Dynamic Coherent Diffractive Imaging with Modulus Enforced Probe and Low Spatial Frequency Constraints
Abstract
:1. Introduction
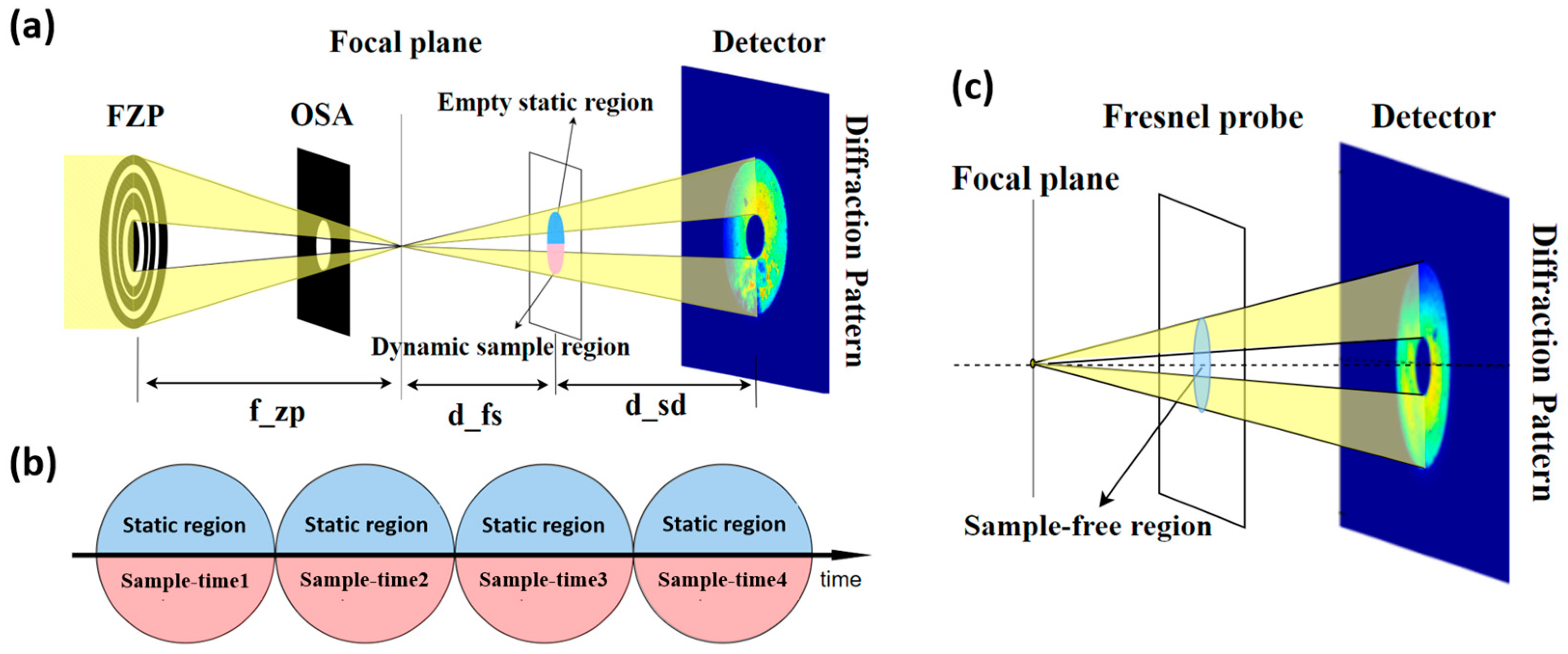
2. Methods
2.1. Static Region Constraint
2.2. Modulus Enforced Probe
2.3. Transfer of Low-Frequency Information
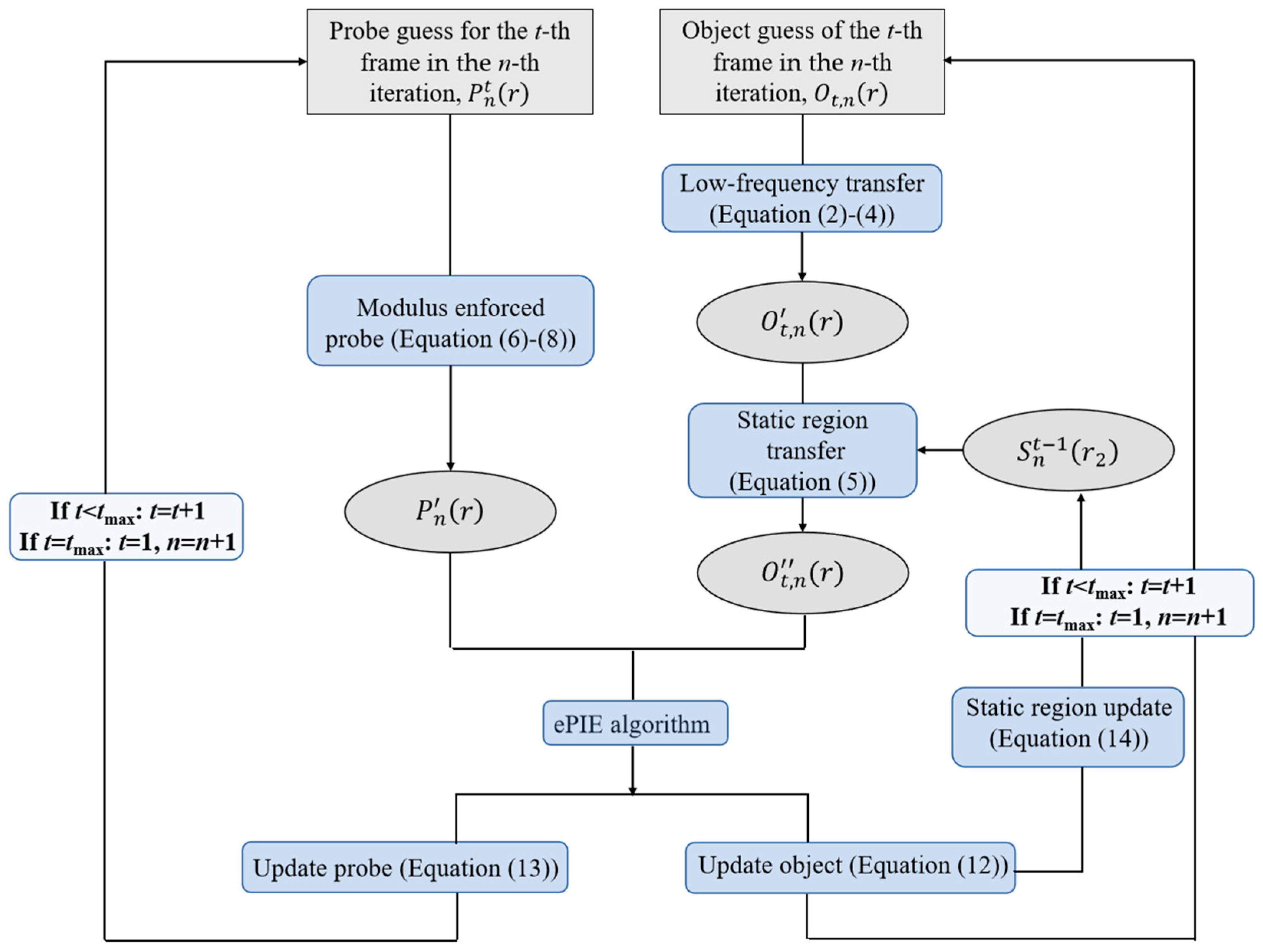
2.4. DynCDI Phase Retrieval Algorithm
3. Results
3.1. Simulation
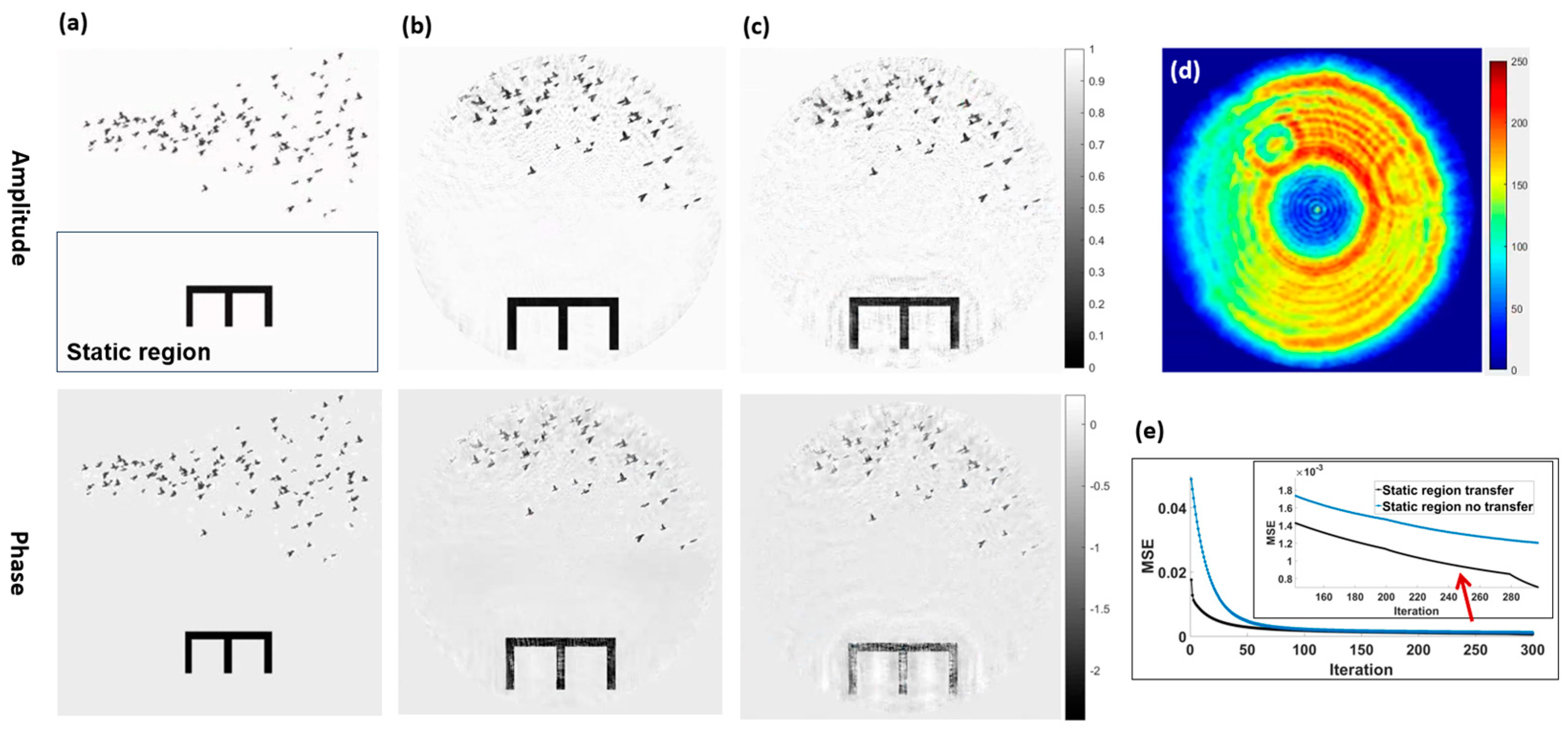
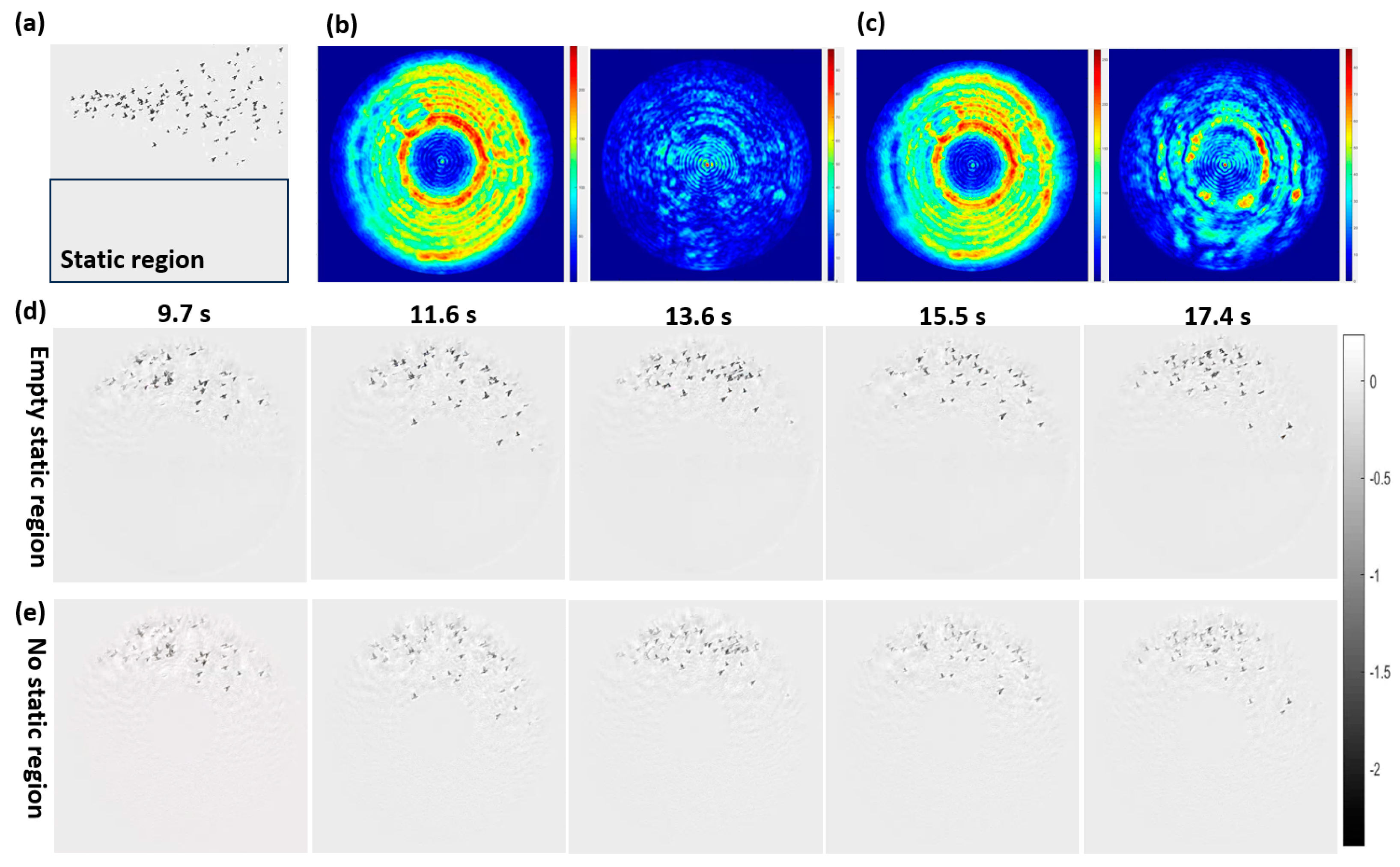
3.1.1. Verification of Information Transfer in Static Region
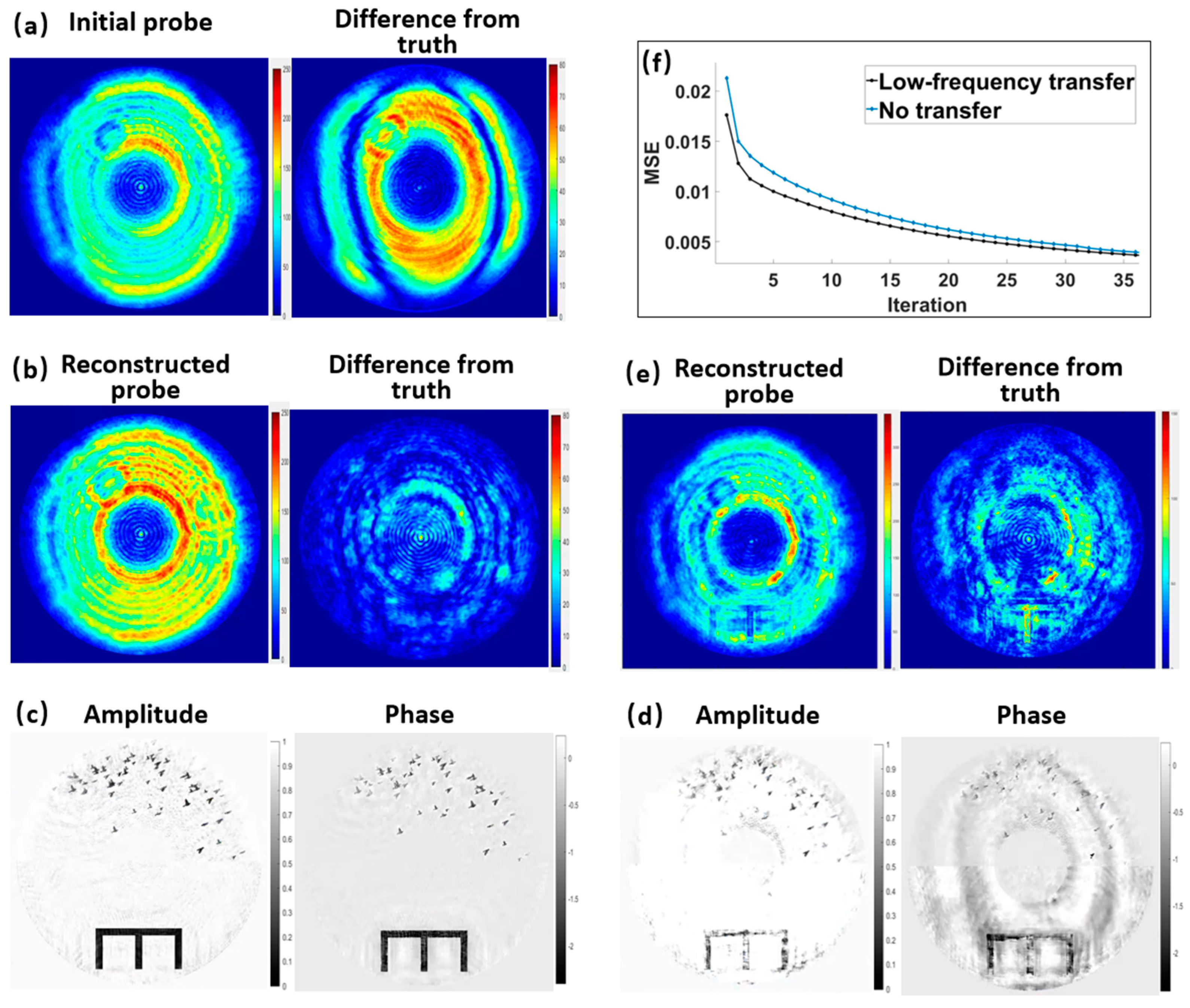
3.1.2. Verification of the MEP Constraint and Low-Frequency Information Transfer
3.1.3. Verification of Structureless Static Region Constraint
3.2. Experiment
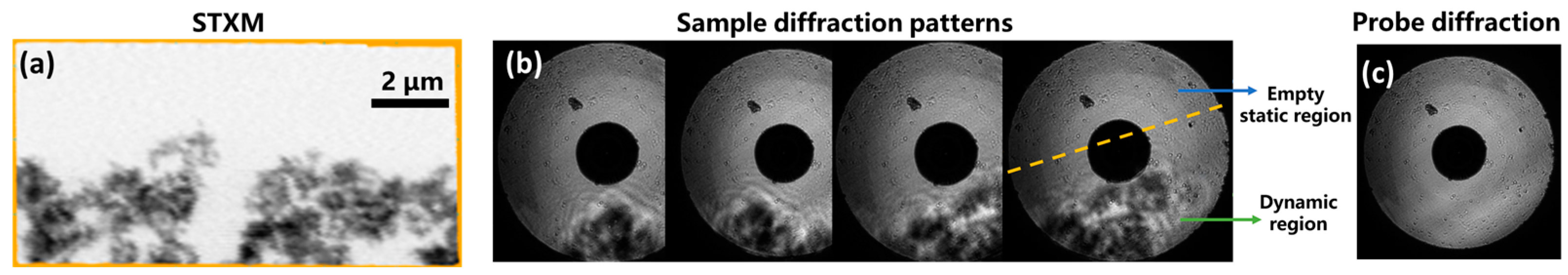
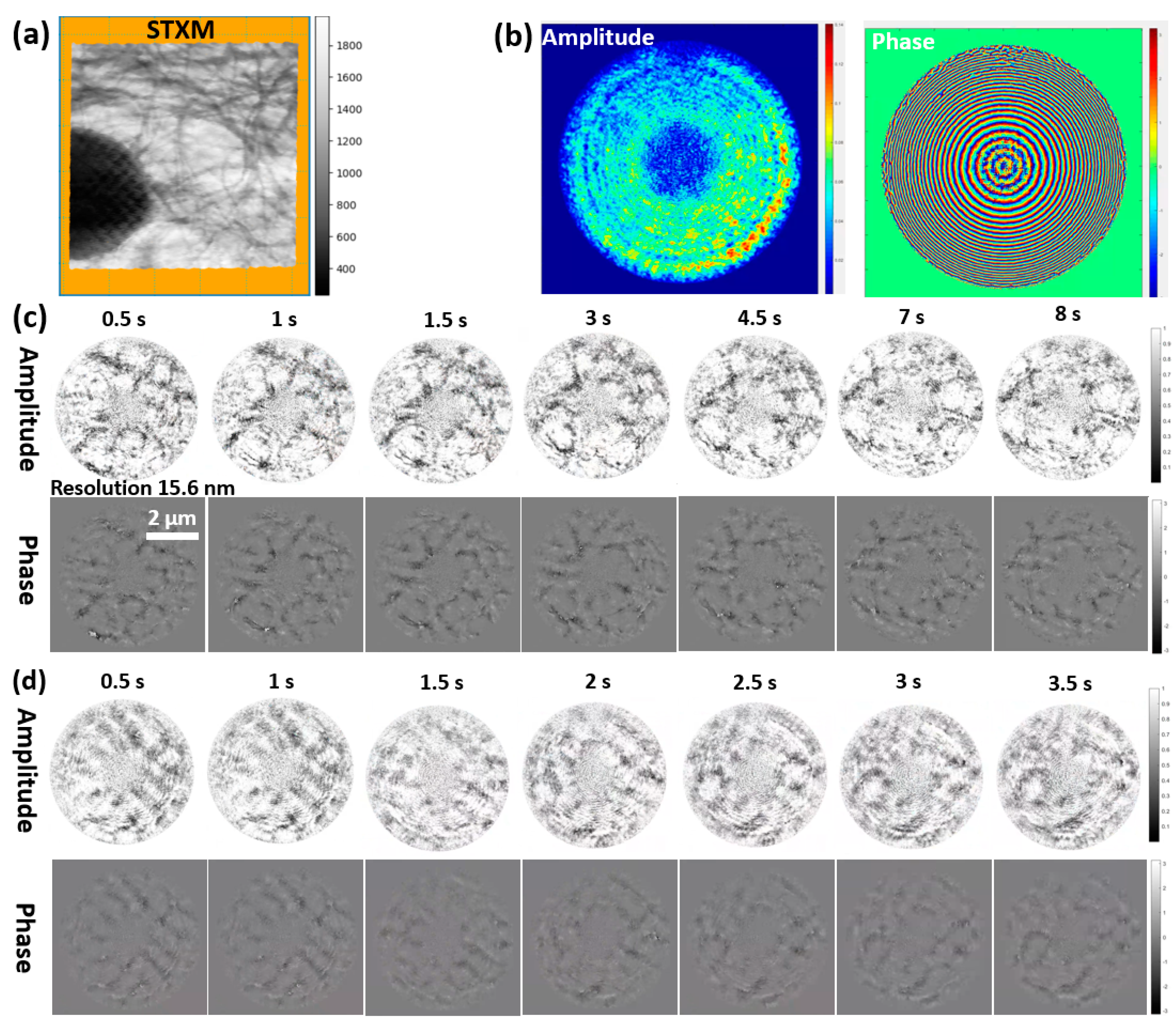
3.2.1. Verification Experiment of DynCDI with a Moving Object
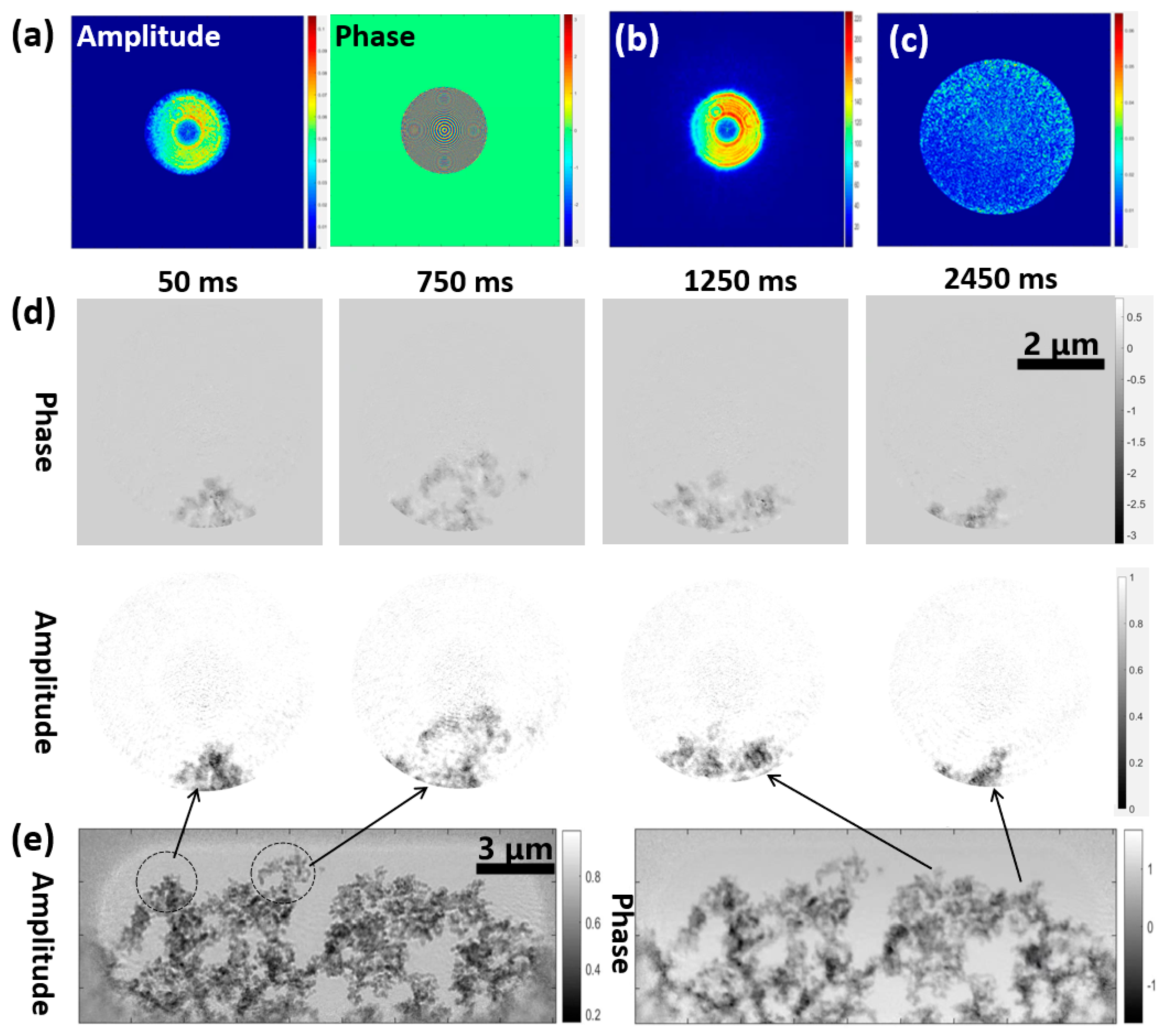
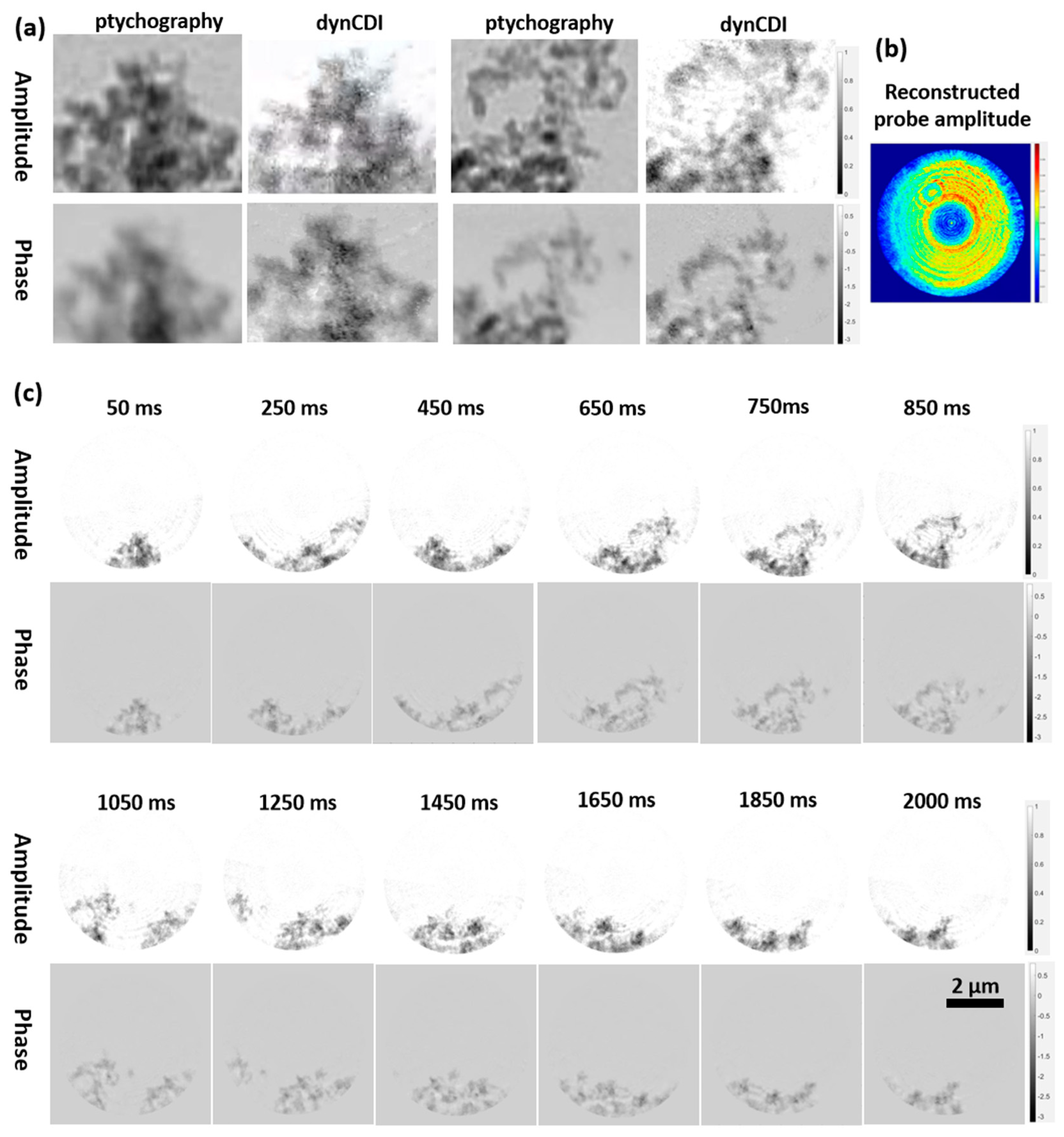
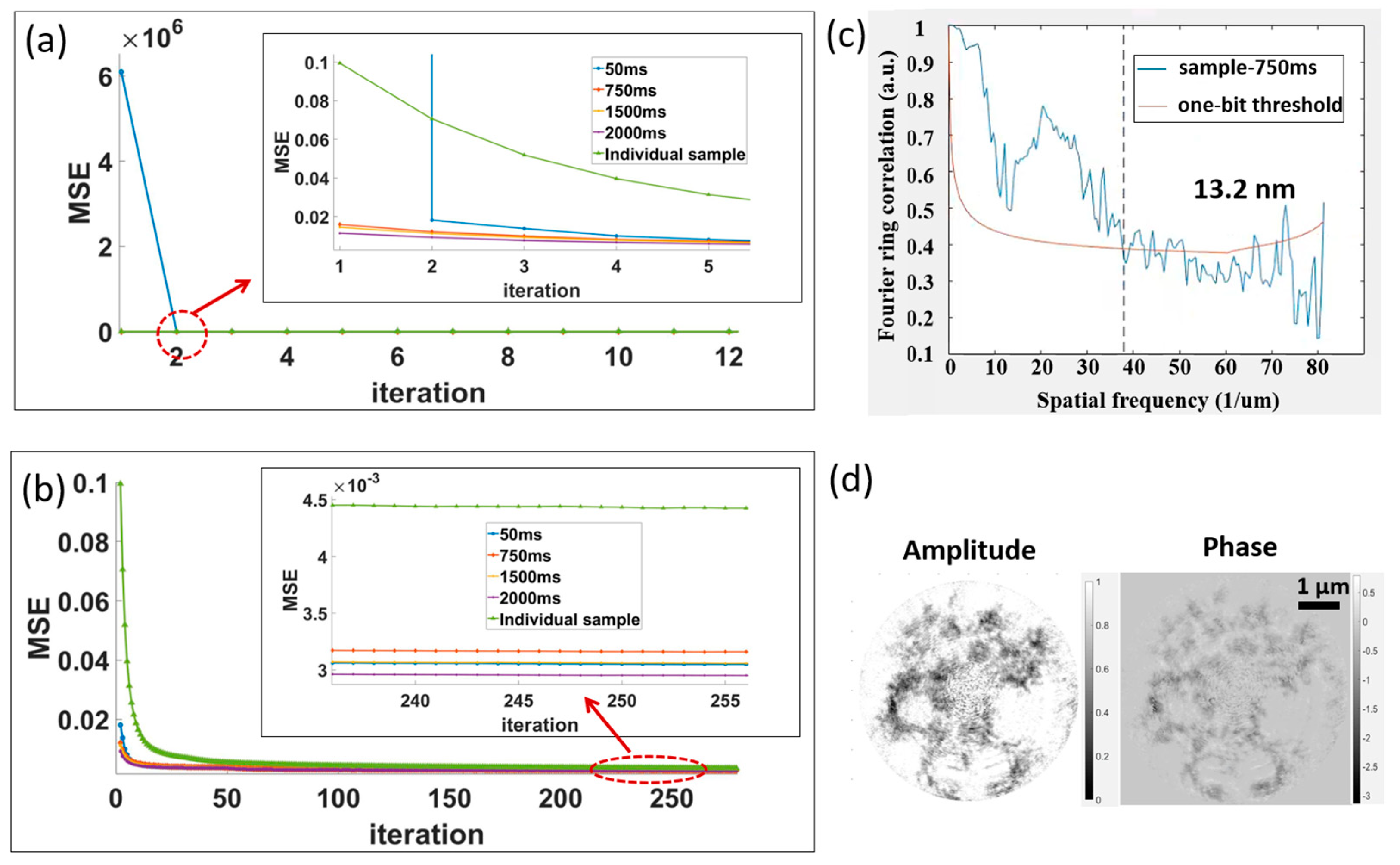
3.2.2. Application Experiment: Stretching a Carbon Fiber Film
3.2.3. Application Experiment: Heating Fe3O4 Nanoparticles Attached to a Copper Grid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uesugi, K.; Mayama, H.; Morishima, K. Analysis of Rowing Force of the Water Strider Middle Leg by Direct Measurement Using a Bio-Appropriating Probe and by Indirect Measurement Using Image Analysis. Cyborg. Bionic Syst. 2023, 4, 0061. [Google Scholar]
- Fan, Z.; Liu, Y.; Ye, Y.; Liao, Y. Functional Probes for the Diagnosis and Treatment of Infectious Diseases. Aggregate 2024, 5, e620. [Google Scholar] [CrossRef]
