A Multi-Modal Light Sheet Microscope for High-Resolution 3D Tomographic Imaging with Enhanced Raman Scattering and Computational Denoising
Abstract
:1. Introduction
2. Materials and Methods
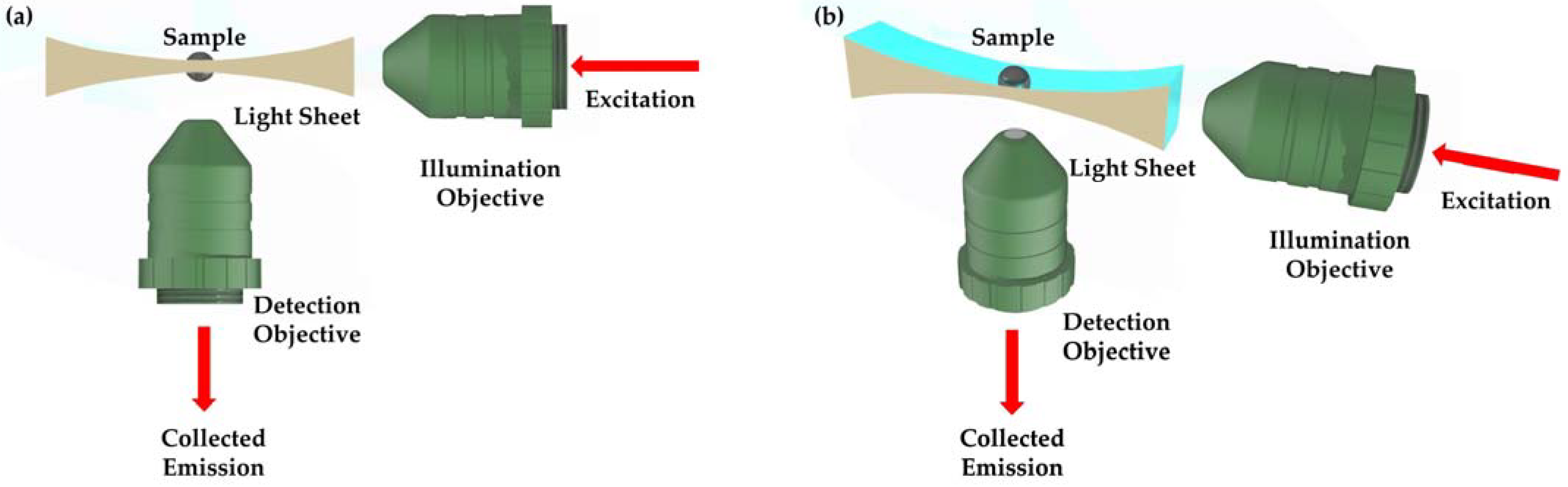
2.1. Measurement Setup Design
2.1.1. Excitation Lasers
2.1.2. Beam Shaping and Light Sheet Formation
2.1.3. Spectral Filtering and Detection System
2.1.4. Calibration and System Validation
2.2. Sample Preparation and Alignment
2.2.1. Sample Preparation
2.2.2. Hydrogel Embedding
2.2.3. Positioning System
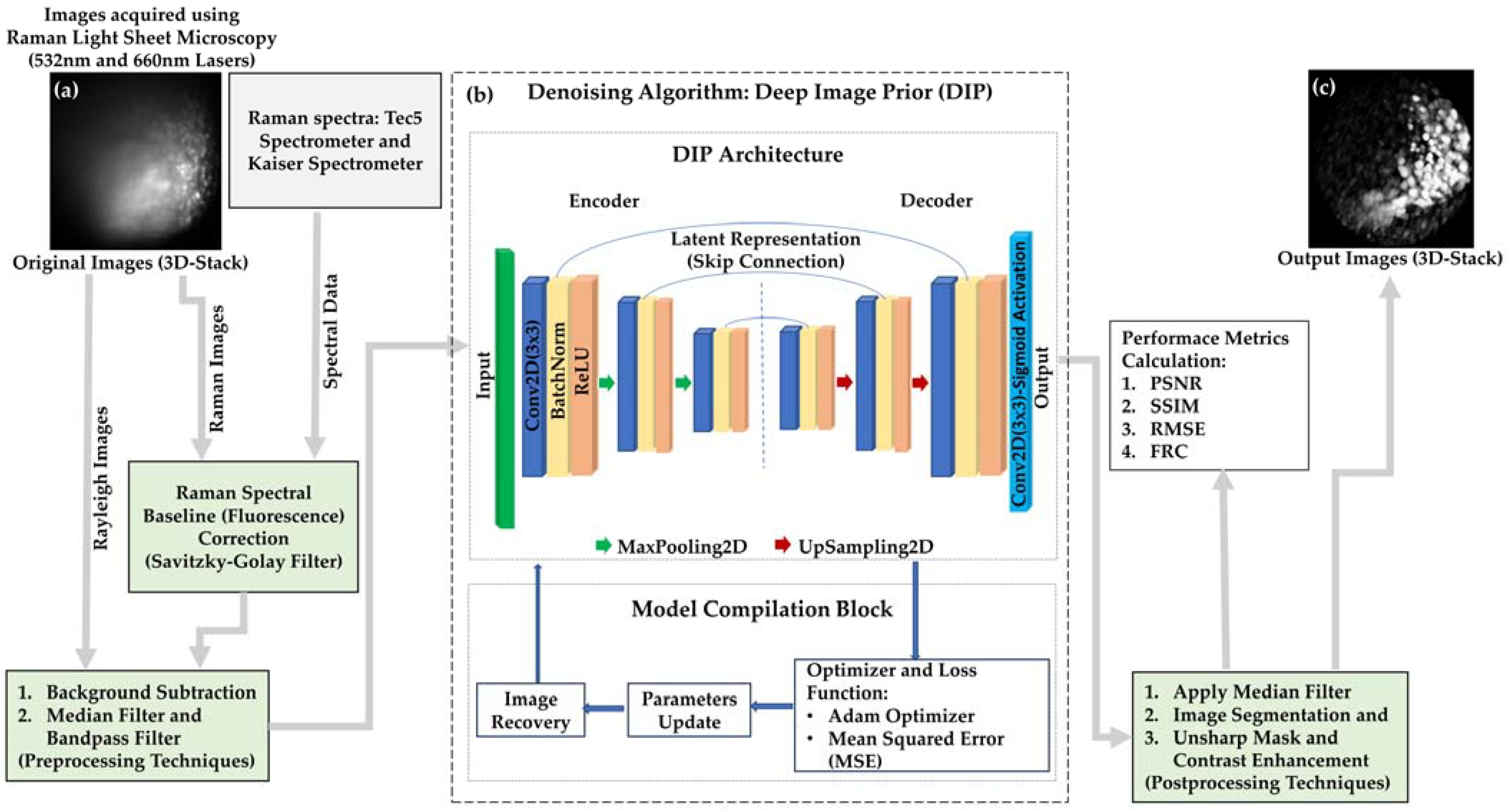
2.3. Imaging Workflow and Computational Processing
3. Results
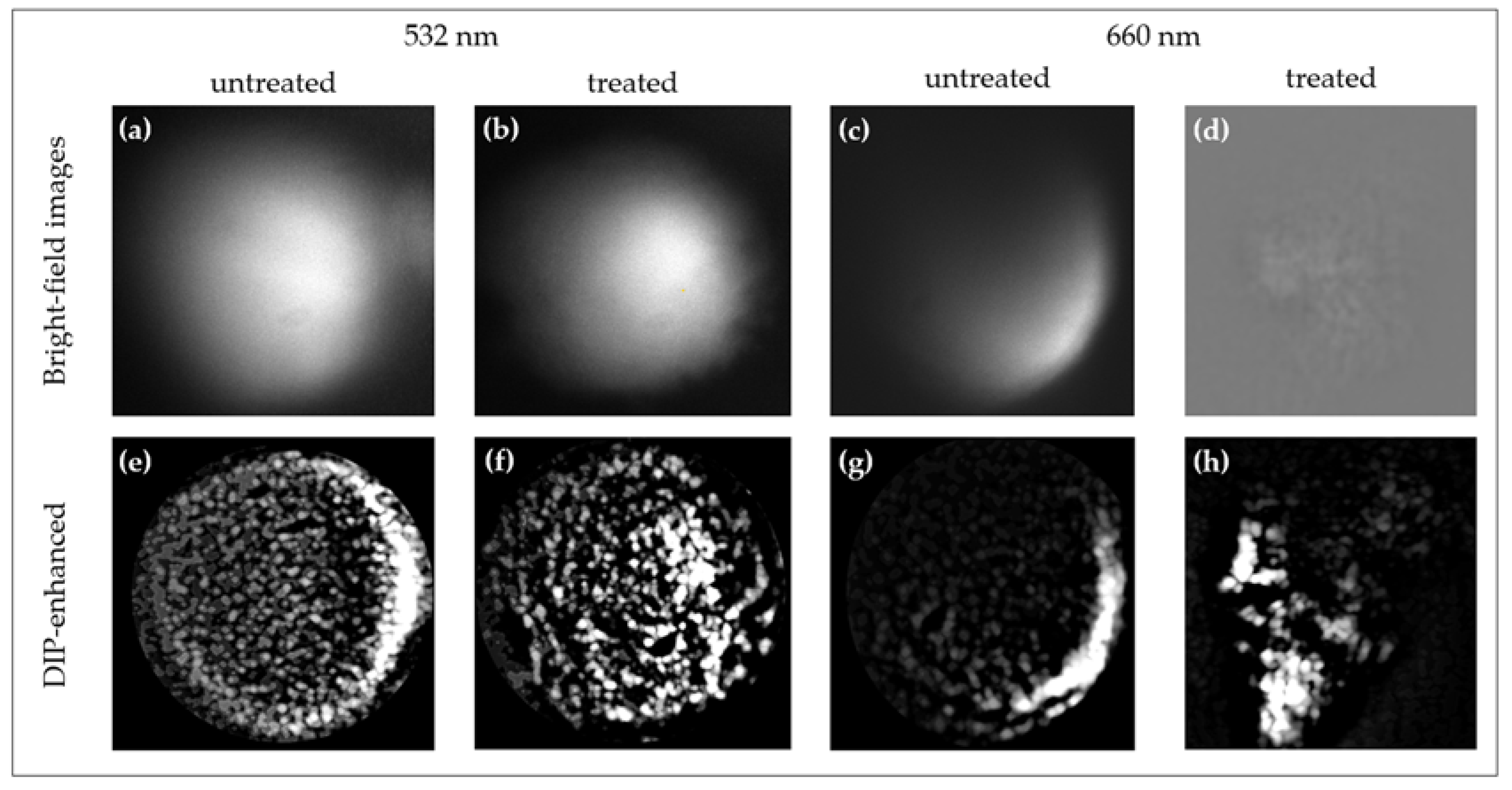
3.1. Comparative Analysis of Cisplatin-Induced Structural and Molecular Alterations in Spheroids Using 660 nm and 532 nm Excitation
3.2. Improved 3D Molecular Imaging in Visible-Range Raman Light Sheet Microscopy
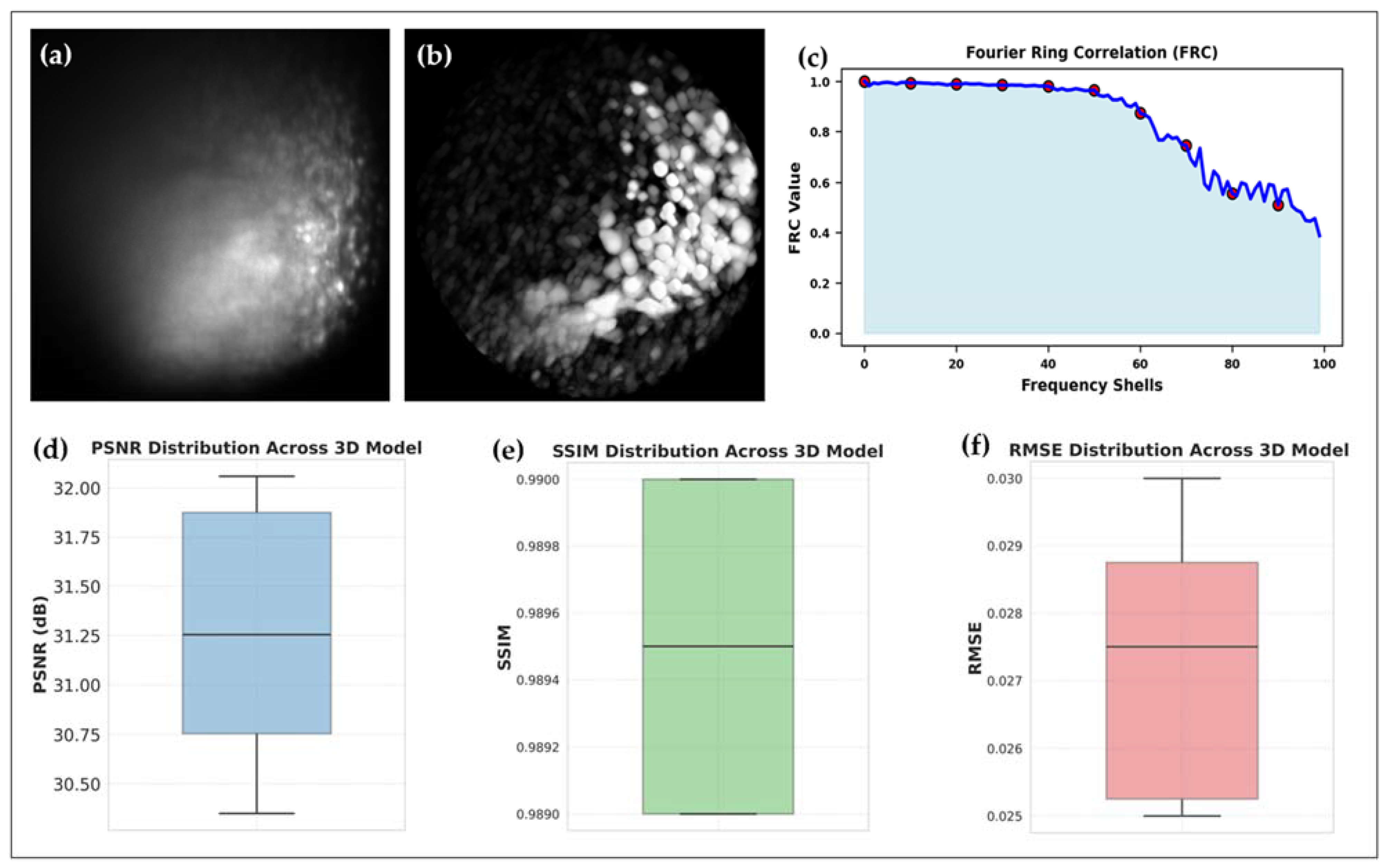
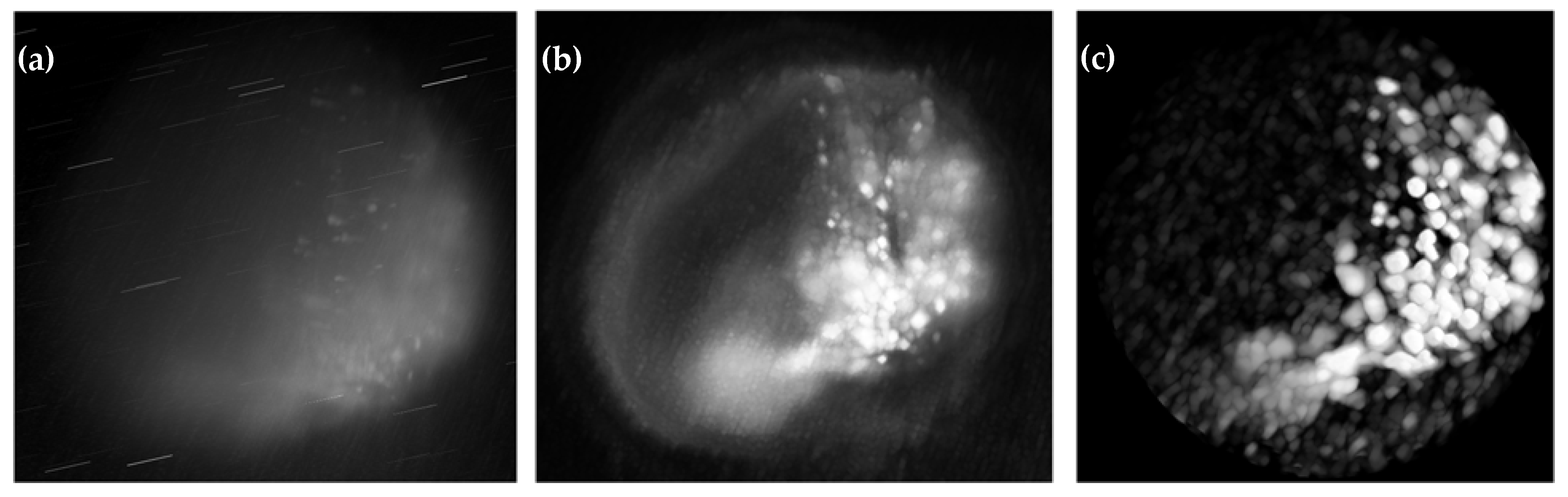
3.2.1. Volumetric Reconstructions: Pre- and Post-DIP-Enhanced 3D Reconstruction of HT29 and UMSCC-14C Spheroids
3.2.2. Fluorescence-Reduced 3D Raman Imaging of Cisplatin-Treated UMSCC-14C Spheroids
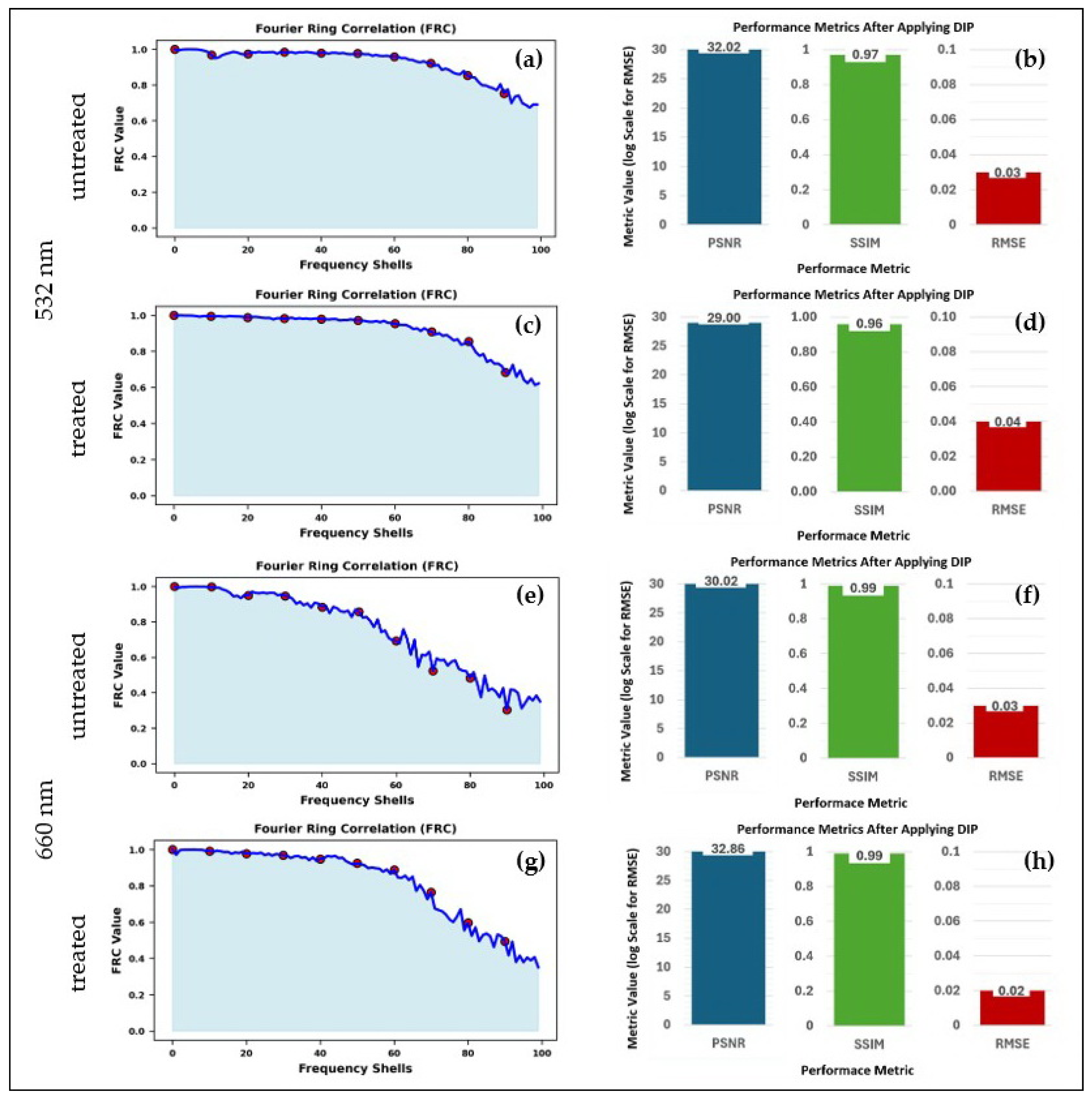
3.3. Evaluating the Role of Pre- and Post-Processing in 3D Raman Light Sheet Reconstruction
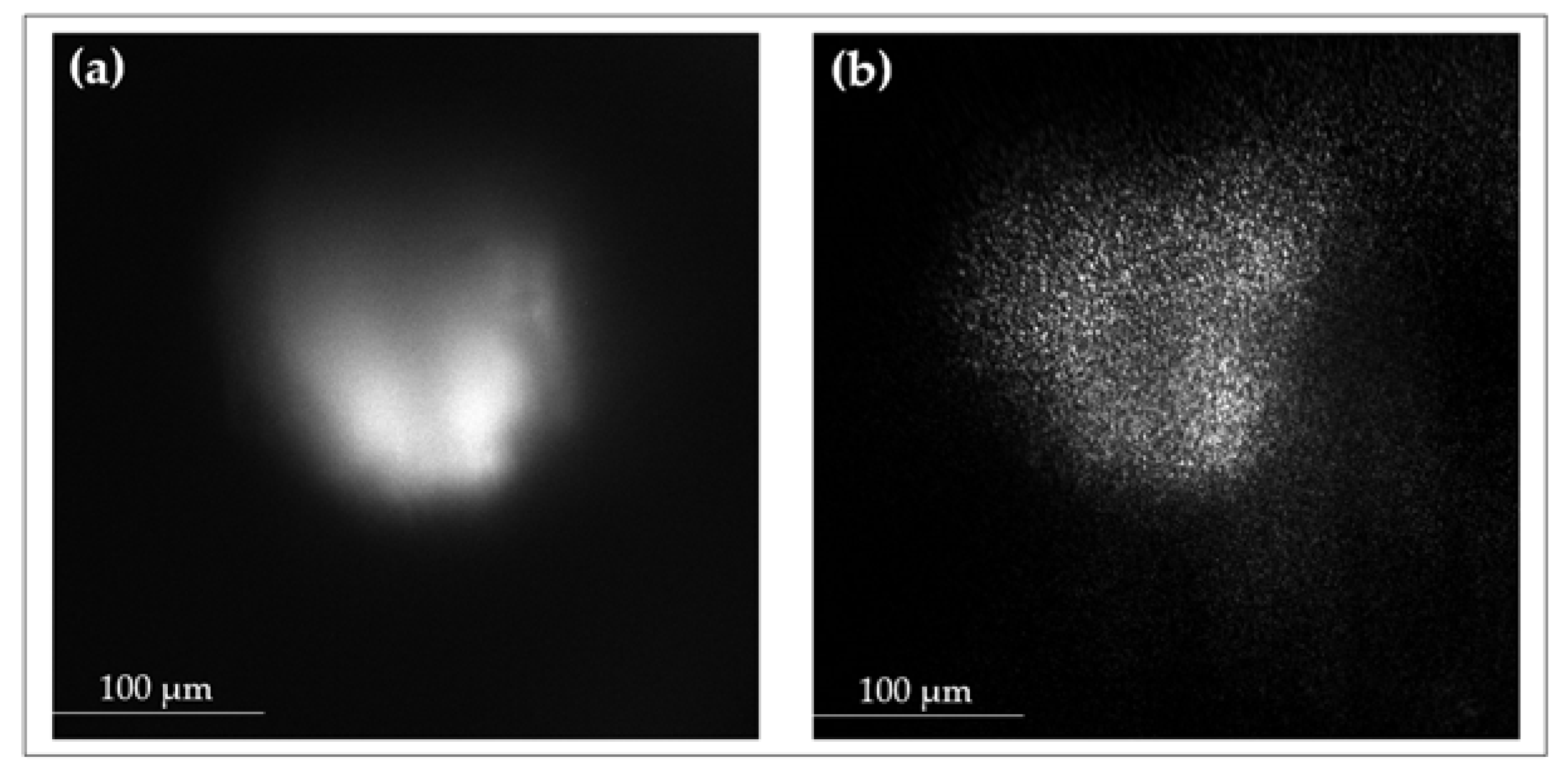
3.4. Complementary Modalities: Rayleigh and Fluorescence Imaging
4. Discussion
4.1. Advancing Raman Imaging with Visible-Wavelength Excitation
4.2. DIP Algorithm: Overcoming Limitations of Visible Excitation
4.3. Biochemical and Structural Insights from Cisplatin-Treated Spheroids
4.4. Comparative Performance and Multi-Modal Integration
