, C.L. Guiding task-oriented gait training after stroke or spinal cord injury (SCI) by means of a biomechanical gait analysis. In Progress in Brain Research; Green, A.M., Chapman, C.E., Kalaskav, J., Lepore, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 161–180. [Google Scholar]
- Harris, G.F.; Benson, L.; Riedel, S.; Acharaya, K.; Matesi, D.; Millar, E.A. Biomechanical Assessment Techniques in Rehabilitation. In Proceedings of the 1990 IEEE Colloquium in South America, Argentina, Brazil, Chile, Uruguay, 31 August–15 September 1990; pp. 31–34. [Google Scholar]
- Chen, C.J. Gait Analysis—Comparison of Wearable and Non-Wearable Device Systems. Available online: https://mag.longgood.com.tw/ (accessed on 30 September 2022).
- Tunca, C.; Pehlivan, N.; Ak, N.; Arnrich, B.; Salur, G.; Ersoy, C. Inertial Sensor-Based Robust Gait Analysis in Non-Hospital Settings for Neurological Disorders. Sensors 2017, 17, 825. [Google Scholar] [CrossRef]
- Lee, H.; Guan, L.; Burne, J.A. Human Gait and Posture Analysis for Diagnosing Neurological Disorders. In Proceedings of the 2000 IEEE International Conference on Image Processing, Vancouver, BC, Canada, 10–13 September 2000; Volume 2, pp. 435–438. [Google Scholar]
- Lau, H.; Tong, K.; Zhu, H. Support Vector Machine for Classification of Walking Conditions of Persons after Stroke with Dropped Foot. Hum. Mov. Sci. 2009, 28, 504–514. [Google Scholar]
- Scheffer, C.; Cloete, T. Inertial Motion Capture in Conjunction with an Artificial Neural Network Can Differentiate the Gait Patterns of Hemiparetic Stroke Patients Compared with Able-bodied Counterparts. Comp. Methods Biomech. Biomed. Eng. 2012, 15, 285–294. [Google Scholar] [CrossRef]
- Zhou, Y.; Romijnders, R.; Hansen, C.; van Campen, J.; Maetzler, W.; Hortobágyi, T.; Lamoth, C.J.C. The Detection of Age Groups by Dynamic Gait Outcomes Using Machine Learning Approaches. Sci. Rep. 2020, 10, 4426. [Google Scholar]
- Iosa, M.; Capodaglio, E.; Pelà, S.; Persechino, B.; Morone, G.; Antonucci, G.; Paolucci, S.; Panigazzi, M. Artificial Neural Network Analyzing Wearable Device Gait Data for Identifying Patients with Stroke Unable to Return to Work. Front. Neurol. 2021, 12, 561. [Google Scholar]
- Seo, J.W.; Kim, S.G.; Kim, J.I.; Ku, B.; Kim, K.; Lee, S.; Kim, J.U. Principal Characteristics of Affected and Unaffected Side Trunk Movement and Gait Event Parameters during Hemiplegic Stroke Gait with IMU Sensor. Sensors 2020, 20, 7338. [Google Scholar] [CrossRef]
- Anwary, A.R.; Yu, H.; Vassallo, M. An Automatic Gait Feature Extraction Method for Identifying Gait Asymmetry Using Wearable Sensors. Sensors 2018, 18, 676. [Google Scholar] [CrossRef]
- Luo, B.; Wang, Z.; Wang, D.; Chen, L.; Ma, X. Algorithm for Gait Parameters Estimation Based on Heel-Mounted Inertial Sensors. IEEE Sens. J. 2024, 24, 24723–24736. [Google Scholar]
- Lin, C.H.; Peng, C.W.; Lee, I.J.; Wu, C.M.; Lin, B.S. Analysis of Gait Data Based on Deep Learning to Predict Multiple Balance Scale Scores. IEEE Sens. J. 2024, 24, 920–930. [Google Scholar]
- Rokhmanova, N.; Pearl, O.; Kuchenbecker, K.J.; Halilaj, E. IMU-Based Kinematics Estimation Accuracy Affects Gait Retraining Using Vibrotactile Cues. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 1005–1012. [Google Scholar] [PubMed]
- Guo, Y.; Hutabarat, Y.; Owaki, D.; Hayashibe, M. Speed-Variable Gait Phase Estimation During Ambulation via Temporal Convolutional Network. IEEE Sens. J. 2024, 24, 5224–5236. [Google Scholar]
- Sole Motion. Available online: https://solemotion.com.au/the-video-gait-analysis/ (accessed on 11 January 2025).
- NGIMU. Available online: https://x-io.co.uk/ngimu/ (accessed on 11 January 2025).