- Wang, B.; Chen, W.; Qian, J. Single-Shot Super-Resolved Fringe Projection Profilometry (SSSR-FPP): 100,000 Frames-Per-Second 3D Imaging with Deep Learning. Light Sci. Appl. 2025, 14, 70. [Google Scholar] [PubMed]
- Wang, S.; Wu, X.; Zhang, Y.; Zhang, D.; Xie, B.; Pan, Z.; Peng, T. Discovery of a Highly Efficient Nitroaryl Group for Detection of Nitroreductase and Imaging of Hypoxic Tumor Cells. Org. Biomol. Chem. 2021, 19, 3469–3478. [Google Scholar]
- Olbinado, M.P.; Cantelli, V.; Mathon, O.; Pascarelli, S.; Grenzer, J.; Pelka, A.; Roedel, M.; Prencipe, I.; Garcia, A.L.; Helbig, U.; et al. Ultra High-Speed X-ray Imaging of Laser-Driven Shock Compression Using Synchrotron Light. J. Phys. D. Appl. Phys. 2018, 51, 055601. [Google Scholar]
- Xu, X.; Luo, Q.; Wang, J.; Song, Y.; Ye, H.; Zhang, X.; Shi, G. Large-Field Objective Lens for Multi-Wavelength Microscopy at Mesoscale and Submicron Resolution. Opto-Electron. Adv. 2024, 7, 230212. [Google Scholar]
- Ma, N.; Fang, X.; Zhang, Y.; Xing, B.; Duan, L.; Lu, J.; Ma, D. Enhancing the Sensitivity of Spin-Exchange Relaxation-Free Magnetometers Using Phase-Modulated Pump Light with External Gaussian Noise. Opt. Express 2024, 32, 33378–33390. [Google Scholar]
- Luan, S.; Yu, X.; Lei, S.; Ma, C.; Wang, X.; Xue, X.; Zhu, B. Deep Learning for Fast Super-Resolution Ultrasound Microvessel Imaging. Phys. Med. Biol. 2023, 68, 245023. [Google Scholar]
- Prosekov, P.A.; Nosik, V.L.; Blagov, A.E. Methods of Coherent X-Ray Diffraction Imaging. Crystallogr. Rep. 2021, 66, 867–882. [Google Scholar] [CrossRef]
- Miao, J.; Ishikawa, T.; Robinson, I.K.; Murnane, M.M. Beyond Crystallography: Diffractive Imaging Using Coherent X-Ray Light Sources. Science 2015, 348, 530–535. [Google Scholar]