4.5. Broader Implications and Future Directions
4.6. Challenges and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikla, V.I.; Mikla, V.V. Medical Imaging Technology; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef] [PubMed]
- Procházka, M. Surface-Enhanced Raman Spectroscopy: Bioanalytical, Biomolecular, and Medical Applications; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Lui, H.; Zhao, J.; McLean, D.; Zeng, H. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012, 72, 2491–2500. [Google Scholar] [CrossRef]
- Zhao, J.; Lui, H.; McLean, D.I.; Zeng, H. Integrated real-time Raman system for clinical in vivo skin analysis. Skin Res. Technol. 2008, 14, 484–492. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, H.; Hamzavi, I.; McLean, D.I.; Lui, H. Rapid near-infrared Raman spectroscopy system for real-time in vivo skin measurements. Opt. Lett. 2001, 26, 1782–1784. [Google Scholar] [CrossRef]
- Huisken, J.; Stainier, D.Y.R. Selective plane illumination microscopy techniques in developmental biology. Development 2009, 136, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Ashok, P.C.; Praveen, B.B.; Dholakia, K.; Herrington, C.S. Multi-modal approach using Raman spectroscopy and optical coherence tomography for the discrimination of colonic adenocarcinoma from normal colon. Biomed. Opt. Express 2013, 4, 2179–2186. [Google Scholar] [CrossRef]
- Tatarkovič, M.; Synytsya, A.; Setnička, V. The minimizing of Fluorescence background in Raman optical activity and Raman spectra of human blood plasma. Anal. Bioanal. Chem. 2015, 407, 1335–1342. [Google Scholar] [CrossRef]