- Daly, J.J.; McCabe, J.P.; Gor-García-Fogeda, M.D.; Nethery, J.C. Update on an Observational, Clinically Useful Gait Coordination Measure: The Gait Assessment and Intervention Tool (G.A.I.T.). Brain Sci. 2022, 12, 1104. [Google Scholar] [CrossRef]







| Subject # | Score by Doctor | Score by IMU |
|---|---|---|
| 1 | 0 | 0.2732 |
| 2 | 4 | 0.7906 |
| 3 | 5 | 0.7266 |
| 4 | 3 | 0.3859 |
| 5 | 3 | 0.3620 |
| 6 | 5 | 0.5506 |
| 7 | 10 | 1.1644 |
| 8 | 6 | 1.1418 |
| 9 | 3 | 0.5846 |
| 10 | 3 | 0.4490 |
| 11 | 9 | 4.0168 |
| 12 | 9 | 2.3792 |
| 13 | 3 | 0.7701 |
| 14 | 0 | 0.3567 |
| 15 | 0 | 0.1239 |
| 16 | 0 | 0.2664 |
| 17 | 4 | 0.4509 |
| 18 | 0 | 0.1600 |
| 19 | 3 | 0.3820 |
| 20 | 0 | 0.2640 |
| 21 | 5 | 0.7700 |
| 22 | 0 | 0.2070 |
| 23 | 7 | 0.8810 |
| 24 | 3 | 0.4000 |
| 25 | 0 | 0.1640 |
| 26 | 0 | 0.1860 |
| Mean | Std. Deviation | |
|---|---|---|
| Score by doctor | 3.35 | 3.032 |
| Score by IMU | 0.7174 | 0.83552 |
| Score by Doctor | Score by IMU | ||
|---|---|---|---|
| Score by doctor | Pearson Correlation | 1.000 | 0.752 |
| Sig. (2-tailed) | 0.000 | ||
| Score by IMU | Pearson Correlation | 0.752 | 1.000 |
| Sig. (2-tailed) | 0.000 |
| Score by Doctor | Score by IMU | |||
|---|---|---|---|---|
| Spearman’s rho | Score by doctor | Correlation Coefficient | 1.000 | 0.933 |
| Sig. (2-tailed) | 0.000 | |||
| Score by IMU | Correlation Coefficient | 0.933 | 1.000 | |
| Sig. (2-tailed) | 0.000 |
| Left Foot (std. dev., Mean) | Right Foot (std. dev., Mean) | |
|---|---|---|
| Gait Cycle Time | (0.08744, 1.35300) | (0.03945, 1.34750) |
| One Step Move Time | (0.04715, 0.61750) | (0.01795, 0.71833) |
| Gait Cycle Length | (0.05711, 1.20995) | (0.02178, 1.33088) |
| One Step Move Length | (0.10636, 0.58199) | (0.10360, 0.66631) |
| Angle At Stop | (0.53145, 2.75407) | (0.94228, −1.17001) |
| Left Foot (std. dev., Mean) | Right Foot (std. dev., Mean) | |
|---|---|---|
| Gait Cycle Time | (0.12664, 1.85375) | (0.11065, 1.83958) |
| One Step Move Time | (0.07505, 0.86346) | (0.11515, 0.97821) |
| Gait Cycle Length | (0.13804, 0.69266) | (0.07912, 0.63808) |
| One Step Move Length | (0.18417, 0.15301) | (0.19998, 0.47711) |
| Angle At Stop | (3.35361, −11.98528) | (0.54522, −2.18445) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Huang, Y.-J.; Han, C.-C.; Cheng, Y.-Y.; Chang, C.-S. Development of an IMU-Based Post-Stroke Gait Data Acquisition and Analysis System for the Gait Assessment and Intervention Tool. Sensors 2025, 25, 1994. https://doi.org/10.3390/s25071994
Wu Y-C, Huang Y-J, Han C-C, Cheng Y-Y, Chang C-S. Development of an IMU-Based Post-Stroke Gait Data Acquisition and Analysis System for the Gait Assessment and Intervention Tool. Sensors. 2025; 25(7):1994. https://doi.org/10.3390/s25071994
Chicago/Turabian StyleWu, Yu-Chi, Yu-Jung Huang, Chin-Chuan Han, Yuan-Yang Cheng, and Chao-Shu Chang. 2025. "Development of an IMU-Based Post-Stroke Gait Data Acquisition and Analysis System for the Gait Assessment and Intervention Tool" Sensors 25, no. 7: 1994. https://doi.org/10.3390/s25071994
APA StyleWu, Y.-C., Huang, Y.-J., Han, C.-C., Cheng, Y.-Y., & Chang, C.-S. (2025). Development of an IMU-Based Post-Stroke Gait Data Acquisition and Analysis System for the Gait Assessment and Intervention Tool. Sensors, 25(7), 1994. https://doi.org/10.3390/s25071994