- Williams, G.J.; Quiney, H.M.; Dhal, B.B.; Tran, C.Q.; Nugent, K.A.; Peele, A.G.; Paterson, D.; de Jonge, M.D. Fresnel Coherent Diffractive Imaging. Phys. Rev. Lett. 2006, 97, 025506. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.J.; Quiney, H.M.; Peele, A.G.; Nugent, K.A. Fresnel Coherent Diffractive Imaging: Treatment and Analysis of Data. New J. Phys. 2010, 12, 035020. [Google Scholar] [CrossRef]
- Abbey, B.; Nugent, K.A.; Williams, G.J.; Clark, J.N.; Peele, A.G.; Pfeifer, M.A.; Jonge, D.M.; Mcnuity, I. Keyhole Coherent Diffractive Imaging. Nat. Phys. 2008, 4, 394–398. [Google Scholar] [CrossRef]
- Miao, J.; Charalambous, P.; Kirz, J.; Sayre, D. Extending the Methodology of X-ray Crystallography to Allow Imaging of Micrometre-Sized Non-Crystalline Specimens. Nature 1999, 400, 342–344. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, J.; Zhao, M.; Yao, J.; Liu, X.; Chen, S.; Shi, L.; Tsai, D.P.; Geng, Z.; Chen, M.K. Metalenses Phase Characterization by Multi-Distance Phase Retrieval. Light Sci. 2024, 13, 182. [Google Scholar]
- Rodenburg, J.M.; Faulkner, H.M.L. A Phase Retrieval Algorithm for Shifting Illumination. Appl. Phys. Lett. 2004, 85, 4795–4797. [Google Scholar]
- Pfeiffer, F. X-ray Ptychography. Nat. Photonics 2018, 12, 9–17. [Google Scholar]
- Lee, S.Y.; Park, E.; Kim, S.; Jo, E.; Lee, S.Y.; Choi, J.W.; Song, C. Off-Axis X-Ray Vortex Beam Ptychography. ACS Photonics 2024, 11, 3804–3810. [Google Scholar]
- Donnelly, C.; Guizar-Sicairos, M.; Scagnoli, V.; Gliga, S.; Holler, M.; Raabe, J.; Heyderman, L.J. Three-Dimensional Magnetization Structures Revealed with X-Ray Vector Nanotomography. Nature 2017, 547, 328–331. [Google Scholar] [CrossRef]