- Beier, B.D.; Berger, A.J. Method for automated background subtraction from Raman spectra containing known contaminants. Analyst 2009, 134, 1198. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2014, 230, 16–26. [Google Scholar] [CrossRef]
- Macleod, M.; Michie, S.; Roberts, I.; Dirnagl, U.; Chalmers, I.; Ioannidis, J.; Salman, R.; Chan, A.; Glasziou, P. Biomedical reseach: Increasing value, reducing waste. Lancet 2014, 383, 101–104. [Google Scholar] [CrossRef]
- Pampaloni, F.; Chang, B.-J.; Stelzer, E. Light sheet-based fluorescence microscopy (LSFM) for the quantitative imaging of cells and tissues. Cell Tissue Res. 2015, 360, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Olarte, O.; Andilla, J.; Gualda, E.; Loza-Alvarez, P. Light-sheet microscopy: A tutorial. Adv. Opt. Photonics 2018, 10, 111–179. [Google Scholar] [CrossRef]
- Girkin, J.; Carvalho, M. The light-sheet microscopy revolution. J. Opt. 2018, 20, 053002. [Google Scholar] [CrossRef]
- Weber, M.; Mickoleit, M.; Huisken, J. Chapter 11—Light sheet microscopy. Methods Cell Biol. 2014, 123, 193–215. [Google Scholar]
- Mueller, W.; Kielhorn, M.; Schmitt, M.; Popp, J.; Heintzmann, R. Light Sheet Raman micro-spectroscopy. Optica 2016, 3, 452–457. [Google Scholar] [CrossRef]
- Kubitscheck, U. Fluorescence Microscopy: From Principles to Biological Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KgaA: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Kumari, P.; Keck, S.; Sohn, E.; Kern, J.; Raedle, M. Advanced Imaging Integration: Multi-modal Raman Light Sheet Microscopy Combined with Zero-Shot Learning for Denoising and Super-Resolution. Sensors 2024, 24, 7083. [Google Scholar] [CrossRef]