- Gao, Z.; Odstrcil, M.; Bücklein, S.; Palagin, D.; Holler, M.; Sanchez, D.F.; Krumeich, F.; Menzel, A.; Stampanoni, M.; Mestl, G. Sparse Ab Initio X-Ray Transmission Spectrotomography for Nanoscopic Compositional Analysis of Functional Materials. Sci. Adv. 2021, 7, eabf3079. [Google Scholar] [CrossRef]
- Górecki, R.; Polo, C.C.; Kalile, T.A.; Miqueles, E.X.S.; Tonin, Y.R.; Upadhyaya, L.; Meneau, F.; Nunes, S.P. Ptychographic X-Ray Computed Tomography of Porous Membranes with Nanoscale Resolution. Commun. Mater. 2023, 4, 68. [Google Scholar] [CrossRef]
- Shapiro, D.A.; Babin, S.; Celestre, R.S.; Chao, W.; Conley, R.P.; Denes, P.; Enders, B.; Enfedaque, P.; James, S.; Joseph, J.M. An Ultra-High-Resolution Soft X-Ray Microscope for Quantitative Analysis of Chemically Heterogeneous Nanomaterials. Sci. Adv. 2020, 6, eabc4904. [Google Scholar] [CrossRef]
- Aidukas, T.; Phillips, N.W.; Diaz, A.; Poghosyan, E.; Müller, E.; Levi, A.F.J.; Aeppli, G.; Guizar, S.; Holler, M. High-Performance 4-nm-Resolution X-Ray Tomography Using Burst Ptychography. Nature 2024, 632, 81–88. [Google Scholar] [CrossRef]
- Bozzini, B.; Kourousias, G.; Gianoncelli, A. In Situ Observation of Dynamic Electrodeposition Processes by Soft X-Ray Fluorescence Microspectroscopy and Keyhole Coherent Diffractive Imaging. J. Phys. D Appl. Phys. 2017, 50, 124001. [Google Scholar] [CrossRef]
- Mathiesen, R.H.; Arnberg, L.; Mo, F.; Weitkamp, T.; Snigirev, A. Time-Resolved X-Ray Imaging of Dendritic Growth in Binary Alloys. Phys. Rev. Lett. 1999, 83, 5062–5065. [Google Scholar] [CrossRef]
- Zhao, C.; Fezzaa, K.; Cunningha, R.W.; Wen, H.; Carlo, F.D.; Chen, L.; Rollett, A.D.; Sun, T. Real-Time Monitoring of Laser Powder Bed Fusion Process Using High-Speed X-Ray Imaging and Diffraction. Sci. Rep. 2017, 7, 3602. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Shoya, R.; Matsuo, T.; Sato, W.; Nakamura, T.; Takeuchi, A.; Uesugi, M. X-Ray Nanoimaging of a Transversely Embedded Carbon Fiber in Epoxy Matrix Under Static and Cyclic Loads. Sci. Rep. 2022, 12, 8843. [Google Scholar] [CrossRef] [PubMed]
- Baier, S.; Wittstock, A.; Damsgaard, C.D.; Diaz, A.; Reinhardt, J.; Shi, J.; Scherer, T.; Wang, D.; Kübel, C.; Schroerfi, C.G.; et al. Influence of Gas Atmospheres and Ceria on the Stability of Nanoporous Gold Studied by Environmental Electron Microscopy and In Situ Ptychography. RSC Adv. 2016, 6, 83031–83043. [Google Scholar] [CrossRef]
- Clark, J.N.; Huang, X.; Harder, R.J.; Robinson, I.K. Dynamic Imaging Using Ptychography. Phys. Rev. Lett. 2014, 112, 113901.1–113901.5. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, Y.; Klug, J.A.; Wojcik, M.; Maxey, E.R.; Sirica, N.S.; Roehrig, C.; Cai, Z.H.; Vogt, S.; Lai, B.; et al. Multi-Beam-Ray Ptychography for High-Throughput Coherent Diffraction Imaging. Sci. Rep. 2020, 10, 19550. [Google Scholar] [CrossRef]
- Hirose, M.; Higashino, T.; Ishiguro, N.; Takahashi, Y. Multibeam Ptychography with Synchrotron Hard X-Rays. Opt. Express 2020, 28, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Nashed, Y.S.G.; Chen, S.; Phillips, N.W.; Peterka, T.; Ross, R.; Vogt, S.; Jacobsen, C.; Vine, D.J. Continuous Motion Scan Ptychography: Characterization for Increased Speed in Coherent X-Ray Imaging. Opt. Express 2015, 23, 5438. [Google Scholar] [CrossRef]
- Pelz, P.M.; Guizar-Sicairos, M.; Thibault, P.; Johnson, I.; Holler, M.; Menzel, A. On-the-Fly Scans for X-Ray Ptychography. Appl. Phys. Lett. 2014, 105, 251101. [Google Scholar] [CrossRef]