- Manser, S.; Keck, S.; Vitacolonna, M.; Wühler, F.; Rudolf, R.; Raedle, M. Innovative Imaging Techniques: A Conceptual Exploration of Multi-modal Raman Light Sheet Microscopy. Micromachines 2023, 14, 1739. [Google Scholar] [CrossRef]
- Kumari, P.; Kern, J.; Raedle, M. Self-Supervised and Zero-Shot Learning in Multi-Modal Raman Light Sheet Microscopy. Sensors 2024, 24, 8143. [Google Scholar] [CrossRef]
- Meyer, L.; Maier, A.; Deserno, T.M.; Handels, H.; Maier-Hein, K.; Palm, C.; Tolxdorff, T. Deep Image Prior for Spatio-Temporal Fluorescence Microscopy Images DECO-DIP. In Bildverarbeitung für die Medizin 2024; BVM 2024. Informatik aktuell; Springer Vieweg: Wiesbaden, Germany, 2024. [Google Scholar] [CrossRef]
- Qiao, C.; Zeng, Y.; Meng, Q.; Chen, X.; Chen, H.; Jiang, T.; Wei, R.; Guo, J.; Fu, W.; Lu, H.; et al. Zero-shot learning enables instant denoising and super-resolution in optical Fluorescence microscopy. Nat. Commun. 2024, 15, 4180. [Google Scholar] [CrossRef]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P.C. Super-resolution microscopy demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, S.; Li, L.; Huang, X.; Xing, S.; Zhang, Y.; Qiu, G.; Han, Z.; Shang, Y.; Sun, D.-E.; et al. Sparse deconvolution improves the resolution of live-cell super-resolution Fluorescence microscopy. Nat. Biotechnol. 2022, 40, 606–617. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, J.; Liu, L.; Gao, Y.; Yu, J.; Ye, S.; Li, H.; Zheng, W. Self-supervised deep-learning two-photon microscopy. Photon. Res. 2023, 11, 1–11. [Google Scholar] [CrossRef]
- Edwards, H.G.M. Spectra–Structure Correlations in Raman Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Peike, C.; Kaltenbach, T.; Weiß, K.-A.; Koehl, M. Non-destructive degradation analysis of encapsulants in PV modules by Raman Spectroscopy. Sol. Energy Mater. Sol. Cells 2011, 95, 1686–1693. [Google Scholar] [CrossRef]