- Clark, J.N.; Huang, X.; Harder, R.J.; Robinson, I.K. Continuous Scanning Mode for Ptychography. Opt. Lett. 2014, 39, 6066–6069. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Wu, Z.; Tao, X.; Tian, L.; Liu, G.; Tian, Y.; Guan, Y. Large Step Size Fly-Scan Ptychography Using Local Sample Separation Reconstruction. Opt. Lasers Eng. 2024, 180, 107950. [Google Scholar] [CrossRef]
- Kharitonov, K.; Mehrjoo, M.; Ruiz-Lopez, M.; Keitel, B.; Kreis, S.; Gang, S.G.; Pan, R.; Marras, A.; Correa, J.; Wunderer, C.B. Single-Shot Ptychography at a Soft X-Ray Free-Electron Laser. Sci. Rep. 2022, 12, 14430. [Google Scholar] [CrossRef]
- Pan, X.; Liu, C.; Zhu, J. Single-shot ptychographical iterative engine based on multi-beam illumination. Appl. Phys. Lett. 2013, 103, 171105. [Google Scholar] [CrossRef]
- Tao, X.; Xu, Z.; Liu, H.; Wang, C.; Xing, Z. Spatially correlated coherent diffractive imaging method. Appl. Opt. 2018, 57, 6527–6533. [Google Scholar] [CrossRef]
- Lo, Y.H.; Zhao, L.; Gallagher, J.M.; Rana, A.; Lodico, J.J.; Xiao, W.; Regan, B.C.; Miao, J. In situ coherent diffractive imaging. Nat. Commun. 2018, 9, 1826. [Google Scholar] [CrossRef]
- Zhang, F.C.; Chen, B.; Morrison, G.R.; Vila, C.J.; Robinson, I.K. Phase retrieval by coherent modulation imaging. Nat. Commun. 2016, 7, 13367. [Google Scholar] [CrossRef]
- Takayama, Y.; Fukuda, K.; Kawashima, M.; Aoi, Y.; Kagoshima, Y. Dynamic nanoimaging of extended objects via hard X-ray multiple-shot coherent diffraction with projection illumination optics. Commun. Phys. 2021, 4, 48. [Google Scholar] [CrossRef]