- Hill, I.; Levin, I. Vibrational spectra and carbonhydrogen stretching mode assignments for a series of n-alkyl carboxylic acids. J. Chem. Phys. 1979, 70, 842–851. [Google Scholar] [CrossRef]
- Blanchet, S.; Moisan, L. An explicit sharpness index related to visual image quality. Signal Process. Image Commun. 2006, 21, 487–509. [Google Scholar]
- You, C.; Li, G.; Zhang, Y.; Zhang, X.; Shan, H.; Li, M. CT Super-resolution GAN constrained by the identical, residual, and cycle learning ensemble (GAN-CIRCLE). IEEE Trans. Med. Imaging 2020, 39, 188–203. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, W.; Zhang, L. FFDNet: Toward a fast and flexible solution for CNN-based image denoising. IEEE Trans. Image Process. 2018, 27, 4608–4622. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gao, J.; He, K. Deepl0ck: A deep denoising model based on self-similarity learning. Int. J. Adv. Res. Artif. Intell. 2019, 8, 48–54. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Chheda, R.R.; Priyadarshi, K.; Muragodmath, S.M.; Dehalvi, F.; Kulkarni, U.; Chikkamath, S. EnhanceNet: A Deep Neural Network for Low-Light Image Enhancement with Image Restoration. In Proceedings of the 4th International Conference on Recent Trends in Machine Learning, IoT, Smart Cities and Applications, ICMISC 2023; Gunjan, V.K., Zurada, J.M., Eds.; Lecture Notes in Networks and Systems; Springer: Singapore, 2024; Volume 873. [Google Scholar]
- Pelt, D.M.; Sethian, J.A. A mixed-scale dense convolutional neural network for image analysis. Proc. Natl. Acad. Sci. USA 2018, 115, 254–259. [Google Scholar] [CrossRef]
- Ulyanov, D.; Vedaldi, A.; Lempitsky, V. Deep Image Prior. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 9446–9454. [Google Scholar]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sharma, N. Denoising of magnetic resonance images using discriminative learning-based deep convolutional neural network. Technol. Health Care 2022, 30, 145–160. [Google Scholar] [CrossRef]
- Cheng, Y.; Grigorieff, N. FRC-based criterion for the resolution of cryo-EM maps. J. Struct. Biol. 2015, 186, 199–203. [Google Scholar]
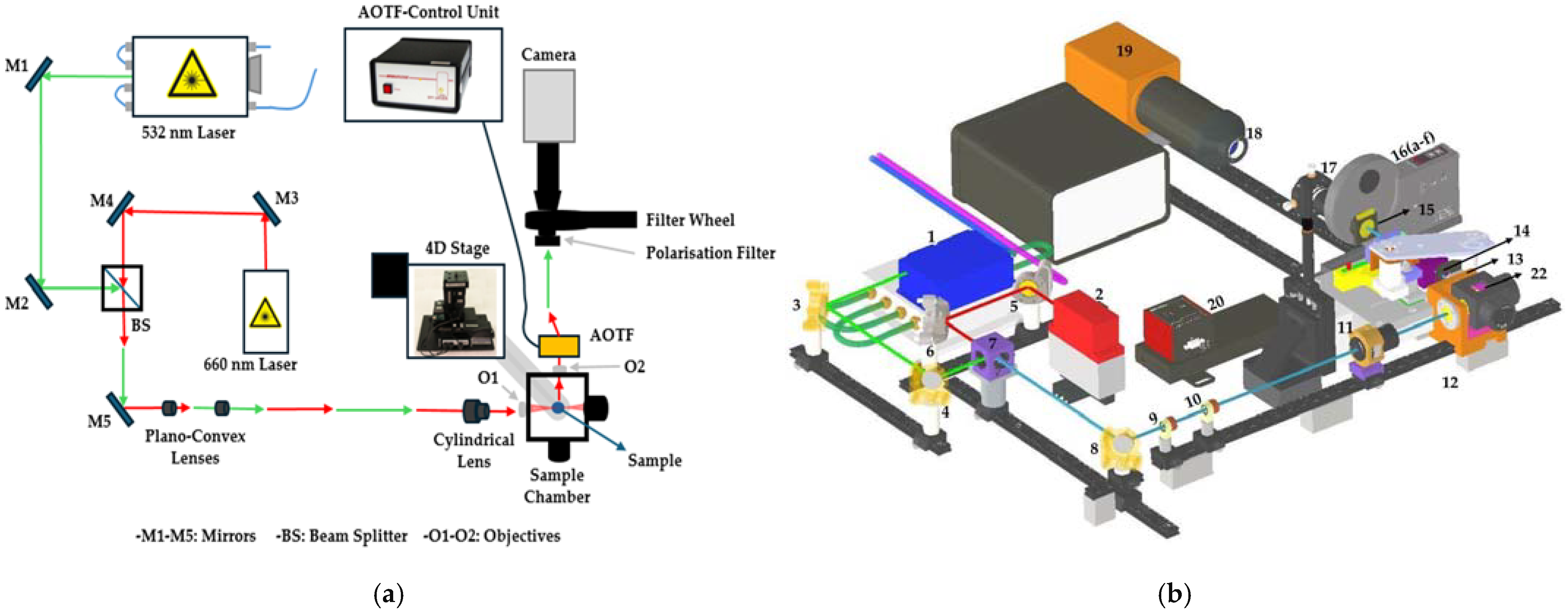


| No. | Component Specification | Manufacturer |
|---|---|---|
| Illumination Components | ||
| 1 | Gem Laser 532 nm, adjustable laser power 0.5–2000 mW | Novanta Inc. |
| 2 | LuxX Laser 660 nm, adjustable laser power 0.5–130 mW | Omicron GmbH |
| 3, 4, 8 | Broadband mirror, Ø25.4 mm, EO2 coated, mounted in Polaris K1 Kinematic Mirror Mount | Thorlabs GmbH (Lübeck, Germany) |
| 5, 6 | Broadband mirror, Ø25.4 mm, EO3 coated, mounted in Polaris K1 Kinematic Mirror Mount | Thorlabs GmbH |
| 7 | BrightLine laser dichroic beam splitter, 25.2 × 36.6 mm, reflection band 350–532 nm, transmission band 545.3–1200 nm | Semrock (New York, NY, USA) |
| 9 | Mounted achromatic doublet lens, Ø12.7 mm, focal length 25 mm, anti-reflex coating 400–1100 nm | Thorlabs GmbH |
| 10 | Mounted achromatic doublet lens, Ø12.7 mm, focal length 50 mm, anti-reflex coating 400–1100 nm | Thorlabs GmbH |
| 11 | Mounted cylindrical achromatic doublet lens, Ø25.4 mm, focal length 50 mm, anti-reflex coating 350–700 nm | Thorlabs GmbH |
| 12 | UMPLFLN10XW water dipping objective, magnification 10×, numerical aperture 0.3, working distance 3.5 mm | Evident (Hamburg, Germany) |