- Clark, J.N.; Putkunz, C.T.; Curwood, E.K.; Curwood, D. Dynamic sample imaging in coherent diffractive imaging. Opt. Lett. 2011, 36, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.F.; Tanksalvala, M.; Shanblatt, E.R.; Zhang, X.; Galloway, B.R.; Porter, C.L.; Karl, R., Jr.; Bevis, C.; Adams, D.E.; Kapteyn, H.C.; et al. Subwavelength coherent imaging of periodic samples using a 13.5 nm tabletop high-harmonic light source. Nat. Photonics 2017, 11, 259–263. [Google Scholar] [CrossRef]
- Thelen, B.J.; Fienup, J.R.; Crimmins, T.R. Improved bounds on object support from autocorrelation support and application to phase retrieval. J. Opt. Soc. Am. A 1990, 7, 3–13. [Google Scholar]
- Marchesini, S.; He, H.; Chapman, H.N.; Hauriege, S.P.; Noy, A.; Howells, M.R.; Weierstall, U.; Spence, J.C.H. X-ray image reconstruction from a diffraction pattern alone. Phys. Rev. B 2003, 68, 140101. [Google Scholar] [CrossRef]
- Nugent, K.A.; Peele, A.G.; Quiney, H.M.; Chapman, H.N. Diffraction with wavefront curvature: A path to unique phase recovery. Acta Crystallogr. 2005, 61, 373–381. [Google Scholar]
- Maiden, A.M.; Rodenburg, J.M. An improved ptychographical phase retrieval algorithm for diffractive imaging. Ultramicroscopy 2009, 109, 1256–1262. [Google Scholar] [CrossRef]
- Wang, C.P.; Xu, Z.J.; Liu, H.G.; Tao, X.; Tai, R.Z. Soft X-ray ptychography method at SSRF. Nucl. Sci. Tech. 2017, 28, 9. [Google Scholar] [CrossRef]
- Qin, Z.; Xu, Z.; Li, R.; Liu, H.; Liu, S.; Wen, Q.; Chen, X.; Zhang, X.; Tai, R. Initial probe function construction in ptychography based on zone-plate optics. Appl. Opt. 2023, 62, 3542–3550. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, K.L.; Ahn, K.; Yoon, T.; Lee, T.I.; Kim, T.S. Thermal expansion behavior of thin films expanding freely on water surface. Sci. Rep. 2019, 9, 7071. [Google Scholar] [CrossRef]
- Vu, T.-S.; Ha, M.-Q.; Bachtiar, A.M.; Dao, D.-A.; Tran, T.; Kino, H.; Takazawa, S.; Ishiguro, N.; Sasaki, Y.; Abe, M.; et al. PID3Net: A Deep Learning Approach for Single-Shot Coherent X-ray Diffraction Imaging of Dynamic Phenomena. Npj. Comput. Mater. 2025, 11, 66. [Google Scholar]
- Van Der Schot, G.; Svenda, M.; Maia, F.R.N.C.; Hantke, M.F.; DePonte, D.P.; Seibert, M.M.; Aquila, A.; Schulz, J.; Kirian, R.; Liang, M.; et al. Imaging Single Cells in a Beam of Live Cyanobacteria with an X-ray Laser. Nat. Commun. 2015, 6, 5704. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xu, Z.; Zhao, B.; Zhang, X.; Li, R.; Chen, S.; Wu, S. Dynamic Coherent Diffractive Imaging with Modulus Enforced Probe and Low Spatial Frequency Constraints. Sensors 2025, 25, 2323. https://doi.org/10.3390/s25072323
Zhang Y, Xu Z, Zhao B, Zhang X, Li R, Chen S, Wu S. Dynamic Coherent Diffractive Imaging with Modulus Enforced Probe and Low Spatial Frequency Constraints. Sensors. 2025; 25(7):2323. https://doi.org/10.3390/s25072323
Chicago/Turabian StyleZhang, Yingling, Zijian Xu, Bo Zhao, Xiangzhi Zhang, Ruoru Li, Sheng Chen, and Shuhan Wu. 2025. "Dynamic Coherent Diffractive Imaging with Modulus Enforced Probe and Low Spatial Frequency Constraints" Sensors 25, no. 7: 2323. https://doi.org/10.3390/s25072323
APA StyleZhang, Y., Xu, Z., Zhao, B., Zhang, X., Li, R., Chen, S., & Wu, S. (2025). Dynamic Coherent Diffractive Imaging with Modulus Enforced Probe and Low Spatial Frequency Constraints. Sensors, 25(7), 2323. https://doi.org/10.3390/s25072323