| Detection Components | ||
| 13 | UMPLFLN20XW water dipping objective, magnification 20×, numerical aperture 0.5, working distance 3.5 mm | Evident |
| 14 | Acousto-Optic Tunable Filter (AOTF), spectral range 400–1000 nm | Brimrose |
| 15 | Polarization Filter | Thorlabs GmbH |
| 16 16a 16b 16c 16d 16e 16f | 6-position motorized filter wheel: from position 1 to 6 in 16a–16f Filter 1: Longpass filter, 660 nm Filter 2: Notch filter, 660 nm Filter 3: Bandpass filter, 532 nm Filter 4: Shortpass filter, 660 nm Filter 5: No filter Filter 6: Longpass filter, 532 nm | Thorlabs GmbH Semrock Semrock Semrock Semrock / Semrock |
| 17 | Tube lens U-TLU and C-mount (U-TV0.5XC-3) | Evident |
| 18 | Aspheric condenser lens, Ø25 mm, focal length 20.1 mm, anti-reflex coating | Thorlabs GmbH |
| 19 | sCMOS camera ORCA Flash 4.0 LT+ | Hamamatsu (Herrsching, Germany) |
| 20 | CXY1 two-axis translating lens mount, Ø550 µm optic fiber | Thorlabs GmbH |
| 21 | USB-4D stage (X, Y, Z, R) | Picard-Industries (Albion, NY, USA) |
| 22 | Sample chamber, aluminum mounting frame, acrylic water chamber | CeMOS Research and Transfer Center (Mannheim, Germany) |
| 23 | MultiSpec®Raman spectrometer | tec5 GmbH (Steinbach, Germany) |
| 24 | Kaiser spectrometer | Kaiser Optical Systems (Germany) |
| Incident Wavelength | Modality | Optical Power | Exposure Time | AOTF Wavelength | Filter Wheel Selection | Detected Wavelength |
|---|---|---|---|---|---|---|
| 532 nm | Rayleigh Scattering | 1 mW | 100 ms | 522 nm | No filter | 532 nm |
| Raman Scattering | 350 mW | 400 ms | 631.6 nm | Longpass filter, 532 nm | 631.6 nm | |
| 660 nm | Rayleigh Scattering | 1 mW | 100 ms | 650 nm | No filter | 660 nm |
| Raman Scattering | 130 mW | 5000 ms | 815 nm | Longpass filter, 660 nm | 815 nm |
| Peak Position/cm−1 | Vibrational Mode | References |
|---|---|---|
| 3000–3400 | CH stretch | [27] |
| 2880–2895 | CH2 asymmetric stretching | [28] |
| 2929–2937 | CH3 stretching | [29] |
| Excitation Wavelength | Pre- and Postprocessing | PSNR (dB) | SSIM | RMSE |
|---|---|---|---|---|
| 532 nm (Raw) | No | 19.56 | 0.723 | 0.105 |
| 532 nm (After DIP) | Yes | 29.00 | 0.958 | 0.0354 |
| 660 nm (Raw) | No | 21.08 | 0.898 | 0.088 |
| 660 nm (After DIP) | Yes | 31.86 | 0.989 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, P.; Van Marwick, B.; Kern, J.; Rädle, M. A Multi-Modal Light Sheet Microscope for High-Resolution 3D Tomographic Imaging with Enhanced Raman Scattering and Computational Denoising. Sensors 2025, 25, 2386. https://doi.org/10.3390/s25082386
Kumari P, Van Marwick B, Kern J, Rädle M. A Multi-Modal Light Sheet Microscope for High-Resolution 3D Tomographic Imaging with Enhanced Raman Scattering and Computational Denoising. Sensors. 2025; 25(8):2386. https://doi.org/10.3390/s25082386
Chicago/Turabian StyleKumari, Pooja, Björn Van Marwick, Johann Kern, and Matthias Rädle. 2025. "A Multi-Modal Light Sheet Microscope for High-Resolution 3D Tomographic Imaging with Enhanced Raman Scattering and Computational Denoising" Sensors 25, no. 8: 2386. https://doi.org/10.3390/s25082386
APA StyleKumari, P., Van Marwick, B., Kern, J., & Rädle, M. (2025). A Multi-Modal Light Sheet Microscope for High-Resolution 3D Tomographic Imaging with Enhanced Raman Scattering and Computational Denoising. Sensors, 25(8), 2386. https://doi.org/10.3390/s25082386









